The sugarcane in Brazil is passing through a management transition that is leading to the abolition of pre-harvest burning. Without burning, large amounts of sugarcane trash is generated, and there is a discussion regarding the utilization of this biomass in the industry versus keeping it in the field to improve soil quality. To study the effects of the trash removal on soil quality, we established an experimental sugarcane plantation with different levels of trash over the soil (0%, 50% and 100% of the original trash deposition) and analyzed the structure of the bacterial and fungal community as the bioindicators of impacts. The soil DNA was extracted, and the microbial community was screened by denaturing gradient gel electrophoresis in two different seasons. Our results suggest that there are no effects from the different levels of trash on the soil chemistry and soil bacterial community. However, the fungal community was significantly impacted, and after twelve months, the community presented different structures among the treatments.
Brazil is the largest sugarcane producer in the world, with a cultivated area of 9millionha that is mainly used for the production of sugar and ethanol.1,2 Traditionally, sugarcane crops are burnt before harvest. However, this procedure results in the emission of particulate matter and smoke, resulting in poor air quality and a problem for public health.3
The government and other organizations proposed the elimination of pre-harvest burning, which resulted in the input of 10,000–30,000kg of drymassha−1 of sugarcane trash over the soil.4–6 This biomass can be used for energy generation,7,8 for cellulosic ethanol or bio-oil production,9,10 or it could be maintained on the field to improve the soil quality.11–13
Several studies have reported the influence of the burnt versus green harvest managements on the soil properties.11–22 They showed that the conversion of burnt to green harvest can positively influence several soil properties, such as C stocks, microbial biomass, soil enzyme activity and soil aggregation. It can also significantly modify the total bacterial community and the nitrifying and denitrifying gene diversity.
However, these studies involved several other factors, such as fire occurrence and the mechanical versus manual harvest, in addition to the sugarcane trash content. Therefore, there is a lack of information regarding the specific impacts of removing versus keeping the sugarcane trash on the soil. The biological attributes of soil, such as the microbial community structure, biomass, diversity, soil enzymes activities, soil respiration and qCO2, are very sensitive to land use changes and crop management. Typically, biological properties change faster when compared to physical or chemical properties,12,19,23–25 and could be used as an early evaluation of some adverse management practices, which also allows the early adoption of corrective practices.
In this context, we studied the influence of the different levels of sugarcane trash over the soil on the structure of soil bacterial and fungal communities in two contrasting seasons. We hypothesized that the sugarcane trash is an important factor influencing the microbial community in the soil, and its removal can significantly impact the structure of both the bacterial and fungal communities.
Materials and methodsField description, experiment design and samplingThe experiment site was located at Cristal Farm, property of Unialco Company, in Dourados, Mato Grosso do Sul state, Brazil. The climate of the region is Cwa, according to Ko¿ppen's classification (temperate humid with hot summer and dry winter), with an annual average temperature of 23°C and an average annual precipitation of 1635mm.
The experiment was set in a randomized block design, with three treatments and three replications. Treatments were defined as different levels of trash left over the soil (0% – all trash on the ground was removed; 50% – half of the trash was maintained; and 100% – all trash was maintained). Each plot had an area of 5m×20m. The treatments were set in January 2009, two years after the sugarcane crop establishment. The area was previously cultivated with corn, wheat and soybean.
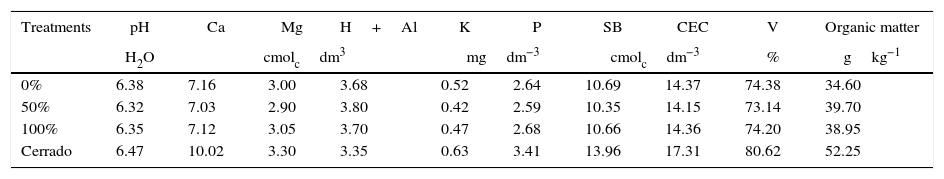
The samplings were performed in January 2010 and July 2010 during the wet and dry seasons, respectively (after 12 and 19 months after treatments implementation, respectively). In each plot, five soil samples (0–10cm) were randomly collected and combined into a composite sample. To compare the community with the natural condition of the soil, in each season, three composite soil samples were collected in an adjacent area under native Cerrado vegetation (Brazilian savana-like vegetation) and similar soil and topographic conditions. The soil of the area was classified as Rhodic Eutrudox, according to Soil Taxonomy,26 and as Latossolo Vermelho Eutroférico, according to the Brazilian System of Soil Classification (SiBCS).27Table 1 presents some soil chemical properties of the sampled soil.
Chemical characterization of the soil samples from the studied areas.
| Treatments | pH | Ca | Mg | H+Al | K | P | SB | CEC | V | Organic matter |
|---|---|---|---|---|---|---|---|---|---|---|
| H2O | cmolcdm3 | mgdm−3 | cmolcdm−3 | % | gkg−1 | |||||
| 0% | 6.38 | 7.16 | 3.00 | 3.68 | 0.52 | 2.64 | 10.69 | 14.37 | 74.38 | 34.60 |
| 50% | 6.32 | 7.03 | 2.90 | 3.80 | 0.42 | 2.59 | 10.35 | 14.15 | 73.14 | 39.70 |
| 100% | 6.35 | 7.12 | 3.05 | 3.70 | 0.47 | 2.68 | 10.66 | 14.36 | 74.20 | 38.95 |
| Cerrado | 6.47 | 10.02 | 3.30 | 3.35 | 0.63 | 3.41 | 13.96 | 17.31 | 80.62 | 52.25 |
* Available P and K; exchangeable Ca, Mg and H+Al; SB, sums of bases; CEC, cation exchange capacity; and V, percent base saturation. The exchangeable Al content was below the detection limit in all of the treatments.
Soil DNA was extracted using the FastDNA Spin Kit for Soil and a FastPrep equipment (Bio 101, CA, USA), according to the manufacturer's instructions. We used the universal primers for the domain bacteria and fungi to analyze the microbial community structure. All of the primers and cycles and PCRs used for this analysis are specified in the supplementary material (Table S1).
The amplified fragments were analyzed via denaturing gradient gel electrophoresis (DGGE),28 as described by Rachid et al.19 The gel concentration and the denaturing gradients were, respectively, 6% and 45–65% for bacteria and 8% and 30–60% for fungi.
Data analysisThe differences in the microbial community structures were analyzed by non-metric multidimensional scaling (NMS) using the PC-ORD statistical package V5 (MjM Software, Gleneden Beach, OR). The DGGE band profiles were digitalized and inserted into the data matrices using the Bionumerics v6.5 package (Applied Maths), according to the manufacturer's instructions.
Each matrix was ordinated by NMS using the Sørensen distance29 and a random initial configuration. The significance of the matrix data structure was assessed with the Monte Carlo test with randomized data. The final result of the NMS analyses was restricted to two dimensions to simplify the data analyses and discussion.
To confirm the existence of the groupings generated by the NMS analysis, we performed a blocked Multi-Response Permutation Procedure (MRPP) that tested the hypothesis that no difference exists between two or more groups of entities.30
Results and discussionThe soil chemical attribute revealed that there were almost no effects on the trash management, probably due the short-term experiment. All of the sugarcane treatments were very similar, despite the different levels of trash over the soil. The organic matter was the only chemical variable that seemed to respond to the trash managements, with a lower value in the treatment with 0% (full removal of the trash). The soil under native vegetation had higher organic matter and nutrients content than the cultivated plots, which corroborated with the natural eutrophic character of the soil sampled and the disruption of C and nutrient cycling (increased nutrient exportation) with sugar cane introduction.
The DGGE profile of the bacterial community revealed a complex and rich banding pattern in both wet and dry seasons (data not shown). The sugarcane areas presented a very similar profile, independently of the level of trash over the soil. The NMS ordination revealed the formation of two groups in taxis 1 (Fig. 1), with significant (MRPP, p<0.03) separation of the sugarcane treatments from the Cerrado, but with no significant differences within the levels of sugarcane trash (MRPP, p>0.37). These results were interesting, and some parts contrasted with those reported by Rachid et al.,19 which demonstrated differences in the bacterial communities of green cane and burnt cane. This result suggested that the differentiation of the bacterial community was more related to the other characteristics of the management (fire occurrence, manual versus mechanical harvest) than the presence of the sugarcane trash over the soil. It was expected that trash removal would influence the soil bacterial community directly (by the presence of absence of a substrate) or indirectly (by changing the soil properties, such as temperature, moisture or fertility); however, this influence was not observed in any season.
NMS ordination of the DGGE profiles of the bacterial community amplified from the soil samples (0–10cm) collected from the treatments 100% (blue, plots with all bagasse), 50% (orange, plots with half of the bagasse), 0% (red, plots with no bagasse) and Ce (green, Cerrado). The fraction of total variance that accounted for each axis is indicated in parentheses: (A) wet season and (B) dry season.
On the contrary, the fungal community had a distinct response to the treatments. The ordination of the samples collected during the wet season showed a significant separation of all of the sugarcane samples from the Cerrado samples along taxis 1 (MRPP, p<0.03). There was a significant separation of the treatment 100% (none trash removed) from the treatment 0% (all trash removed) along axis 2 (MRPP, p<0.04). The treatment 50% was not significantly different from the other sugarcane treatments.
The ordination of the fungal community from the samples collected during the dry season (Fig. 2B) presented a very similar structure to the wet season. However, the variation within each sugarcane treatment was reduced, and each group became more defined, which resulted in the significant differences among all four treatments (MRPP, p<0.03).
NMS ordination of the DGGE profiles of the fungal community amplified from the soil samples (0–10cm) collected from the treatments 100% (blue, plots with all bagasse), 50% (orange, plots with half of the bagasse), 0% (red, plots with no bagasse) and Ce (green, Cerrado). The fraction of total variance that accounted for each axis is indicated in parentheses: (A) wet season and (B) dry season.
Interestingly, the two major microbial groups had distinct responses to the treatments. The bacterial community followed the soil chemistry, and both were not influenced by the sugarcane trash. However, it is important to note that the community evaluation was qualitative; therefore, no conclusions about the effect over the population size can be concluded.
The fungal community showed a clear clusterization in response to the levels of trash on the soil. Because the soil chemistry was not changed, the effect of the sugarcane trash on the community was direct and not indirect (by transforming the environment). It was likely that the different responses were related to the capacity to use the sugarcane trash as a substrate. The sugarcane trash was a poor quality material, with a C:N ratio>70 and high C chains with up to 25% of the lignin content.6,31 Moreover, it is well known that fungi are much more efficient than bacteria in breaking long and complex C chains from recalcitrant substrates, such as sugarcane trash.32 Therefore, the fungal community was probably affected because they could use the sugarcane trash to grow, decomposing the material. From a broad point of view, the treatments with or without the trash means there were different sources of substrate for the fungal community, but not necessarily for the bacterial community, and this could explain the changes only in the one group during the timeframe of this research.
The more acute effect on the fungal community seen during the dry season in comparison to the wet season can be related to the seasonal variation,33 or it may be the result of the longer time after the treatments implementation (19 months in the dry season in contrast to 12 months in the wet season).
The difference in the microbial communities between the cultivated area in relation to the natural Cerrado area was expected, and this result highlighted the impacts of the conversion of natural vegetation to agriculture in the soil microbiology, as described before by other authors.19,20,34,35
Our results demonstrated that the sugarcane trash management significantly impacted the fungal community, but not the bacterial community. We encourage more studies evaluating these and other factors over the long-term for a deeper understanding of microbial ecology and nutrient cycling under these Cerrado conditions.
In memmoriamOn the 28th of February, 2015, Heitor L.C. Coutinho an Embrapa Soils Researcher died, a victim of cancer. He is sorely missed by his family, friends and the wider scientific community who knew and respected him. With a PhD in Biology from the University of Bristol in England, he was brilliant in diverse areas of soil ecology. Most recently, aware of his illness, he dedicated his efforts to knowledge transfer about soil quality in Brazil and Africa. Those who knew and worked closely with Heitor, admired his intelligence, his gift with words and his wisdom, especially in challenging situations. Always an optimist, always loyal, he always looked to include people and ideas, to aggregate value. We know, wherever he may be, that he is happy; we know because he was always happy when with us and this brings us comfort. Thank you for teachings, the opportunities and the example. Rest in peace our friend.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Embrapa Agropecuária Oeste for opening the experimental area to this research activity. This work received funding from the Embrapa (Macroprogram Project n. 03.09.06.011.00.005: Impact of total and partial remove of trash from sugarcane field crops under soil carbon and nitrogen dynamics, Dourados, MS, Brazil), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).