Currently, neonatal electrical cardiac stimulation is done using generators designed for adults. This increases the difficulty of the initial implantation, making it a high-risk procedure due to the risk of infection and erosion.
In 2022, a small pediatric pacemaker was designed (Pediatric IPG). This device is the result of a modification of the Micra leadless model for adults. This modification allows the pacemaker to be implanted in the epicardium.
We present a 3-month-old patient with a large interventricular communication (VSD) and congenital lobar emphysema (CLE). A VSD closure was performed with the patient weighing 2.8kg, resulting in a post-surgical complete atrioventricular block. Due to severe malnutrition and with prior authorization, a Pediatric IPG pacemaker was implanted. This pacemaker was maintained for 4 weeks with basic stimulation, which allowed for growth despite an unstable threshold. These unstable thresholds persisted even after changing the generator. With the suspicion of a mechanical interaction between the CLE and the correct function of the leads, a medial right lobectomy was performed at the age of 4 months, after which the threshold values normalized and the patient remained asymptomatic.
This is the first case in the literature to describe an interaction between CLE and the correct function of the leads of a pacemaker.
We also describe the safety and usefulness of the Pediatric IPG pacemaker. Designs like these are important in stimulating pediatric patients in the first months of life, for whom we currently do not have adequate devices.
Los dispositivos de los que disponemos para estimulación cardiaca en el periodo neonatal son generadores de adultos que implantamos con dificultad y suponen un alto riesgo de infección y erosión.
En 2022, se ha fabricado un marcapasos de pequeño tamaño para la edad pediátrica (Pediatric IPG); dicho dispositivo es el resultado de la modificación del marcapasos modelo Micra™ sin cables diseñado para adultos, cuya adaptación permite a este generador de pequeño tamaño estimular de forma epicárdica.
Paciente de 3 meses de edad con comunicación interventricular (CIV) grande y enfisema lobar congénito (ELC). Tras intervención cardiaca con 2,8kg de peso, precisa implante de marcapasos por bloqueo aurículo-ventricular postquirúrgico. Debido a la desnutrición, tras la autorización pertinente, decidimos implantarle un marcapasos Pediatric IPG. Mantuvimos durante 4 semanas el marcapasos con una estimulación básica, con adecuada ganancia de peso, pero con inestabilidad en los umbrales que se mantuvo a pesar del cambio de generador. Con la sospecha de que el ELC podía estar interfiriendo en el funcionamiento del electrodo, se decide realizar lobectomía media derecha a los 4 meses de edad. Posteriormente, permanece asintomática y se normaliza la función del marcapasos.
Es la primera vez en la literatura que se describe la interacción del ELC con el funcionamiento de un electrodo.
Además, observamos que la estimulación con el marcapasos Pediatric IPG es una estimulación básica, pero segura, y destacamos que es importante la iniciativa de diseñar dispositivos de pequeño tamaño para cardioestimular a pacientes en los primeros meses de vida, para los que no tenemos dispositivos adecuados.
There is a lack of small devices for electrical cardiac stimulation in the neonatal period. The devices currently available are adult generators, which are implanted with great difficulty in the abdominal wall.1
Pediatric patients are at risk of complete atrioventricular (AV) block, due to an alteration of the conduction system in the fetal period or due to damage to the AV node during cardiac surgery. Despite advances in perioperative and intraoperative care, around 2% of patients will require a pacemaker after cardiac surgery.2
In recent years, adult pacemaker profiles have been reduced, but they are still large enough to pose a risk of infection and erosion when implanted in the first years of life.3 In children below 15kg, due to limited vascular access, epicardial implantation of pacemakers is the norm. The electrodes and generators available for these patient profiles are the same as those used in adult patients, adapted for basic stimulation. There have been no advances in electrical cardiac stimulation for young patients in their first months of life.4
The quality of life and life expectancy in pediatric patients with cardiac stimulation is similar to the general population. As per preliminary studies, specific adapted electrostimulation can reduce the number of interventions and better preserve cardiac function. Recently, in 2022, a small permanent pacemaker for the pediatric population was manufactured. This device is the result of a modification of the Micra leadless Medtronic® model designed for adults.5,6 This pacemaker was initially designed for endocardial stimulation without leads. It has been adapted for epicardial stimulation with the incorporation of a polymer case, which allows an IS-1 4968 Medtronic® connection. This allows for basic epicardial VVI electrostimulation. This design is known as the Pediatric IPG (implantable pulse generator).7
There is a considerable size difference compared to conventional VVI permanent pacemakers: the profile of a Pediatric IPG device is 27.5mm×15.3mm×8.15mm, weighs 5g, and has a volume of 3.5cm3. These characteristics allow for easy implantation. In our case, a 4mm incision in the lateral abdominal wall was sufficient. Regarding the software, the device uses the same platform as the Micra with the same basic parameters. However, there is no possibility for special advanced algorithms.
We present our experience with the first Pediatric IPG permanent pacemaker implanted in our institution and the second implanted in Spain. In our case, the indication was made after a literature review and as a compassionate use with prior authorization from the Spanish Agency of Medicines and Medical Products. Our patient is a 2.5-month-old infant, referred from another center with a diagnosis of large, hemodynamically relevant interventricular communication (VSD) and congenital lobar emphysema (CLE). At 3 months of age and weighing 2.8kg, a VSD patch closure was performed. The patient developed a postoperative complete AV block. Ten days after the initial surgery, a Pediatric IPG was implanted via re-sternotomy with the placement of a bipolar electrode on the anterior surface of the right ventricle. In the operating room, a stable threshold of 2V was registered with correct and stable impedance. After the pacemaker implant, there was an improvement in the symptoms of cardiac insufficiency; however, the patient still needed oxygen therapy due to the large CLE. During the postoperative period, the patient progressively developed unstable stimulation thresholds, especially during feeding. Despite this, and under constant monitoring with telemetry, the patient presented adequate electrostimulation. During the following 35 days, the patient gained weight with mixed feeding: breastfeeding complemented with a hypercaloric formula administered via a nasogastric tube.
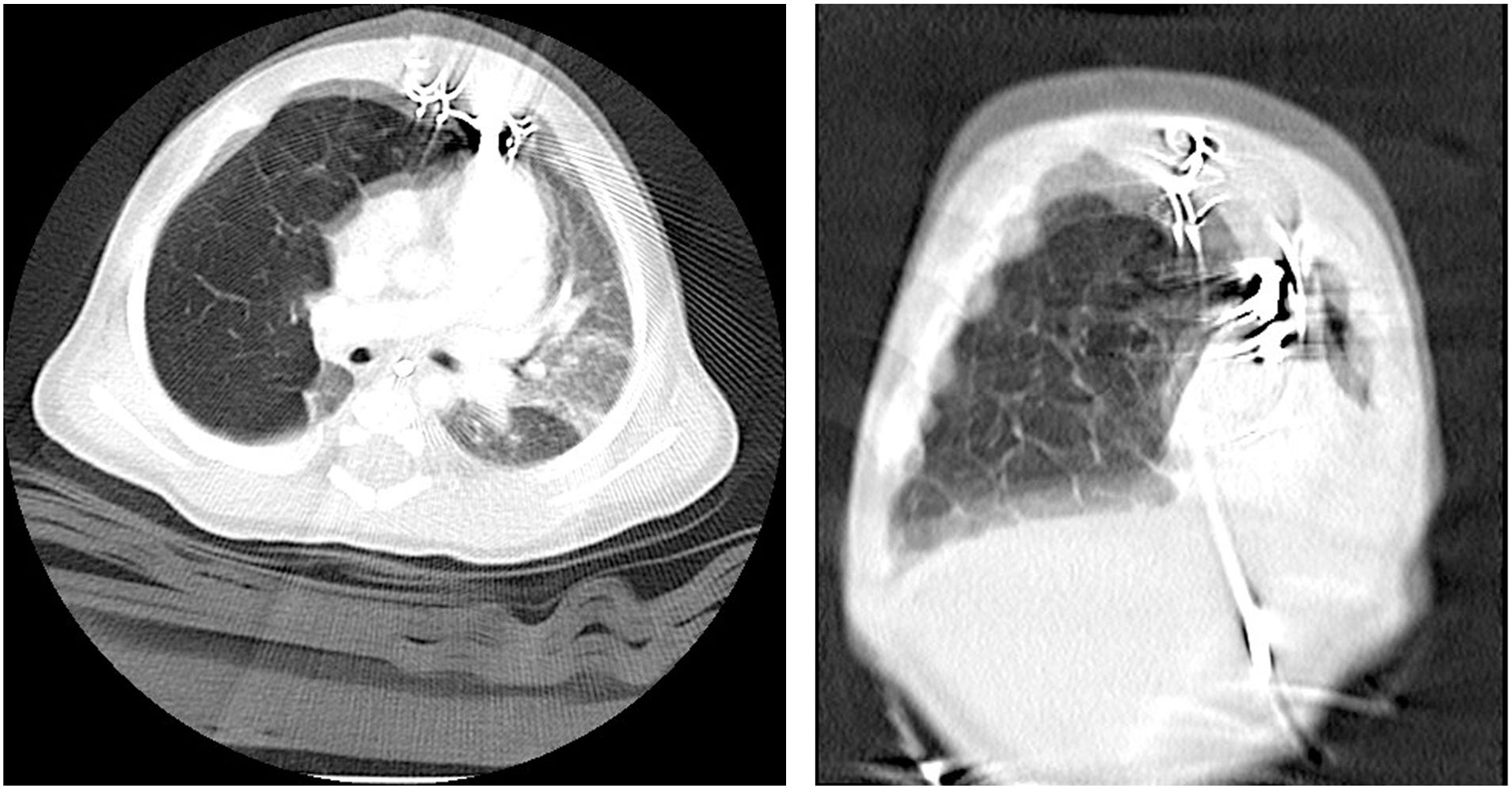
The thresholds continued to increase, reaching output thresholds of 4V (Figs. 1 and 2). A CT scan after the cardiac surgery revealed that the CLE was in contact with the epicardial electrodes. To eliminate confounding factors before the CLE surgery, and at a weight of 4.5kg, the Pediatric IPG generator was replaced with a Medtronic® Sensia generator, maintaining the original epicardial leads. Despite the generator replacement, the electrode values were similar, with a gradual increasing threshold trend. Forty days after the initial VSD correction, a lobectomy of the right medial lobe was performed via a right thoracotomy without any incidents. Immediately after the procedure, we observed a normalization of the threshold values, reaching values of 1V. The patient was discharged 5 days after the CLE resection.
At 12-month follow-up, the patient was asymptomatic, thriving, and with good generator and lead parameters.
To this day, there is no similar case in the literature where a CLE altered the normal function of an epicardial pacemaker, which was resolved by the CLE resection.8
Despite the limitations of an initial experience, the Pediatric IPG device allows safe electrostimulation in small children. It enables the patient to reach an adequate size where stimulation can be continued using adult-profile permanent pacemakers. During our experience with the Pediatric IPG permanent pacemaker, we observed that the battery life is less than the Micra model implanted in adults. This is probably due to the epicardial mode of stimulation, which requires more energy than the endocardial mode, explaining the higher thresholds. We also have to consider that pediatric patients have a higher cardiac frequency compared to adults, which increases the stimulation rate.
Currently, we do not have an adequate permanent pacemaker for newborns. The use of leadless pacemakers in adult patients has allowed us to obtain smaller-profile permanent pacemakers, which, when adapted for epicardial use, can be employed safely in patients weighing less than 4kg. This allows basic cardiac electrostimulation but does not allow for remote monitoring or auto-capture of thresholds. Another limitation is the inability to stimulate using unipolar leads, this leads to the need to implant a bipolar lead in a small heart, which could be challenging in certain neonatal cases. Moreover, the Pediatric IPG, does not allow a reliable stimulation beyond 5V, this was the reason in our case for changing the generator when thresholds reached 4V. Finally, the device is not compatible with magnetic resonance imaging (MRI). Although the case is made from high-quality thermoplastic polyether ether ketone, this material and the epicardial leads have not been approved for MRI. Thus, this type of imaging cannot be recommended for these patients.9
A salient feature of our case report is the rare association of a hemodynamically relevant VSD with a respiratory relevant CLE. Moreover, we report a mechanical interaction of the CLE, which could negatively affect the normal function of epicardial leads and should be taken into account when encountering unstable epicardial lead thresholds in these patients.
Regardless of our initial experience with small-sized permanent pacemakers, patients like the one presented here make a case for innovation and investigation in reducing the profile of these devices, especially in the neonatal period. This allows for safe electrostimulation, reducing profile-related serious complications.
Ethical considerationsConsent was obtained from the parents to publish the article.
Conflict of interestThe authors declare that they have no conflict of interest.