Transcatheter aortic valve implantation has revolutionized the management of aortic stenosis and is currently an alternative to surgical aortic valve replacement. Some studies have found costs of percutaneous procedure to be greater than those of surgical replacement. However, these published studies are based on pooled data from clinical registries. The objective of the present study was to compare costs of both treatments during the hospital phase in a Spanish public hospital.
MethodsA hospital phase cost study consisted of patients diagnosed and treated with aortic stenosis in a Spanish tertiary hospital between 1st January 2019 and 29th February 2020. An individual analysis was conducted in relation to real costs pertaining to materials, staffing and hospital stay. Several baseline characteristics were controlled for.
ResultsMean costs per patient from procedure through to discharge were 26,684.1 € in the percutaneous group and 14,939.5 € in the surgical group (p<0.001). Independent of comorbidities, the cost of transcatheter aortic valve implantation was, on average, 8636.6 € greater than that of surgical aortic valve replacement (95% CI 6086.3–11,186.9; p < 0.001).
ConclusionsAortic stenosis treatment costs in hospital phase were greater when percutaneous option was used in comparison to surgery.
El implante transcatéter de la válvula aórtica ha revolucionado el tratamiento de la estenosis aórtica, siendo actualmente la única alternativa terapéutica a la cirugía de recambio valvular. Algunos estudios han encontrado que los costes del procedimiento percutáneo son superiores al quirúrgico. No obstante, los trabajos publicados utilizan datos agregados procedentes de registros clínicos. El objetivo de este estudio es comparar el coste de ambos procedimientos durante la fase hospitalaria en un hospital del sistema sanitario público español.
MétodosSe estudiaron los costes de la fase hospitalaria de los pacientes diagnosticados y tratados de estenosis aórtica mediante procedimiento percutáneo o quirúrgico en un hospital terciario español entre el 1 de enero de 2019 y el 29 de febrero de 2020. Se analizaron de forma individual los costes relacionados con el material utilizado, la estancia hospitalaria y el personal interviniente.
ResultadosEl coste medio desde la intervención hasta el alta fue de 26.684,1€ para el grupo percutáneo y de 14.939,5€ para el grupo quirúrgico (p<0,001). De forma independiente a las comorbilidades, el coste de la opción percutánea fue de media 8636.6€ superior al de la cirugía (IC 95%: 5.528-11.053,9; p<0,001).
ConclusionesEl coste del tratamiento de la estenosis aórtica en la fase hospitalaria es superior utilizando la técnica percutánea que la quirúrgica.
Transcatheter aortic valve implantation (TAVI) has revolutionized the management of aortic stenosis (AoS). Clinical trials comparing outcomes of TAVI versus surgical aortic valve replacement (SAVR) in differing surgical risk groups have established percutaneous approach as the technique of choice for high-risk patients and a solid alternative for intermediate-risk patients.1,2 With a view to prescribing TAVI in progressively numbers of patients, aspects such as economic impact of the two techniques are increasingly important, especially as different studies have consistently evidenced that TAVI is more expensive than surgery. Regarding the comparison of TAVI with medical treatment in inoperable patients, several cost and cost-effectiveness analyses have already demonstrated the superiority of the percutaneous option.3–5 Nevertheless, studies comparing TAVI and SAVR are based on national databases and registries that make use of pooled data and calculate the costs according to mean values per patient rather than on an individual basis. Furthermore, the aforementioned studies were conducted in United States, a healthcare system which differs greatly to many other countries.6–9 In this respect, Spanish healthcare system is characterized by universal, cost-free coverage of the entire population. In addition, remuneration for healthcare professionals is lower in Spain than in other European countries,10 this makes the contextual evaluation both interesting and novel. No studies to date have compared cost of TAVI versus surgical replacement in Spain. The present study compared costs of TAVI versus open surgery, using individual data, in a Spanish tertiary hospital.
MethodsStudy design and populationThe study comprised of patients previously diagnosed with severe degenerative AoS, who were subjected to either TAVI or SAVR in our Center between 1st January 2019 and 29th February 2020. Procedure date for each patient was taken as study day 0.
Inclusion criteria: Patients previously diagnosed with severe degenerative AoS and subjected to TAVI or SAVR on a native aortic valve. Patients subjected to TAVI or SAVR on a previously degenerated surgical valve were also included.
Exclusion criteria: Patients diagnosed with concomitant severe aortic regurgitation. Patients with aortic valve disease of congenital origin. Patients undergoing combined procedures (coronary bypass/SAVR or coronary angioplasty/TAVI). Patients with active infection. Patients who were considered inoperable (where TAVI was the only choice possible).
Patient baseline characteristics and intra- and postoperative clinical variables were registered on a prospective basis in hospital electronic database. The cost was individually calculated for each patient and recorded in the electronic database. Surgical aortic valve replacement was performed in an operating theater located within a surgical block, whereas TAVI was carried out in the Hemodynamics Laboratory. Use of these facilities, which included the need for staff who had been trained to perform the procedures, generated imputable costs. The Department of Economic Evaluation informed us about the cost to the Health System of a professional in ordinary working hours (7h a day) in terms of basic salary, allowances, Social Security, etc. (we received the amount for each professional category involved). The duration of each procedure is recorded in the patient's electronic medical record by the nursing staff. To calculate the cost of the staff involved per procedure, the cost/minute of each professional was multiplied by the duration in minutes of the procedure.
Cost of each patient stay within the hospital was based on the number of days spent in Intensive Care Unit (ICU), Advanced Cardiological Care Unit (ACCU), Hospitalization Unit or Rehabilitation Center (for those patients who needed, for instance due to a disability stroke). Individual costs for staying in ICU and ACCU were 1152.6 € and 858 € per day of hospital stay, respectively. In order to calculate this parameter, center's Financial Evaluation Department provided the costs attributed to human resources, material resources and the number of stays within each destination unit.
Costs of devices, items and materials were simply what the Health System pays for.
With regards to materials used during the procedures, there was a proprietary fixed materials list (which were used in each of interventions according to whether the patient was in TAVI or SAVR group) and another variable materials list which was in accordance with individual characteristics of each patient.
Fixed materials: Global products used in each case produced a cost of 629.39 € for each TAVI procedure and 527.69 € for each SAVR procedure.
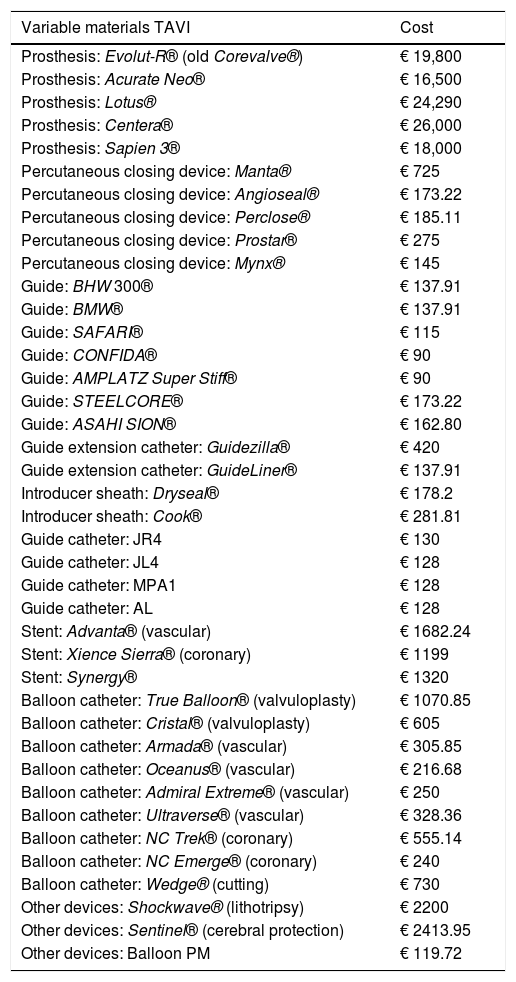
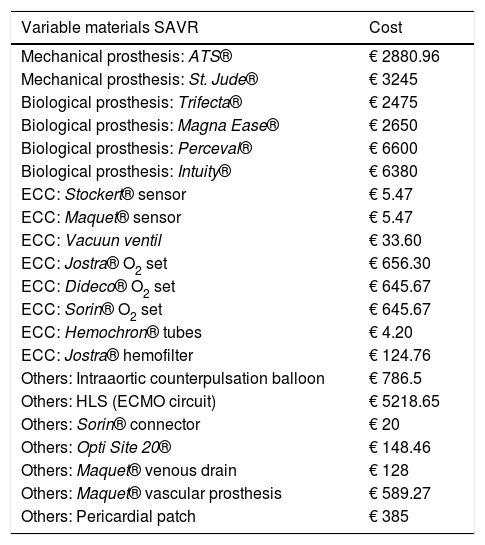
Variable materials: Variable materials mostly referred to the valve model used in each procedure, those used as guides and catheters for the TAVI, or extracorporeal circulation consumables for SAVR. Calculation was made individually for each patient according to what was actually consumed. Tables 1 and 2 reflect costs of each device used according to the therapeutic option involved.
Variable material costs for TAVI. All of these costs were taken into account.
| Variable materials TAVI | Cost |
|---|---|
| Prosthesis: Evolut-R® (old Corevalve®) | € 19,800 |
| Prosthesis: Acurate Neo® | € 16,500 |
| Prosthesis: Lotus® | € 24,290 |
| Prosthesis: Centera® | € 26,000 |
| Prosthesis: Sapien 3® | € 18,000 |
| Percutaneous closing device: Manta® | € 725 |
| Percutaneous closing device: Angioseal® | € 173.22 |
| Percutaneous closing device: Perclose® | € 185.11 |
| Percutaneous closing device: Prostar® | € 275 |
| Percutaneous closing device: Mynx® | € 145 |
| Guide: BHW 300® | € 137.91 |
| Guide: BMW® | € 137.91 |
| Guide: SAFARI® | € 115 |
| Guide: CONFIDA® | € 90 |
| Guide: AMPLATZ Super Stiff® | € 90 |
| Guide: STEELCORE® | € 173.22 |
| Guide: ASAHI SION® | € 162.80 |
| Guide extension catheter: Guidezilla® | € 420 |
| Guide extension catheter: GuideLiner® | € 137.91 |
| Introducer sheath: Dryseal® | € 178.2 |
| Introducer sheath: Cook® | € 281.81 |
| Guide catheter: JR4 | € 130 |
| Guide catheter: JL4 | € 128 |
| Guide catheter: MPA1 | € 128 |
| Guide catheter: AL | € 128 |
| Stent: Advanta® (vascular) | € 1682.24 |
| Stent: Xience Sierra® (coronary) | € 1199 |
| Stent: Synergy® | € 1320 |
| Balloon catheter: True Balloon® (valvuloplasty) | € 1070.85 |
| Balloon catheter: Cristal® (valvuloplasty) | € 605 |
| Balloon catheter: Armada® (vascular) | € 305.85 |
| Balloon catheter: Oceanus® (vascular) | € 216.68 |
| Balloon catheter: Admiral Extreme® (vascular) | € 250 |
| Balloon catheter: Ultraverse® (vascular) | € 328.36 |
| Balloon catheter: NC Trek® (coronary) | € 555.14 |
| Balloon catheter: NC Emerge® (coronary) | € 240 |
| Balloon catheter: Wedge® (cutting) | € 730 |
| Other devices: Shockwave® (lithotripsy) | € 2200 |
| Other devices: Sentinel® (cerebral protection) | € 2413.95 |
| Other devices: Balloon PM | € 119.72 |
PM: Pacemaker.
Variable material costs for SAVR. All of these costs were taken into account.
| Variable materials SAVR | Cost |
|---|---|
| Mechanical prosthesis: ATS® | € 2880.96 |
| Mechanical prosthesis: St. Jude® | € 3245 |
| Biological prosthesis: Trifecta® | € 2475 |
| Biological prosthesis: Magna Ease® | € 2650 |
| Biological prosthesis: Perceval® | € 6600 |
| Biological prosthesis: Intuity® | € 6380 |
| ECC: Stockert® sensor | € 5.47 |
| ECC: Maquet® sensor | € 5.47 |
| ECC: Vacuun ventil | € 33.60 |
| ECC: Jostra® O2 set | € 656.30 |
| ECC: Dideco® O2 set | € 645.67 |
| ECC: Sorin® O2 set | € 645.67 |
| ECC: Hemochron® tubes | € 4.20 |
| ECC: Jostra® hemofilter | € 124.76 |
| Others: Intraaortic counterpulsation balloon | € 786.5 |
| Others: HLS (ECMO circuit) | € 5218.65 |
| Others: Sorin® connector | € 20 |
| Others: Opti Site 20® | € 148.46 |
| Others: Maquet® venous drain | € 128 |
| Others: Maquet® vascular prosthesis | € 589.27 |
| Others: Pericardial patch | € 385 |
ECC: extracorporeal circulation, O2: oxygen.
In addition, costs of possible complications, such as the need for a permanent pacemaker or a repair of a vascular complication, were also taken into account. Complications were defined according to VARC-2 criteria.11
In order to establish costs at 6 months, number of visits to the emergency room and hospital admissions were taken into account, including duration of hospital stay and the type of unit to which the patient was admitted.
ObjectivesPrimary objective: To compare global cost of AoS treatment using either TAVI or SAVR during admission to a Spanish tertiary hospital.
Secondary objective: To compare costs of using either the TAVI or SAVR techniques during the first 6 months of recovery.
Statistical analysisAll the collected data were entered in a Microsoft Excel® spreadsheet, with statistical analysis undertaken using the STATA® version 16 package (StataCorp, College Station, TX, USA). Qualitative variables were reported as n (%), whilst quantitative variables were reported as mean±standard deviation in presence of a normal distribution, and as median [interquartile range] in absence of a normal distribution. Normal data distribution was checked using Shapiro–Wilk test. Qualitative variables were compared using the Fisher's exact test, except for ordinal variables where a linear trend test was used. Student t-tests were used to compare quantitative variables for independent groups in presence of a normal distribution, whereas Mann–Whitney U-test was applied for data following a non-normal distribution. Student t-test took into account equal or different variances, as applicable. Equality of variances was assessed with the Levene robust test.
Due to the observational nature of the study, it was necessary to control for all baseline characteristics that could have made an impact on the outcome (cost). Therefore, multivariate linear regression analysis was used to assess the influence of the technique employed (TAVI or SAVR) upon the total cost. A maximum model was initially generated. In this model, dependent variable was the total cost per patient, the exposure or study variable was TAVI/SAVR, and the remaining independent variables (or controlled confounding factors) were: age, sex, cardiovascular risk factors (arterial hypertension, diabetes mellitus, dyslipidemia, smoking status, obesity), comorbidities (coronary disease), left ventricular ejection fraction (LVEF), atrioventricular block, left or right bundle branch block and laboratory test parameters (hemoglobin and estimated glomerular filtration rate). After establishing the maximum model, and in an attempt to simplify the model and make it more precise (with less standard error), the possibility of eliminating confounding factors was evaluated. Two simultaneous conditions were required in order to eliminate a confounding factor: (1) elimination of the variable did not result in important modifications to the impact of the technique (TAVI or SAVR) in relation to the total economic cost: [| (coefficient b−coefficient b-adjusted)/coefficient b-adjusted | <0.10]; and (2) the new standard error of coefficient b of the variable TAVI/SAVR was required to be equal to, or smaller than, the previous error. This more simple and precise model was referred to as the reduced model.12 Statistical significance was assessed with Wald test. This procedure was carried out automatically using “confound” command for STATA®.13 Coefficient beta was interpreted as difference in total cost between TAVI and SAVR techniques, the result was reported with the corresponding 95% confidence interval (95% CI). Assumptions referred to normality of residuals, linearity, homogeneity of variances and absence of collinearity were tested. Statistical significance was considered as p<0.05 in two-tailed testing.
Ethics committeeThe study was approved by Research Ethics Committee of Asturias (Spain; project number: 2020.235). Due to the nature of this study, informed consent was not considered necessary.
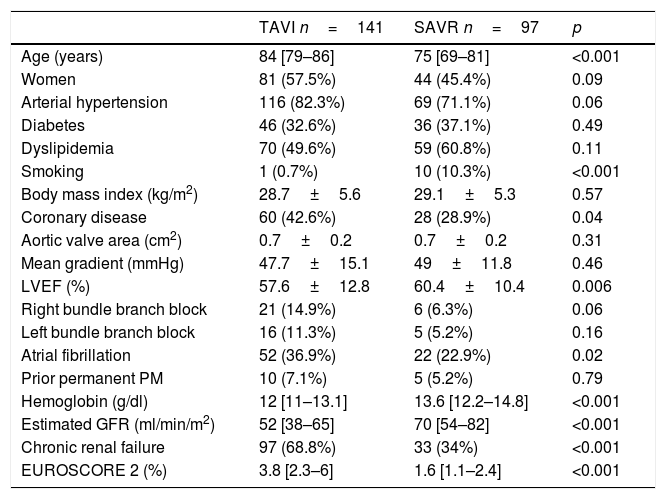
ResultsSampleA total of 238 patients met inclusion criteria during the study period. Of these, 141 underwent TAVI and 97 were subjected to SAVR. Mean patient age in TAVI and SAVR groups were 84 [79–86] and 75 years [69–81] (p<0.001), respectively. Women accounted for 57.5% (n=81) of patients in TAVI group and 45.4% (n=44) of patients in SAVR group (p=0.09).
The results from remaining baseline characteristics within the two treatment groups are shown in Table 3.
Baseline characteristics of the study sample.
| TAVI n=141 | SAVR n=97 | p | |
|---|---|---|---|
| Age (years) | 84 [79–86] | 75 [69–81] | <0.001 |
| Women | 81 (57.5%) | 44 (45.4%) | 0.09 |
| Arterial hypertension | 116 (82.3%) | 69 (71.1%) | 0.06 |
| Diabetes | 46 (32.6%) | 36 (37.1%) | 0.49 |
| Dyslipidemia | 70 (49.6%) | 59 (60.8%) | 0.11 |
| Smoking | 1 (0.7%) | 10 (10.3%) | <0.001 |
| Body mass index (kg/m2) | 28.7±5.6 | 29.1±5.3 | 0.57 |
| Coronary disease | 60 (42.6%) | 28 (28.9%) | 0.04 |
| Aortic valve area (cm2) | 0.7±0.2 | 0.7±0.2 | 0.31 |
| Mean gradient (mmHg) | 47.7±15.1 | 49±11.8 | 0.46 |
| LVEF (%) | 57.6±12.8 | 60.4±10.4 | 0.006 |
| Right bundle branch block | 21 (14.9%) | 6 (6.3%) | 0.06 |
| Left bundle branch block | 16 (11.3%) | 5 (5.2%) | 0.16 |
| Atrial fibrillation | 52 (36.9%) | 22 (22.9%) | 0.02 |
| Prior permanent PM | 10 (7.1%) | 5 (5.2%) | 0.79 |
| Hemoglobin (g/dl) | 12 [11–13.1] | 13.6 [12.2–14.8] | <0.001 |
| Estimated GFR (ml/min/m2) | 52 [38–65] | 70 [54–82] | <0.001 |
| Chronic renal failure | 97 (68.8%) | 33 (34%) | <0.001 |
| EUROSCORE 2 (%) | 3.8 [2.3–6] | 1.6 [1.1–2.4] | <0.001 |
GFR: glomerular filtration rate; LVEF: left ventricle ejection fraction; PM: Pacemaker.
The team involved in TAVI consisted of two hemodynamists and three nurses (minimalist technique), except for transaxillary approach. For this technique, orotracheal intubation was used and an anesthesiologist was present. Transaxillary approach was only used in 12 (8.51%) patients. Patients who underwent general anesthesia were generally extubated in the Cath Lab and had a post-procedure routine similar to patients with a minimalist approach.
The team involved in SAVR consisted of two surgeons, an anesthetist, three nurses and a perfusionist. Mean duration of TAVI intervention was 158min [148–160], which increased to 261min [248–263] for SAVR procedure (p<0.001).
106 patients (75.2%) in TAVI group received Evolut® and 25 (17.7%) received Acurate®.
In the surgical group, a biological prosthesis was implanted in 87 patients (89.7%), and the most frequently used prosthesis was Trifecta® (33 cases; 34%). In addition, sutureless prostheses were used in 31 (32%) patients of whom 20 were female (64.5%) with a median age of 78 [73–83] years.
Post-procedure periodIn-hospital mortality was similar between both groups: 5 (3.6%) vs 3 (3.1%), (p=1) for TAVI and SAVR, respectively. Myocardial infarction rates were 0 (0%) and 3 (3.1%) (p=0.07) for TAVI and SAVR, respectively.
There were 1 (0.7%) life-threatening or disabling bleeding in TAVI group and 4 (4.1%) in SAVR group (p=0.16).
In surgical group, the most frequent complication was new onset atrial fibrillation, which occurred in 21 (21.7%) patients. A post-procedure left bundle block was more frequently observed in TAVI group: 59 (42.1%) versus 14 (14.4%), (p<0.001), and patients in TAVI group also required more permanent pacemaker implantation: 21 (14.9%) vs 6 (6.2%), (p=0.04).
TAVI group presented higher hemoglobine (10.1mg/dl [9–11.2] versus 8.3mg/dl [7.8–9.2]; p<0.001) and lower estimated glomerular filtration rate values (55ml/min/m2 [41–77] versus 75ml/min/m2 [60–90]; p<0.001) than the SAVR group.
Following procedure, red cell concentrates were required by 16 patients in TAVI group (11.4%), and 49 patients of the SAVR group (50.5%) (p<0.001).
Hospital stay was 6 days [4–8] versus 11 days [9–15] (p<0.001) for the TAVI and SAVR group, respectively. UCI stay was 0 days versus 3 days [2–4] for TAVI and SAVR (p<0.001), respectively. ACCU stay was 0.5 days [0.5–1] versus 0 [0–0] days (p<0.001), respectively. Hospital ward stay was 5 days [3.5–7.5] versus 8 days [6–11] (p<0.001), respectively. Finally, the stay in a rehabilitation unit was 0.8 days±4.7 versus 0.9 days±4.9 (p=0.82).
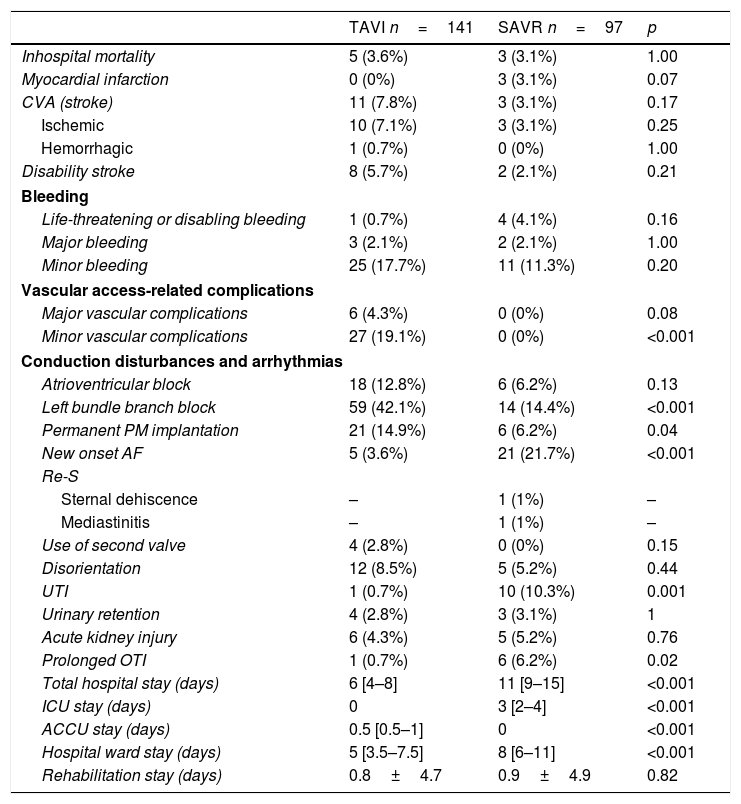
Table 4 shows the rest of variables related to post-procedure period.
Post-procedure variables.
| TAVI n=141 | SAVR n=97 | p | |
|---|---|---|---|
| Inhospital mortality | 5 (3.6%) | 3 (3.1%) | 1.00 |
| Myocardial infarction | 0 (0%) | 3 (3.1%) | 0.07 |
| CVA (stroke) | 11 (7.8%) | 3 (3.1%) | 0.17 |
| Ischemic | 10 (7.1%) | 3 (3.1%) | 0.25 |
| Hemorrhagic | 1 (0.7%) | 0 (0%) | 1.00 |
| Disability stroke | 8 (5.7%) | 2 (2.1%) | 0.21 |
| Bleeding | |||
| Life-threatening or disabling bleeding | 1 (0.7%) | 4 (4.1%) | 0.16 |
| Major bleeding | 3 (2.1%) | 2 (2.1%) | 1.00 |
| Minor bleeding | 25 (17.7%) | 11 (11.3%) | 0.20 |
| Vascular access-related complications | |||
| Major vascular complications | 6 (4.3%) | 0 (0%) | 0.08 |
| Minor vascular complications | 27 (19.1%) | 0 (0%) | <0.001 |
| Conduction disturbances and arrhythmias | |||
| Atrioventricular block | 18 (12.8%) | 6 (6.2%) | 0.13 |
| Left bundle branch block | 59 (42.1%) | 14 (14.4%) | <0.001 |
| Permanent PM implantation | 21 (14.9%) | 6 (6.2%) | 0.04 |
| New onset AF | 5 (3.6%) | 21 (21.7%) | <0.001 |
| Re-S | |||
| Sternal dehiscence | – | 1 (1%) | – |
| Mediastinitis | – | 1 (1%) | – |
| Use of second valve | 4 (2.8%) | 0 (0%) | 0.15 |
| Disorientation | 12 (8.5%) | 5 (5.2%) | 0.44 |
| UTI | 1 (0.7%) | 10 (10.3%) | 0.001 |
| Urinary retention | 4 (2.8%) | 3 (3.1%) | 1 |
| Acute kidney injury | 6 (4.3%) | 5 (5.2%) | 0.76 |
| Prolonged OTI | 1 (0.7%) | 6 (6.2%) | 0.02 |
| Total hospital stay (days) | 6 [4–8] | 11 [9–15] | <0.001 |
| ICU stay (days) | 0 | 3 [2–4] | <0.001 |
| ACCU stay (days) | 0.5 [0.5–1] | 0 | <0.001 |
| Hospital ward stay (days) | 5 [3.5–7.5] | 8 [6–11] | <0.001 |
| Rehabilitation stay (days) | 0.8±4.7 | 0.9±4.9 | 0.82 |
AF: atrial fibrillation; ACCU: advanced cardiac care unit; CVA: cerebrovascular accident; ICU: intensive care unit; OTI: orotracheal intubation; PM: pacemaker; Re-S: repeat surgery; UTI: urinary tract infection.
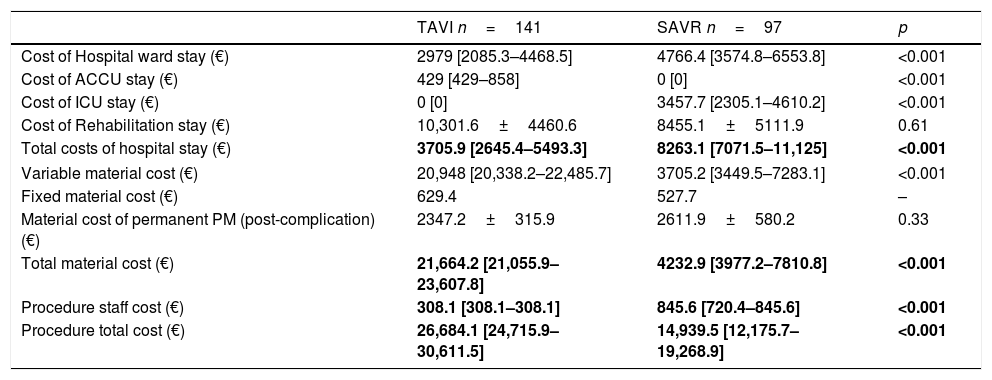
Variable material costs were 20,948 € [20,338.2–22,485.7] for percutaneous procedure and 3705.2 € [3449.5–7283.1] for surgical procedure (p<0.001). Fixed material costs were 629.4 € for each TAVI case and 527.7 € for each SAVR case. The total material costs per procedure were 21,664.2 € [21,055.9–23,607.8] in TAVI group and 4232.9 € [3977.2–7810.8] in surgical group. In turn, staff costs were 308.1 € [308.1–308.1] for percutaneous procedure and 845.6 € [720.4–845.6] for surgical procedure (p<0.001). Costs referring to the hospital stays were 3705.9 € [2645.4–5493.3] for TAVI group and 8263.1 € [7071.5–11,125] for SAVR group per patient (p<0.001).
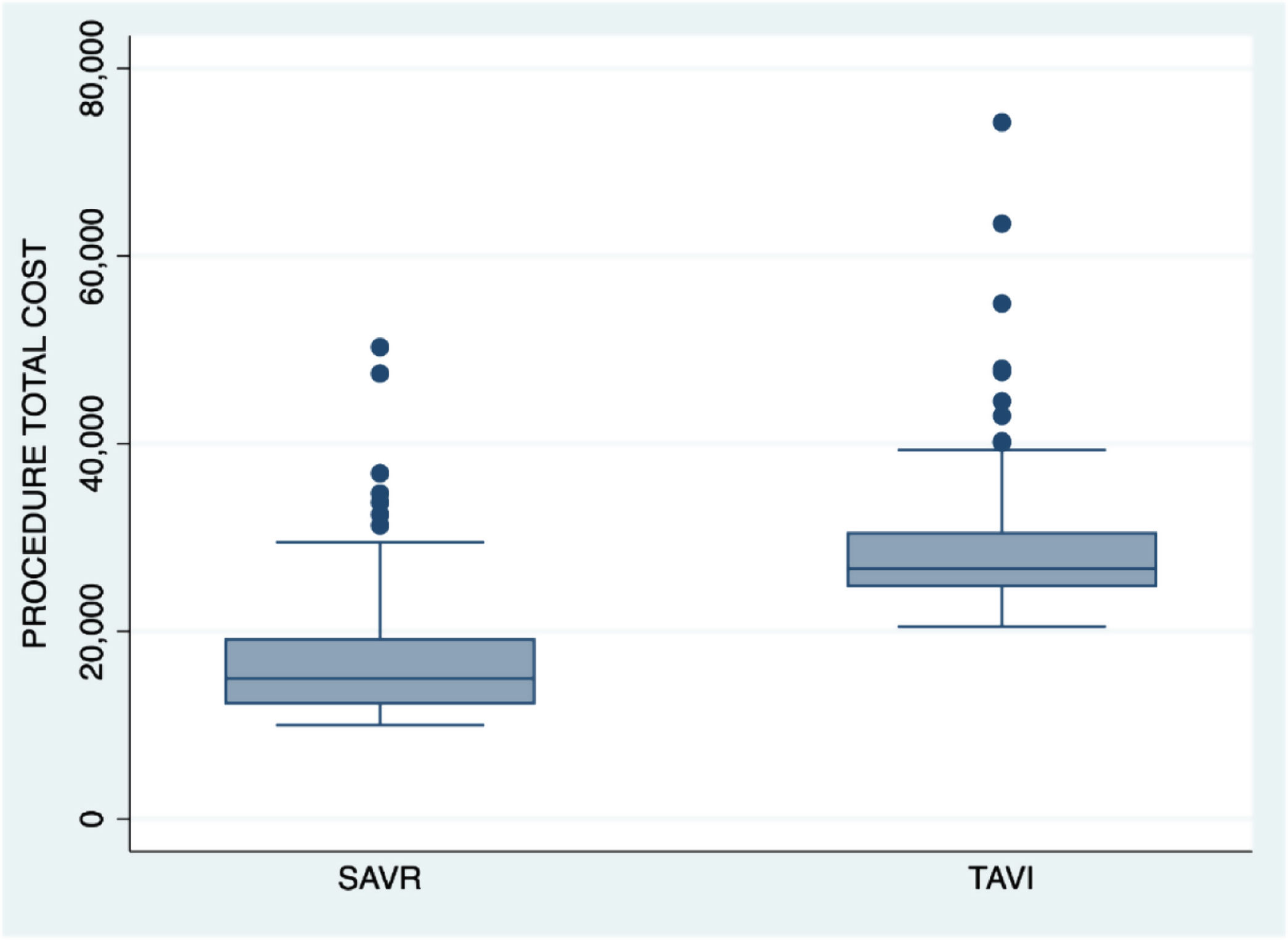
As a result of the above costs, total cost from intervention to discharge was 26,684.1 € [24,715.9–30,611.4] per patient within the TAVI group and 14,939.5 € [12,175.7–19,268.9] per patient within the SAVR group (p<0.001). When sutureless prostheses were used, the total cost was 22,163.1 € [16,245.7–26,365.6].
Table 5 shows remaining variables related to the cost of the procedures.
Economic variable costs.
| TAVI n=141 | SAVR n=97 | p | |
|---|---|---|---|
| Cost of Hospital ward stay (€) | 2979 [2085.3–4468.5] | 4766.4 [3574.8–6553.8] | <0.001 |
| Cost of ACCU stay (€) | 429 [429–858] | 0 [0] | <0.001 |
| Cost of ICU stay (€) | 0 [0] | 3457.7 [2305.1–4610.2] | <0.001 |
| Cost of Rehabilitation stay (€) | 10,301.6±4460.6 | 8455.1±5111.9 | 0.61 |
| Total costs of hospital stay (€) | 3705.9 [2645.4–5493.3] | 8263.1 [7071.5–11,125] | <0.001 |
| Variable material cost (€) | 20,948 [20,338.2–22,485.7] | 3705.2 [3449.5–7283.1] | <0.001 |
| Fixed material cost (€) | 629.4 | 527.7 | – |
| Material cost of permanent PM (post-complication) (€) | 2347.2±315.9 | 2611.9±580.2 | 0.33 |
| Total material cost (€) | 21,664.2 [21,055.9–23,607.8] | 4232.9 [3977.2–7810.8] | <0.001 |
| Procedure staff cost (€) | 308.1 [308.1–308.1] | 845.6 [720.4–845.6] | <0.001 |
| Procedure total cost (€) | 26,684.1 [24,715.9–30,611.5] | 14,939.5 [12,175.7–19,268.9] | <0.001 |
ACCU: advanced cardiological care unit; ICU: intensive care unit; PM: pacemaker.
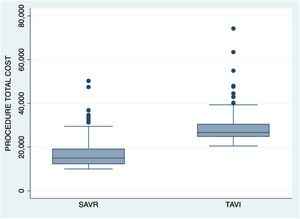
Fig. 1 shows total cost per procedure at time of hospital discharge.
After controlling for all comorbidities and confounding factors, multivariate regression model showed the per patient cost for TAVI group patients (independently of the mentioned factors) to be 8636.6 € (95% CI 6086.3–11,186.9; p<0.001) more than costs for SAVR patients.
Costs at 6 monthsThere were no losses at follow-up. With the exception of patients that died, all other patients concluded at the 6-month follow-up period. From discharge up until 6 months after procedure, a total of 36 patients (25.5%) in TAVI group reported to emergency room, versus 21 patients (21.6%) in the surgical treatment group (p=0.54). Sixteen patients (11.4%) in TAVI group required hospital admission, versus 15 patients (15.5%) in SAVR group (p=0.43). Among patients requiring admission, duration of hospital stay from discharge to 6 months was 10.6 days±11 in TAVI group and 9.3 days±5.3 in SAVR group (p=0.61).
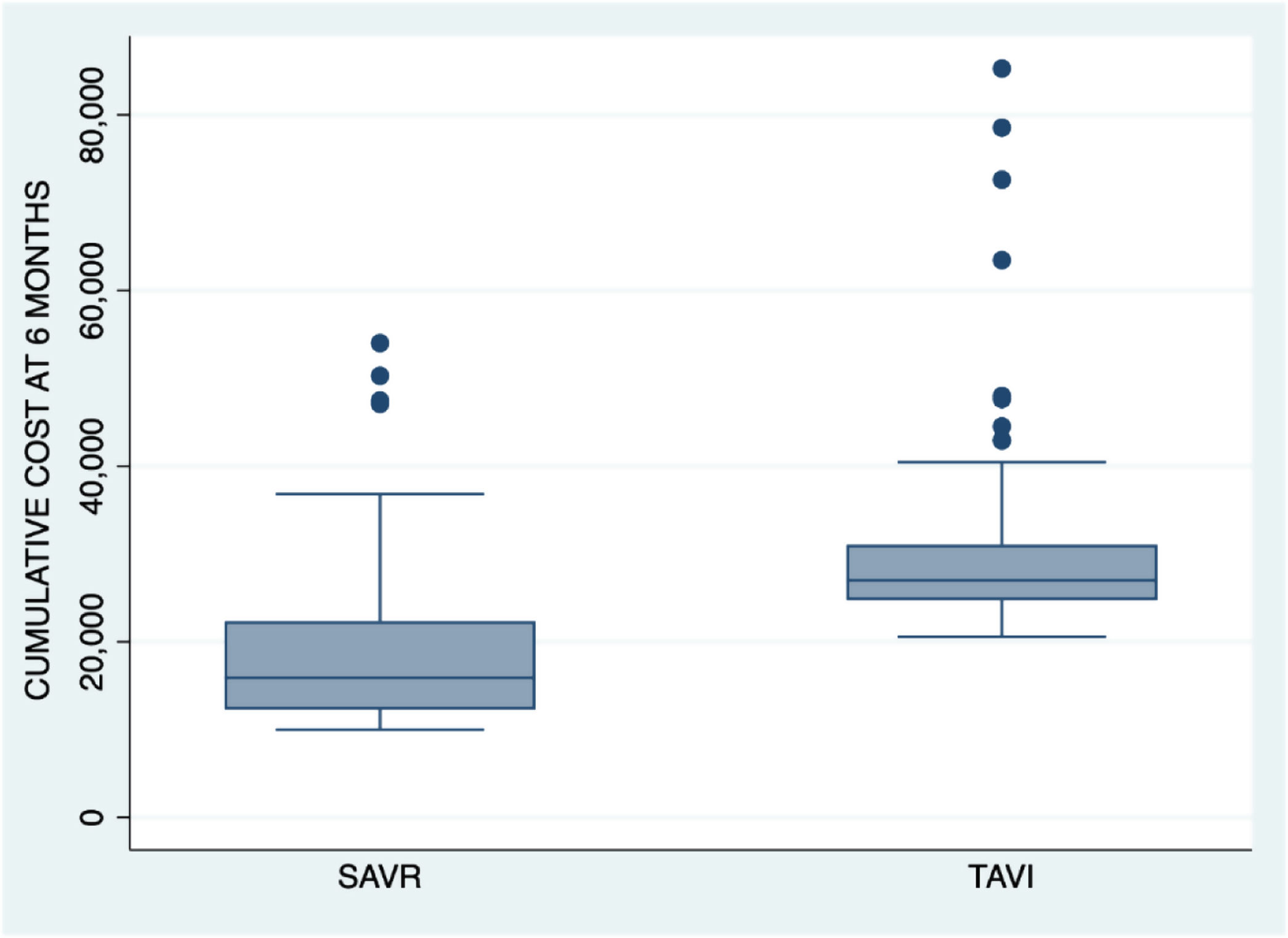
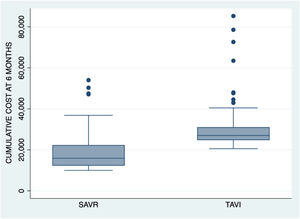
The cumulative cost up until month 6 was an average of 29,726.1 €±9530 for TAVI group patients and 19,058.5 €±9329.3 for surgical group patients (p<0.001). Fig. 2 shows the cumulative cost at 6 months for both procedures.
After controlling for all comorbidities and confounding factors, regression model showed the cost for TAVI patients (independently of mentioned factors) to be 8528.7 € (95% CI 6083.8–10,983.5; p<0.001) more than the average SAVR group patient.
DiscussionMain finding of the present study was that global cost of treatment for AoS in our center is currently higher for patients receiving TAVI technique than when surgical technique was employed. This comparative cost increment associated with TAVI was persistently noted from the procedure up to patient discharge, and 6 months post procedure. Furthermore, when the two procedures were assessed independently of comorbidities or baseline characteristics of the sample, the cost of the percutaneous option remained approximately 8000 € higher than the cost of the surgical treatment option.
Studies published to date on this issue had been based on aggregate data taken from clinical registries. Costs were therefore analyzed according to averages and not by calculating individual costs. Furthermore, since databases intended for clinical application were used, it was not possible to control for certain confounding factors that nevertheless are of importance when assessing costs.7,8,14,15 Studies involving real and individual costs, such as our own, are very few in number, and sample sizes have been similar to, or smaller than, those in our present study.6,16 Furthermore, the previously published data were from the United States, and therefore based on a healthcare system (Medicare) that is entirely different from Spanish system.6,14
Staff costsMean duration of intervention was 158min in TAVI group and 261min in SAVR group (p<0.001). Our literature search yielded only one publication that considered the time spent by the professionals in carrying out the two techniques for calculating staff costs. Osnabrugge et al. reported that the mean percutaneous times were 229min±79 versus 294min±76 for the surgical group (p<0.001).16 The comparatively shorter times in our cohort, particularly within the TAVI group, may be related to the data compilation period involved (2019 in our case versus 2006–2010 in the study carried out in The Netherlands). The tendency toward minimalism in TAVI, greater experience of the operators, and the use of prostheses that have become increasingly simplified with regards to implantation methods are factors that may have contributed toward this time difference and should be noted between the two studies.
The series published by Potter et al. recorded TAVI times, but not surgical times. Their mean staff cost for TAVI was about 45 $ per minute,17 whereas in our study, the comparable cost was 2 € when no anesthetist was required, rising to 2.4 € per minute when an anesthetist was needed. The aforementioned study did not detail costs of intervening professionals. In depth comparisons of the costs cannot therefore be made, however the evidence indicating lower salaries among healthcare professionals in Spain would undoubtedly account for this immense financial difference.10
Staff cost in our sample was about 300 € for the percutaneous procedure, and over twice that much (800 €) for the surgical procedure. In the series published by Osnabrugge et al., staff cost was 2300 $ for TAVI and 2400 $ for SAVR.16 In the study by Sunner et al. in Canada, these figures increased to 6400 $ and 6900 $, respectively.18 Here again, differences in Canadian and Spanish salaries, in addition to the need for a larger number of professionals in carrying out the two procedures in differing countries (10 individuals for TAVI and 8 for SAVR in the study carried out in The Netherlands) could explain these differences.
Material costsIn our series, total material cost per procedure was approximately 22,000 € in TAVI group and 4000 € in surgical group. Other similar studies only specified total costs and did not detail individual costs in relation to materials, length of type of unit the patients stayed in, or the professionals required. Only Osnabrugge et al., who analyzed 84 patients, reported an isolated material cost of 22,055 € for TAVI and 5162 € for SAVR. It is noteworthy that material costs between the two European studies are similar, and that the cost of the percutaneous procedure was consistently far higher than that of surgery. The higher price of the percutaneous valve is likely to be most responsible for this difference. In effect, the most commonly used valve in our series was the Evolut® versus the Corevalve® (its predecessor) which was preferred in the Dutch cohort, with an assigned price of 19,800 € and 17,590 €, respectively. In our center, the most frequently used surgical prosthesis was the Trifecta®, while in the Erasmus Medical Center it was the Magna Ease®, equating to a cost of 2475 € versus 2700 €.16
Costs of hospital stayOnce again, the literature yielded no studies that individually assessed costs of hospital stay in a separate manner to total costs. Osnabrugge et al. study did report a total cost of hospital stay of 8500 € for TAVI versus 17,000 € for SAVR patients. In our series, total cost of the hospital stay (3700 € for TAVI group and 8300 € for SAVR group) was far lower than in the study by Osnabrugge et al.16 This difference is likely attributable to a lower cost rate per day, shorter hospital stays within our center, and use of the ACCU for immediate TAVI patient care – thus avoiding the more expensive ICU costs.
Total cost to dischargeIn our series, regardless baseline characteristics, mean cost from procedure to discharge was about 27,000 € for each patient in TAVI group and 15,000 € for patients in SAVR group. McCarthy et al. analyzed a total of 20,613 patients in United States (4083 in TAVI group and 16,530 in SAVR group) and calculated a total cost at discharge of 50,700 $ for the percutaneous approach and 39,400 $ for the surgical procedure. Of this total cost, value of the percutaneous valve represented approximately 80% of total TAVI expenditure. The authors offered no information relating to the relative cost impact of surgical prosthesis, and costs associated with the remaining resources were not detailed. This large study was also based on administrative data sources; therefore, real costs of care could not be specified, as the authors themselves recognized.8
Osnabrugge et al. reported a total cost of about 40,800 € for TAVI and 33,400 € for SAVR. As with our study, authors considered individual costs and, as mentioned, their study offered more data, and was more detailed and rigorous than previously published work.16
In 2016, Ailawadi et al. published a study containing 1701 patients in the United States. These authors recorded a total cost of 81,000 € for TAVI and 44,000 € for SAVR, although the distribution of the total cost was not detailed, and only a fraction of the patients was used to conduct an individual cost analysis – not the entire cohort.6
Our results evidence costs considerably lower than those reported in all of the studies published to date. The important difference between the health system in United States and Spanish healthcare system (access to service, cost-free coverage, etc.) may explain the aforementioned differences.10 Compared to the study published by Osnabrugge et al. (the only other European publication), difference still remains substantial, but is smaller than the remaining studies.16
In contrast to most of data found in the literature, Patel et al. recorded lower global costs for TAVI than for SAVR (57,300 € versus 61,800 €). Unfortunately, the reasons for this price differential cannot be analyzed as the costs for their procedures were not subdivided according to categories.14
Total cost at 6 monthsFrom discharge to 6 months after the procedure, 25% of the patients in the TAVI group and 22% of those in the surgical treatment group had reported to the emergency room. Eleven percent of the patients in the TAVI group required hospital admission, versus 15% in the SAVR group.
Cumulative cost up until month 6 was about 30,000 € in the percutaneous treatment group and 19,000 € in the surgical group. This represents an increase versus the initial costs of about 3000 € and 4100 €, respectively.
The study published by Goldsweig et al., which involved the largest patient sample to date in relation to cost analyses (190,563 individuals), reported a cost at 6 months of 59,743 $ for TAVI and 64,395 $ for SAVR patients – of which 7455 $ and 5505 $ respectively related to readmission costs. In our study this increase was smaller, yet proportional when compared against the differing global costs in both publications.7 Osnabrugge et al. also published results at one year, with an associated cost increase of 5400 € for TAVI and 2200 € for SAVR patients.16
SARS-COV-2 pandemic has had a disproportionate economic impact. Only in the “first wave”, it is estimated that COVID cost 13.9 billion euros in Europe.19 Lack of access and the collapse of public healthcare systems was associated with a significant and abrupt reduction (42–64%) in cardiac diagnostic procedures across the globe,20 which has translated into increased mortality in cardiac patients. For example, in Italy, patients diagnosed with STEMI, mortality in the 2nd quarter of 2020 rose from 4.1% to 13.7%.21 Severe funding problems and longer waiting lists are expected in the near future, with an increase in private activity to the detriment of public services, so the adjustment of costs associated with procedures will be more important than ever.22 In this setting, knowing the costs attributed to TAVI or SAVR are more important than ever.
Finally, decision for TAVI or SAVR should not be based on costs but on the individualization of the best clinical results for each patient.
LimitationsThe retrospective design of our study involves biases inherent to publications of this kind. Furthermore, it is a single-center study, therefore it is not clear whether the results can be extrapolated to the rest of Spain. Nevertheless, it is true that material costs and salaries of the professionals are very similar in all hospitals within the Spanish healthcare system.
Cost analyses can be extended infinitely, however the present study, analyzed each patient individually, and has attempted to encompass as many of the associated costs as possible whilst taking into consideration that some are never accounted for such as the cost of implanting a permanent pacemaker or the cost of treating vascular complications in the Hemodynamics laboratory or in the Vascular surgery operating room. However, the costs of other complications such as the use of blood products and the personnel and material costs associated with re-interventions in the surgical group were not taken into account. Hospital stay was not calculated in an hourly basis.
ConclusionsRegardless baseline characteristics, TAVI procedure up to patient discharge costs approximately 9000 € more than SAVR in our center.
Compared with the cost of the staff involved in SAVR, the cost of the team involved in TAVI is less than half. However, this is not enough to balance the cost with SAVR, due mainly to the costs of the materials required for each operation.
FundingThis research received no external funding.
Conflicts of interestDr. Hernández-Vaquero declares that he has received a fee for teaching a course for Abbott®.
Dr. Morís declares that he is proctor for Medtronic® and Boston®.
The rest of authors declare no conflict of interest.