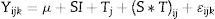In the Southern Hemisphere, ruminants are mostly raised in grazing systems where animals consume forage and are supplemented with low amounts of concentrates. Concentrates are usually given separately and are rapidly ingested. This practice leads to changing rumen environment conditions during the day, may alter the rumen microbial metabolism and could affect host performance. The native ruminal Prevotella bryantii strain 3C5 was administered every 48h to wethers under experimental conditions simulating Southern-Hemisphere feeding to evaluate its potential as a rumen fermentation modulator. The inoculum potential was assessed on day 17. The ammonia nitrogen (NH3-N), volatile fatty acids and ruminal pH were monitored on a 24-h basis 19 days after the beginning of the experiment, and the microbial community structure was assessed by pyrosequencing. The administration of P. bryantii modified the fermentation products and daily pH values compared to the control. The NH3-N concentration in the rumen of treated animals was significantly higher than that of the untreated animals. Modification of the ruminal environment and fermentation pathways was achieved without altering the general structure of the microbial community or the potential methane production. P. bryantii 3C5 could be considered in potential probiotic formulations for ruminants in semi-intensive systems.
Ruminants are raised in intensive and semi-intensive production systems that include the supplementation with protein-energy concentrates. These practices can generate a misbalance of rumen microorganisms’ metabolism, and therefore, production yields are usually affected. To overcome these limitations, feed additives such as antibiotics or ionophores have been extensively used.1 Due to the emergence risks of resistant strains and antibiotic residues in animal products and byproducts, the use of antibiotics as feed additives has been progressively restricted. The use of antibiotics has been banned in the European Union since January 2006 (Directive 1831/2003/CEE, European Commission 2003). Therefore, alternatives to the use of antibiotics have been proposed, including the use of probiotics.2,3
Probiotics, live microorganisms that confer benefits to the host,4 could be designed to modulate the rumen microbiota, improving feed utilization. This goal could be achieved by enhancing fiber and starch digestion, promoting volatile fatty acid (VFA) synthesis and diminishing or buffering lactate accumulation to avoid acidification of ruminal contents.2,5 Some of these issues have been assessed in previous studies using different native and exogenous microorganisms with diverse results.6–8
Prevotella is the most represented bacterial genus in the rumen,9 and in particular, Prevotella bryantii strains have been tested on high-producing dairy cows to evaluate the potential of the strains to prevent subacute acidosis.10,11 Although P. bryantii was not effective at preventing ruminal acidosis, the authors observed that its administration could improve the ruminal environment.
In the Southern Hemisphere, ruminants are mostly raised in grazing systems. Animals usually consume forage all day long and are supplemented with low amounts of concentrates generally provided separately from forage, once or twice a day. This practice leads to daily variations of rumen environment conditions.12,13 Therefore, in these conditions, the use of probiotics could help to stabilize the rumen environment and feed digestion.
The objective of this work was to evaluate the ability of a native P. bryantii strain to modulate the ruminal environment and fermentation and the bacterial microbiota structure in a model based on sheep consuming forage and supplemented twice a day with a high-starch concentrate.
Materials and methodsBacterial cultures and inoculum preparationP. bryantii 3C5, used in the administration trial, was previously isolated from the rumen of an only pasture-consuming cow at the Experimental Station at Libertad (San José, Uruguay), Faculty of Veterinary, University of Uruguay, and identified by sequencing of the 16S ribosomal RNA gene (JQ674698.1). This strain grew in a culture medium containing lactate as the sole carbon source and exhibited antimicrobial activity against Escherichia coli and Streptococcus bovis. Bacterial cultures were grown using a modified rumen-fluid-free broth according to Caldwell and Bryant14 (Table 1). For inoculum preparation, Pb3C5 was inoculated in CO2-gassed culture bottles containing 50mL of broth and incubated at 39°C until a density of 1×108 cells/mL was achieved.
Animals, feeding and preparation of samplesAnimals were cared for and handled according to the procedures approved by the Honorary Commission of Animal Experimentation (CHEA) of the University of Uruguay and by the National Commission of Animal Experimentation (CNEA, Uruguay). Ten eleven-month-old Corriedale wethers (Ovis aries; 33.8±4.3kg) with a rumen cannula were individually housed in metabolic cages at the Experimental Station at Libertad (San José, Uruguay), Faculty of Veterinary, University of Uruguay. Animals had access to water and were fed ad libitum on alfalfa hay. They were supplemented with cracked corn grain (1% metabolic weight basis) in two supplements, at 10 AM and 4 PM. The average intake was 1347g per day, and the forage/concentrate ratio was 4.4/1 on a dry matter basis. Animals were randomly assigned to the Control or Pb3C5 group. Every 48h, wethers in the Pb3C5 group received an intra-ruminal dose of 1×109 Pb3C5 cells in 100mL of culture medium, while animals in the Control group received 100mL of sterile medium. This experimental procedure was designed after Chiquette et al.15
Wethers were adapted to housing, diet and treatments for 16 days. On day 17, samples were taken for inoculum potential experiments. On days 19 and 20, ruminal fluid samples were taken to measure pH, ammonia nitrogen (NH3-N) and VFA for a 24h period. Additionally, on day 19, ruminal fluid was taken from each wether to evaluate the microbial community.
Inoculum potential assay and methane productionThe effect of P. bryantii 3C5 on the fermentative potential of ruminal fluid was assessed using an in vitro fermentation assay based on gas production experiments.16 Experiments were performed in 125mL fermenters containing a substrate, buffer solution,17 reductive solution and fresh ruminal fluid from each wether. The substrate (0.5g per fermenter) was a mix of alfalfa hay (70%) and corn (30%), ground to pass a 1mm sieve, with 90% dry matter, 93.4% organic matter, 34.8% neutral detergent fiber, 21.4% acid detergent fiber, and 14.6% crude protein. This substrate was allowed to hydrate with the buffer solution (38mL) and the reductive solution (2mL) for 18h at 4°C (solutions from Oeztuerk et al.17) inside the fermenters. Then, 10mL of fresh ruminal fluid, obtained from both groups of animals, was added to every fermenter. The headspace was saturated with CO2, and the fermenters were sealed. All incubations were performed in individual batches. To deduct the gas production of the rumen fluid of the donors, two were incubated without substrate addition. Incubation was performed at 39°C, and the internal pressure was measured with a manual manometer D1005PS (Ashcroft®, Stratford, USA) coupled to a 0.6mm needle. Measurements were taken at 2, 4, 8, 10, 12, 18, 24, 48, 72 and 96h after inoculation, and gas was vented after pressure readings. At times of 4, 8 and 24h, headspace gas samples from the fermenters were obtained for measuring methane concentration by GC as described before.18
Ruminal environment measuresOn day 19, ruminal fluid samples were taken every 2h, covering the first 12h of the day and then every 4h, completing the whole 24h period. The pH was measured immediately. For VFA determination, the samples were mixed 1:1 with 0.1M perchloric acid. For NH3-N analysis, rumen fluid samples were preserved with H2SO4, 50% (v/v) in a 100:1 relation. Samples were stored at −20°C until they were analyzed.
Lactic acid and VFA (acetic, propionic and butyric acids) were quantified by HPLC separation.19 The chromatogram peaks were integrated at 210nm.20 The concentration of NH3-N was determined using a selective electrode (Thermo Scientific Orion) according to manufacturer's instructions.
Structure of the ruminal bacterial microbiotaTotal DNA was extracted from 10g of contents from each animal using the method proposed by Zhou et al.21 to assess the ruminal bacterial community of wethers by massive sequencing. Extracted DNA was used to amplify and sequence the 16S rDNA V1-V2 region in a GS FLX Titanium XLR70 as in Allai et al.22 The 16S rDNA sequences generated by pyrosequencing were subsequently analyzed running the Quantitative Insights into Microbial Ecology (QIIME, version 1.8.0) per scripted modules and workflow scripts.23 The sequences were trimmed and then filtered by length (≥150bp), quality (≥25 score), and the content of either one or more ambiguous bases or a long homopolymer (>6). Operational taxonomic units (OTU) were generated by aligning the reads to the GreenGenes database24 and clustered at 97% sequence identity using the PyNAST tool23 and the UCLUST algorithm,25 respectively. An analysis of similarity was performed with QIIME using ANOSIM and ADONIS analysis.
StatisticsCumulated gas volume along time was compared among treatments (control and Pb3C5). For this purpose, the gas produced at a specific time was considered to be dependent on the preceding time. Consequently, this variable was analyzed as a repeated measure over the fermenter substrate, according to the model:
where Yijk is the volume of gas produced, μ is the overall mean, SI is the effect of Pb3C5 treatment (I=Control, Pb3C5) in k replicates (2 fermenters per wether), Tj is the fixed effect of time (j=2, 4, 6, 8, 10, 18, 26, 48, 72 and 96h), (S*T)ij is the interaction between the strain and the time and ɛijk is the residual error.All data sets of pH, NH3-N and VFA in ruminal contents were tested before statistical analysis to ensure that all the assumptions of analysis of variance (additive model, independence of errors, data normality and homoscedasticity) were met. After, they were analyzed using the MIXED procedure as repeated measures, using the wether as the subject for the repeated measurement, according to the following model:
where Yijk is the variable, μ is the general mean, Si the fixed effect of the treatment, hj is the fixed effect of the time of measurement, (S*h)ij is the interaction between treatment and time and ɛik is the residual error.The data were analyzed using SAS software (version 8.2; SAS 185 Institute, Cary, NC, USA), and differences among means with p<0.05 were considered statistically significant.
Family and genus abundance values were compared using the non-parametric Kruskal–Wallis ANOVA test.
ResultsInoculum activity and potential methane productionNo significant differences were observed between the groups in in vitro fermentation gas production associated with the addition of ruminal fluid of treated and untreated animals (ptreatment=0.0622). The methane concentrations in the fermenters were similar in both groups (p>0.05, data not shown).
Influence of added bacteria on the ruminal parameters (VFA, pH, NH3-N)Total VFA levels (considering acetic, propionic and butyric acids) tended to be higher (p=0.0622) in the ruminal fluid of the treated animals. Acetic and butyric acid concentrations were significantly higher in the Pb3C5-treated animals than in the control group (p=0.0187 and p=0.0199, respectively; Fig. 1). No differences were observed when propionic acid levels were compared between the two groups (p>0.05). Lactic acid was not detected in any sample.
The ruminal pH of the treated animals was significantly lower than that of the controls during most of the day (ptreatment=0.006), and the treatment*time interaction was not significant (p>0.05; Fig. 2).
The N-NH3 concentration in the rumen of the animals treated with Pb3C5 was also significantly higher than recorded values in the ruminal fluid of the control animals (p=0.05); the treatment*time interaction was not significant (p>0.05, Fig. 3).
Influence of added bacteria on the structure of ruminal bacterial microbiotaThe microbial community structure, assessed eight hours after the first feed offer on day 19, was similar in both groups of animals (pADONIS=0.837; pANOSIM=0.712), and when comparing the structure at different phylogenetic levels, no differences were found (p>0.05). An average 2588.6 (±645.9) sequences per animal were analyzed and were designated to 1987 OTUs. The microbiota of every animal was dominated by Bacteroidetes, representing more than 65% of all sequences. Firmicutes was the second most represented phylum in both groups of animals, while 15.8% and 18.8% of sequences in the control and treated groups, respectively, could not be classified. Prevotellacea was the most represented family in the rumen of animals in both treatment and control groups, with 56% and 50% of all the sequences, respectively.
DiscussionThe rumen bears a complex ecosystem and, the modulation of ruminal fermentation and the microbiota by probiotic bacteria is a considerable challenge. However, in this work, the ruminal environment was modulated by adding repeated low doses of the native P. bryantii 3C5 strain (Pb3C5). Administration of this strain effectively modified the VFA profile in the treated animals compared to the non-treated controls. The modulation of total VFA in the ruminal contents of treated animals could reflect a fermentative shift associated with the influence of the administered bacteria. This effect was evidenced by a significantly higher concentration of acetic and butyric acids in the Pb3C5-treated animals. It is important to note that butyric acid, which significantly increased in the treated animals, plays an important role in the rumen, being the most energetic VFA along with propionic acid.5 Butyric acid is also metabolized in the rumen mucosae and intestinal epithelium7,26,27 as an energy source and exerts mitotic and trophic effects on the host gut epithelia.28,29 These effects may enhance digestion and absorption efficiency, which may contribute to a better nutrient absorption.30
Animals that belonged to the Pb3C5-treated group showed lower ruminal pH values than those in the control group. This observation could be associated with the increase in VFA concentration. Although ruminal pH values were lower than in the control group, these values were never dangerous or harmful to the animals. Ruminants can show subclinical acidosis signs when pH values are between 5.2 and 5.6 for a long time,31 and they show acute acidosis signs when the ruminal pH reaches values lower than 5.2,1 but in our conditions, the lowest mean ruminal pH observed was 5.9.
Modifications of the rumen environment were also seen in the daily ruminal NH3-N concentration, with a higher daily concentration in the Pb3C5-treated animals than in the control group. These results are similar to those obtained when the probiotic potential of Bacillus subtilis natto was assessed using in vitro and in vivo approaches.6,32 NH3-N is a microbial protein precursor in the rumen; it is necessary for good microbial growth and microbial protein synthesis and is considered the most important source of nitrogen for protein synthesis, especially for fibrolytic bacteria.33 Generally, the NH3-N level is high when protein feedstuffs or good quality young forage are given.34 The modulation induced by Pb3C5 administration could be associated with a better digestibility of the feed and an influence on host nitrogen and protein metabolism.
These ruminal environment modifications were obtained without altering the potential methane production nor the potential based on in vitro gas production experiments.
The structure of the ruminal microbial communities in both groups of animals was similar after the administration of Pb3C5.
The microbiota of all animals was dominated by Bacteroidetes and Firmicutes phyla, and Prevotella was the most abundant genus, which is in concordance with several analyses of the ruminal microbiota performed by other authors.9,35,36
This result could be observed as a positive trait since the diversity of the rumen microbiota was not affected by the treatment. Considering these results, it is not possible to associate the predominance or the absence of certain groups of microorganisms with the changes observed in the rumen environment induced by Pb3C5 administration. In similar studies performed with human beings, researchers observed significant changes at the community transcriptome level, but they did not find any difference in the community structure.37
ConclusionIn this work, the modulation of ruminal fermentation could be achieved by the addition of repeated doses of a native bacterial strain. These doses were small when compared with the whole ruminal microbial community, but a differential fermentation pattern could be observed. Pb3C5 administration modified fermentation products, acetic and butyric acids, which could explain the differences of the daily pH values in the treated animals. Additionally, a differential pattern of NH3-N concentration in the rumen was observed. The modification of ruminal fermentation and environment was achieved by providing repeated low doses of Pb3C5. Although in this approach, the causes of the ruminal fermentation modulation could not be associated with changes in the microbiota structure, the effect may be associated with changes in ruminal microbial metabolism Additional studies should be performed to shed light on the specific activity of Pb3C5 and its possible use in probiotic formulations for ruminants in semi-intensive systems.
FundingThis study was supported by Agencia Nacional de Investigación (ANII, Uruguay) [grants numbers: PR_FMV_2009_1_2799; MOV_CA_2013_1_10852; ANII POS_2011_3374].
Authors’ contributionMF, CC and PZ initiated and designed the study. MF, SF, NP performed the experiments. KP contributed with strains. MF, SF, CC, analyzed the data. MF, CC, PZ wrote the paper. All authors read, contributed and approved the final manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Luis Vázquez, Maximiliano Pastorini, Paola Delgado, Paola San Pedro, Diego Fernández, Cristina Manzzi, Bruno Cremella, Virginia Ramón, Ernesto Rosas and Elena de Torres for their support with the animal experiments and Luis Gustavo Corbellini for helping in statistical analysis