In skeletal muscle, adenosine triphosphate stores decrease during the first 3h of ischemia. In the present study, we performed a comprehensive hemodynamic evaluation during the postoperative period after cardiac surgery and measured skeletal muscle enzyme levels and markers of muscle damage and inflammation. The aim was to determine whether these values change and, if so, whether these changes coincide with the presence of low flow and poor perfusion.
MethodsWe included a cohort of 280 nonconsecutive adults who were monitored in the postoperative period following cardiac surgery. We measured hemodynamic indices repeatedly in the first 24h postoperatively, and we identified differences between the levels of skeletal muscle enzymes and muscle damage markers on admission (0h) and 12 and 24h postoperatively.
ResultsA clinically and statistically significant elevation of creatine kinase (CK) level was observed at 12h postoperatively in patients with low macrocirculatory flow and anaerobic metabolism. Lactate dehydrogenase (LDH) level was significantly elevated in these patients at 24h.
ConclusionsIn the first 24h after cardiac surgery, a state of low macrocirculatory flow and the consequent deficit in flow at the capillary–cell interface in the presence of anaerobic metabolism was associated with clinically and statistically significant elevations of CK level at 12h and LDH level at 24h. These changes may be markers of skeletal muscle ischemia and may provide an additional tool in the monitoring and resuscitation of these critically ill patients.
En el músculo esquelético, las reservas de trifosfato de adenosina disminuyen durante las primeras 3h de isquemia. En el presente estudio, realizamos una evaluación hemodinámica integral durante el período postoperatorio de cirugía cardíaca, y medimos los niveles de enzimas del músculo esquelético y los marcadores de daño muscular. El objetivo fue determinar si estos valores cambian y, en caso afirmativo, coinciden con la presencia de bajo flujo y mala perfusión.
MétodosIncluimos una cohorte de 280 adultos no consecutivos que fueron monitorizados en el postoperatorio de cirugía cardíaca. Medimos los índices hemodinámicos postoperatoriamente, e identificamos diferencias entre los niveles de enzimas del músculo esquelético y los marcadores de daño muscular al ingreso (0h) y a las 12 y 24h, postoperatoriamente.
ResultadosSe observó una elevación clínica y estadísticamente significativa del nivel de creatina cinasa (CK) a las 12h del postoperatorio en los pacientes con bajo flujo macrocirculatorio y metabolismo anaeróbico. El nivel de lactato deshidrogenasa (LDH) estuvo significativamente elevado en estos pacientes a las 24h.
ConclusionesEn las primeras 24h después de una cirugía cardíaca, un estado de bajo flujo macrocirculatorio y el consiguiente déficit de flujo en la interfaz capilar/célula en presencia de metabolismo anaeróbico se asoció con elevaciones clínica y estadísticamente significativas del nivel de CK a las 12h y de la LDH a las 24h. Estos cambios pueden ser marcadores de isquemia del músculo esquelético y pueden proporcionar una herramienta adicional en el seguimiento y en la reanimación de estos pacientes.
In skeletal muscle under resting conditions, oxygen consumption (VO2) is lower than in other organs; for example, the average resting VO2 is 5–10mL/min/100g in skeletal muscle but 60–100mL/min/100g in organs with high metabolic activity, such as the brain or heart. The low rate of blood flow per unit of tissue mass in quiescent skeletal muscles reflects the high basal vascular tone or partial constriction of arterioles.1 In the myofibril at rest, the concentration of cytosolic Ca2+ is maintained at 50nM but can increase by 100 times during muscle contraction. Intracellular Ca2+ concentration depends on the type of fiber. The Ca2+ concentration is higher in slow-twitch red (type I) fibers, which rely more on oxidative metabolism, than in fast-twitch white (type II) fibers, which rely more on glycolytic metabolism.2 In skeletal muscle, the number of mitochondria also depends on the type of fiber and are numerous in oxidative fibers. Mitochondria are found in strategic locations around the nuclei or between bundles of myofilaments, which allows them to communicate with each other and to perform physiological functions through a complex network.3,4
During ischemic events, the nutrient- and oxygen-starved blood supply cannot meet the energy requirements of the muscles, and this deficit leads to numerous ionic and metabolic changes. The accumulated energy is used mainly to maintain the membrane potential and ionic compartmentalization. Reduced perfusion limits the supply of exogenous substrates, particularly oxygen and free fatty acids, which in turn induces increased flux along the anaerobic pathways for energy production and leads to a deficit in adenosine triphosphate (ATP) synthesis. As this inefficient ATP production continues, intracellular pH decreases, an event that inhibits the enzyme phosphofructokinase, which is involved in the glycolytic process.5,6
In skeletal muscle, ATP stores decrease at a very low rate during the first 3h of ischemia, when creatine phosphate (CP) and glycogen stores are high. After 3h of ischemia, the ATP reserve decreases rapidly, and depletion of ATP, CP, and glycogen occurs after 6–7h and correlates with the almost complete death of skeletal muscle.7 The oxidative phosphorylation process and production of reactive oxygen species in mitochondria are affected mainly by ischemia. Cell damage is irreversible after 4h of ischemia. Reperfusion is necessary but should not be started abruptly to preserve skeletal muscle.8
Intense physiological stress occurs during the postoperative period after cardiac surgery and reflects a dysregulated systemic inflammatory response associated with extracorporeal circulation and tissue injury, which lead to an imbalance between tissue VO2 and oxygen delivery (DO2). To compensate, blood flow is redirected to the organs essential for life, such as the heart and brain, by limiting the flow to the kidney, gut, skin, and skeletal muscle. This phenomenon is exacerbated by vasopressor drugs. If a state of stability cannot be restored, multiple organ dysfunction and death may occur.9
During the postoperative period after cardiac surgery, hemodynamic monitoring is used to assess the patient's macrohemodynamic profile, which includes the cardiac index, systemic vascular resistance index (SVRI), and central venous pressure (CVP). The oxygenation-derived indices are used to evaluate the DO2/VO2 ratio (mixed venous oxygen saturation [SvO2], arterial-to-venous O2 difference [a-vO2D], and the oxygen extraction rate [O2ER]). The lactate level is also used to identify poor tissue perfusion.
As in the dysregulated systemic inflammatory response that occurs during sepsis, such dysregulation during the postoperative period after cardiac surgery can result in microcirculatory alterations, such as heterogeneity in capillary flow and decreased functional capillary density. In this context, two indices derived from carbon dioxide (CO2) may provide useful information. The first index is the central venous-to-arterial PCO2 difference (Pv-aCO2), which indicates a state of low flow at the capillary–cell interface when ≥6mmHg.10 The second index is the difference between venous–arterial CO2 and arterial–venous O2 content (Cv-aCO2/a-vO2D ratio), which provides a surrogate marker of the respiratory quotient (calculated as the ratio of CO2 production to VO2). An increase in the respiratory quotient >1 indicates an oxygen deficit and increased anaerobic production of CO2.11,12
In the present study, we comprehensively evaluated the hemodynamic responses in the postoperative period after cardiac surgery, and we measured the levels of skeletal muscle enzymes and markers of muscle damage and inflammation. Our aim was to determine whether these values increase in the postoperative period and whether such changes coincide with the presence of a low flow and poor perfusion state. If so, we reasoned that these may provide markers of ischemia during the state of hemodynamic instability. Our hypothesis was that muscle ischemia and its clinical manifestations of macro- or microcirculatory imbalance would be associated with the consequent redirection of blood flow toward more vital tissues and that these changes would be reflected in changes in some biochemical markers.
MethodsThis cross-sectional study included 280 nonconsecutive adult patients who were admitted following cardiac surgery to the critical care unit at the Instituto Nacional de Cardiología Ignacio Chávez, Mexico City, Mexico, from June 1 to December 30, 2022. In all patients, CVP was measured invasively with a central venous catheter with the tip at the cavoatrial junction. All patients were monitored invasively with a pulmonary artery catheter. We performed serial measurements in the first hours (on admission and at 12 and 24h) of the postoperative period after cardiac surgery. We measured macrocirculatory parameters (cardiac index, SVRI, and CVP), global oxygenation indices (SvO2, a-vO2D, and O2ER), CO2-derived indices (Pv-aCO2 and Cv-aCO2/a-vO2D ratio), and perfusion indices (lactate level). The formulae used to obtain these measurements are presented in Supplemental Table 1.
Levels of creatine kinase (CK), CK-MB, high-sensitivity troponin, lactic dehydrogenase (LDH), aspartate transaminase (AST), leukocytes, and troponin I were measured on admission and at 12 and 24h postoperatively. We performed an individual analysis of all patients with a lactate level ≥2mmol/L and classified them into four hemodynamic profiles based on the analysis of SvO2, Pv-aCO2, Cv-aCO2/a-vO2D ratio, and lactate level as follows: (1) low macrocirculatory flow with anaerobic metabolism; (2) low microcirculatory flow with anaerobic metabolism; (3) cellular (mitochondrial) dysfunction; and (4) alteration in lactate kinetics (clearance).12 We compared differences between the levels of skeletal muscle enzymes and muscle damage markers between these four groups on admission and at 12 and 24h postoperatively. The levels of total leukocytes and C-reactive protein were also measured to evaluate the post-bypass systemic inflammatory response. The local institutional research and ethics committees waived approval for this study.
Statistical analysisWe used the Shapiro–Wilk test of normality for continuous variables and report them as median and interquartile ranges because all were not normally distributed. The Kruskal–Wallis test was used to compare continuous variables. We report categorical variables as frequencies and percentages, and used the Chi-squared or Fisher's exact probability tests, as appropriate, to compare expected values. All statistical analyses were performed using Stata v. 14 (StataCorp LLC, College Station, TX, USA), and P values<0.05 were considered to be significant. Some results are presented as the odds ratio (OR) and 95% confidence interval (CI).
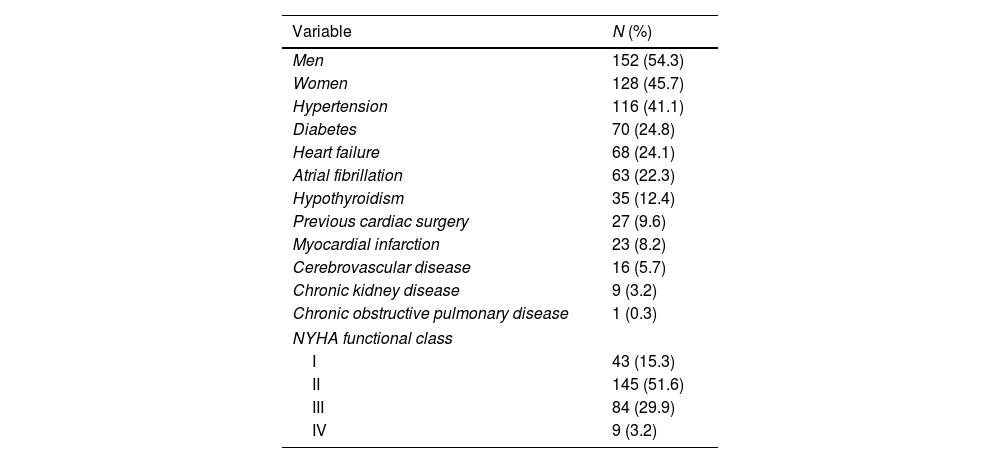
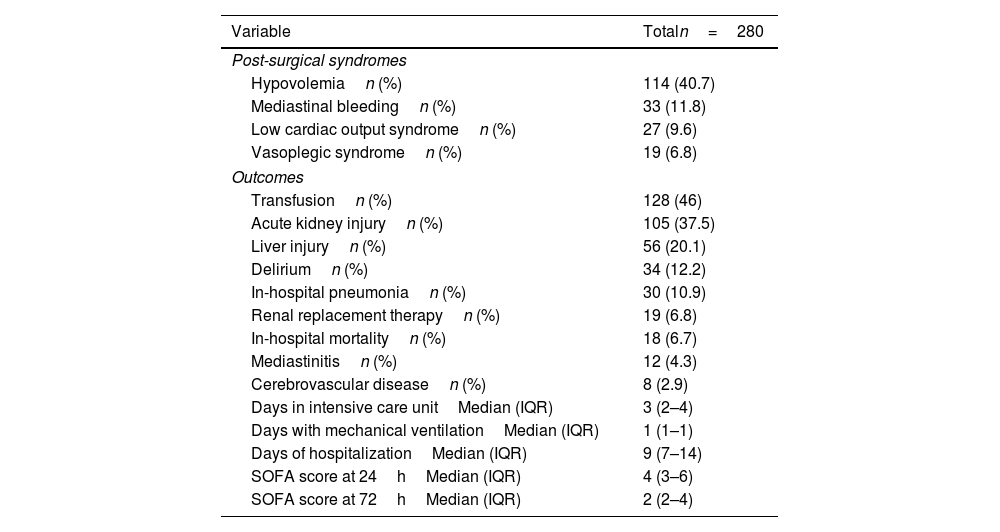
ResultsDemographic and surgical characteristicsMost patients were male (54.3%), and their median age was 57 (range 45–65) years and New York Heart Association functional class II (51.6%). Their most frequent comorbidities were hypertension, diabetes, and atrial fibrillation (41.1%, 24.8%, and 22.3%, respectively), and 9.6% of patients had previous cardiac surgery. The most frequent cardiac surgery was aortic valve replacement (25.9%), followed by coronary artery bypass graft (15.6%). Their median extracorporeal circulation time was 145min, and median aortic clamping time was 101min. The most frequent postsurgical syndrome was hypovolemia (40.8%), followed by mediastinal bleeding (11.7%) (Table 1).
Baseline and surgical characteristics.
| Variable | N (%) |
|---|---|
| Men | 152 (54.3) |
| Women | 128 (45.7) |
| Hypertension | 116 (41.1) |
| Diabetes | 70 (24.8) |
| Heart failure | 68 (24.1) |
| Atrial fibrillation | 63 (22.3) |
| Hypothyroidism | 35 (12.4) |
| Previous cardiac surgery | 27 (9.6) |
| Myocardial infarction | 23 (8.2) |
| Cerebrovascular disease | 16 (5.7) |
| Chronic kidney disease | 9 (3.2) |
| Chronic obstructive pulmonary disease | 1 (0.3) |
| NYHA functional class | |
| I | 43 (15.3) |
| II | 145 (51.6) |
| III | 84 (29.9) |
| IV | 9 (3.2) |
| Variable | Median (IQR) |
|---|---|
| Age (years) | 57 (45–65) |
| Weight (kg) | 68 (59–75) |
| Height (m) | 1.62 (1.55–1.68) |
| Body mass index (kg/m2) | 25.7 (23.2–28.4) |
| Variable | N (%) |
|---|---|
| Aortic valve replacement | 73 (25.9) |
| Coronary artery bypass graft | 44 (15.6) |
| Mitral valve replacement | 27 (9.6) |
| Bentall procedure | 18 (6.4) |
| Aortic valve replacement+mitral valve replacement | 17 (6) |
| Mitral valve replacement+tricuspid valve replacement | 16 (5.7) |
| Coronary artery bypass graft+aortic valve replacement | 12 (4.3) |
| Other surgery | 71 (25.8) |
| Hypovolemia | 115 (40.8) |
| Mediastinal bleeding | 33 (11.7) |
| Low cardiac output syndrome | 27 (9.6) |
| Vasoplegic syndrome | 20 (7.1) |
| Variable | Median (IQR) |
|---|---|
| Extracorporeal circulation time (min) | 145 (113–188) |
| Aortic clamping (min) | 101 (77–126) |
| EuroSCORE II | 1.7 (0.9–3.3) |
NYHA: New York Heart Association; IQR: interquartile range.
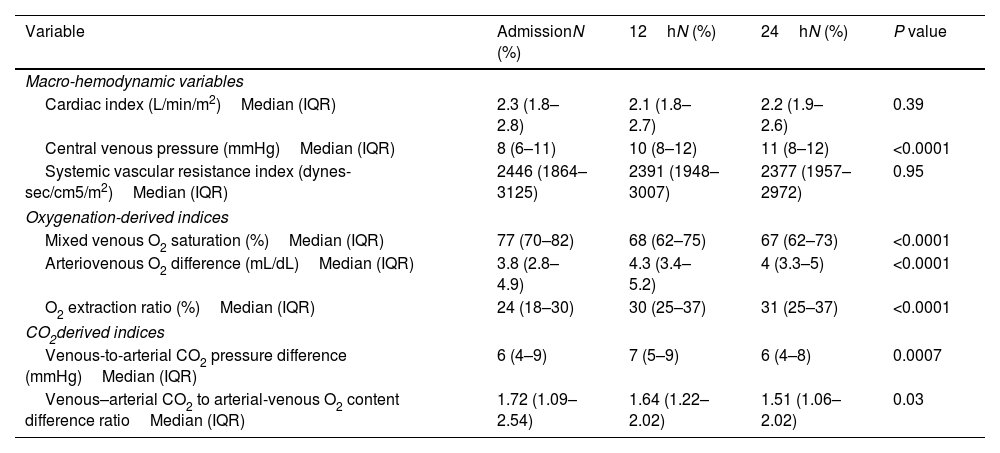
The cardiac index remained >1.8L/min/m2 throughout the first 24h. There was a trend for the CVP to increase throughout the first 24h, and the SVRI was above its maximum reference value (2400dynes-sec/cm5/m2) only during the first 12h. The SvO2, a-vO2D, and O2ER were within normal limits during the first 24h.
Hemodynamic parameters.
| Variable | AdmissionN (%) | 12hN (%) | 24hN (%) | P value |
|---|---|---|---|---|
| Macro-hemodynamic variables | ||||
| Cardiac index (L/min/m2)Median (IQR) | 2.3 (1.8–2.8) | 2.1 (1.8–2.7) | 2.2 (1.9–2.6) | 0.39 |
| Central venous pressure (mmHg)Median (IQR) | 8 (6–11) | 10 (8–12) | 11 (8–12) | <0.0001 |
| Systemic vascular resistance index (dynes-sec/cm5/m2)Median (IQR) | 2446 (1864–3125) | 2391 (1948–3007) | 2377 (1957–2972) | 0.95 |
| Oxygenation-derived indices | ||||
| Mixed venous O2 saturation (%)Median (IQR) | 77 (70–82) | 68 (62–75) | 67 (62–73) | <0.0001 |
| Arteriovenous O2 difference (mL/dL)Median (IQR) | 3.8 (2.8–4.9) | 4.3 (3.4–5.2) | 4 (3.3–5) | <0.0001 |
| O2 extraction ratio (%)Median (IQR) | 24 (18–30) | 30 (25–37) | 31 (25–37) | <0.0001 |
| CO2derived indices | ||||
| Venous-to-arterial CO2 pressure difference (mmHg)Median (IQR) | 6 (4–9) | 7 (5–9) | 6 (4–8) | 0.0007 |
| Venous–arterial CO2 to arterial-venous O2 content difference ratioMedian (IQR) | 1.72 (1.09–2.54) | 1.64 (1.22–2.02) | 1.51 (1.06–2.02) | 0.03 |
IQR: interquartile range.
The Pv-aCO2 was elevated during the first 24h (normal value: <6mmHg) and reached its highest value at 12h. The Cv-aCO2/a-vO2D ratio remained above its reference range of 1 throughout the first 24h and reached its maximum values at admission and 12h (1.72 and 1.64, respectively).
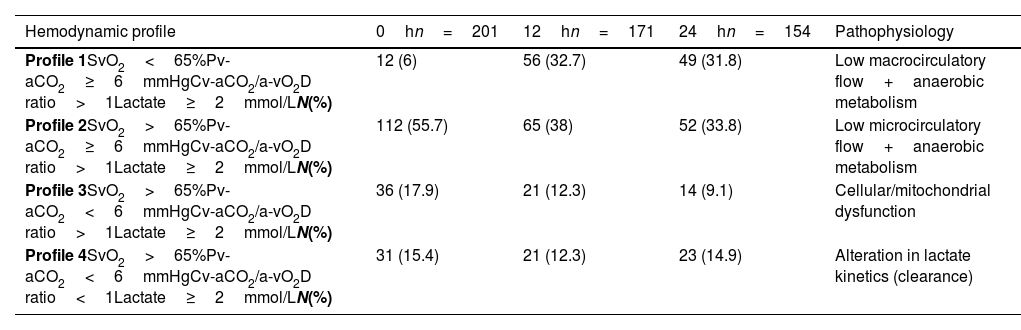
Hemodynamic classificationGiven that a low flow state at the capillary–tissue interface predominated, as shown by elevated Pv-aCO2 and Cv-aCO2/a-vO2D ratio during the first 24h, we decided to classify patients with lactate level ≥2mmol/L (hypoperfused), into four hemodynamic profiles. We used this method to infer the pathophysiological state of the patient based on the analysis of SvO2, Pv-aCO2, Cv-aCO2/a-vO2D ratio, and lactate level, as follows.
- 1.
Low macrocirculatory flow with anaerobic metabolism
- 2.
Low microcirculatory flow with anaerobic metabolism
- 3.
Cellular (mitochondrial) dysfunction
- 4.
Alteration in lactate kinetics (clearance)
Profile 2 predominated, at 0, 12, and 24h (Table 3), which suggested the predominance of a state of low microcirculatory flow.
Hemodynamic profiles and its pathophysiology.
| Hemodynamic profile | 0hn=201 | 12hn=171 | 24hn=154 | Pathophysiology |
|---|---|---|---|---|
| Profile 1SvO2<65%Pv-aCO2≥6mmHgCv-aCO2/a-vO2D ratio>1Lactate≥2mmol/LN(%) | 12 (6) | 56 (32.7) | 49 (31.8) | Low macrocirculatory flow+anaerobic metabolism |
| Profile 2SvO2>65%Pv-aCO2≥6mmHgCv-aCO2/a-vO2D ratio>1Lactate≥2mmol/LN(%) | 112 (55.7) | 65 (38) | 52 (33.8) | Low microcirculatory flow+anaerobic metabolism |
| Profile 3SvO2>65%Pv-aCO2<6mmHgCv-aCO2/a-vO2D ratio>1Lactate≥2mmol/LN(%) | 36 (17.9) | 21 (12.3) | 14 (9.1) | Cellular/mitochondrial dysfunction |
| Profile 4SvO2>65%Pv-aCO2<6mmHgCv-aCO2/a-vO2D ratio<1Lactate≥2mmol/LN(%) | 31 (15.4) | 21 (12.3) | 23 (14.9) | Alteration in lactate kinetics (clearance) |
Cv-aCO2/a-vO2D ratio: venous–arterial CO2 to arterial-venous O2 content difference, Pv-aCO2: central venous-to-arterial PCO2 difference, SvO2: mixed oxygen saturation.
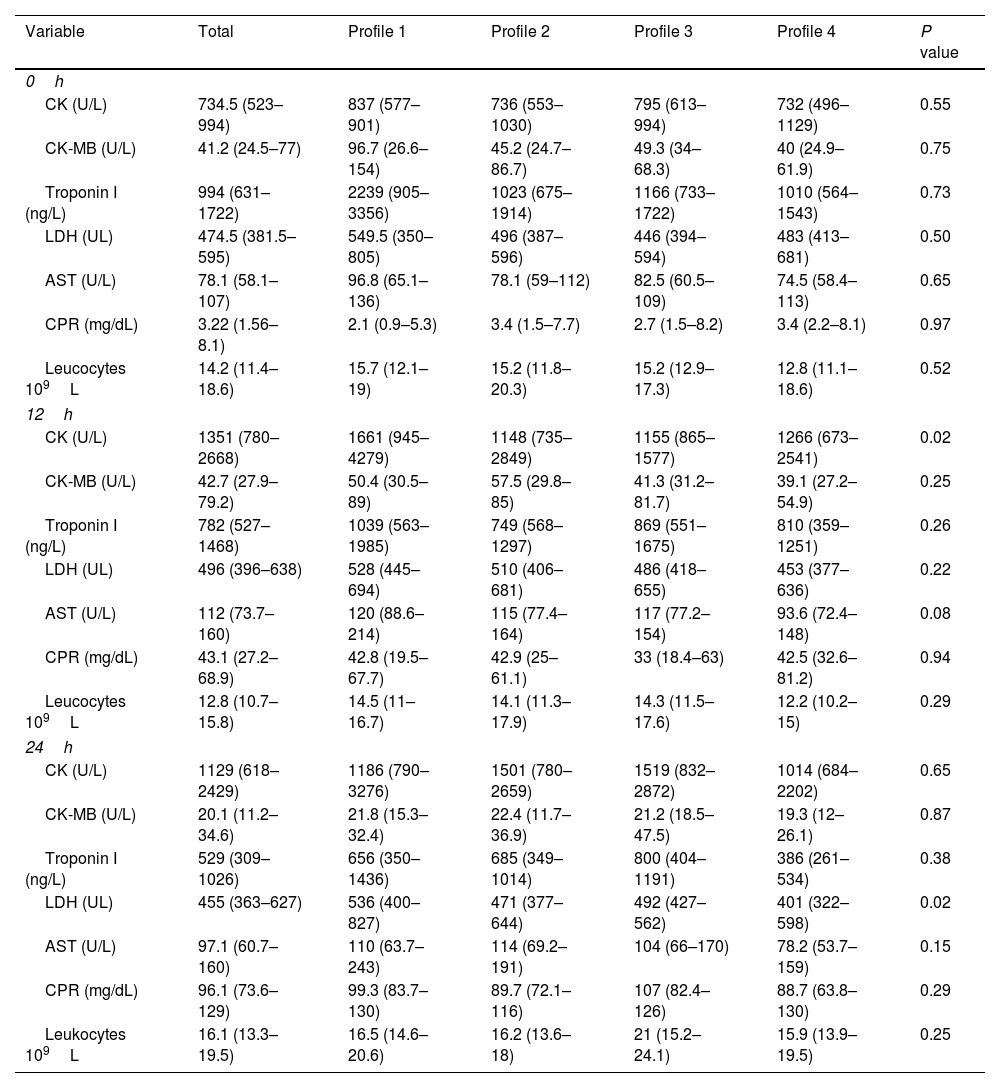
Analysis of the muscle enzyme levels and markers of muscle damage and inflammation was performed on admission and at 12 and 24h after surgery and compared between the four hemodynamic profiles. A clinically and statistically significant elevation of CK at 12h was observed in patients with low macrocirculatory flow and anaerobic metabolism (1661U/L, profile 1) compared with the other profiles (1148, 1155, and 1266U/L for profiles 2, 3, and 4, respectively; P value: 0.02). There was also a significant elevation in the LDH levels in patients with profile 1 (536U/L) compared with the other profiles at 24h (471, 492, and 401U/L for profiles 2, 3, and 4, respectively; P value: 0.02). The levels of the skeletal muscle enzymes and markers of muscle damage and inflammation did not differ significantly between the groups at the three measurement times (on admission and 12 and 24h postoperatively).
Muscle damage and inflammatory markers.
| Variable | Total | Profile 1 | Profile 2 | Profile 3 | Profile 4 | P value |
|---|---|---|---|---|---|---|
| 0h | ||||||
| CK (U/L) | 734.5 (523–994) | 837 (577–901) | 736 (553–1030) | 795 (613–994) | 732 (496–1129) | 0.55 |
| CK-MB (U/L) | 41.2 (24.5–77) | 96.7 (26.6–154) | 45.2 (24.7–86.7) | 49.3 (34–68.3) | 40 (24.9–61.9) | 0.75 |
| Troponin I (ng/L) | 994 (631–1722) | 2239 (905–3356) | 1023 (675–1914) | 1166 (733–1722) | 1010 (564–1543) | 0.73 |
| LDH (UL) | 474.5 (381.5–595) | 549.5 (350–805) | 496 (387–596) | 446 (394–594) | 483 (413–681) | 0.50 |
| AST (U/L) | 78.1 (58.1–107) | 96.8 (65.1–136) | 78.1 (59–112) | 82.5 (60.5–109) | 74.5 (58.4–113) | 0.65 |
| CPR (mg/dL) | 3.22 (1.56–8.1) | 2.1 (0.9–5.3) | 3.4 (1.5–7.7) | 2.7 (1.5–8.2) | 3.4 (2.2–8.1) | 0.97 |
| Leucocytes 109L | 14.2 (11.4–18.6) | 15.7 (12.1–19) | 15.2 (11.8–20.3) | 15.2 (12.9–17.3) | 12.8 (11.1–18.6) | 0.52 |
| 12h | ||||||
| CK (U/L) | 1351 (780–2668) | 1661 (945–4279) | 1148 (735–2849) | 1155 (865–1577) | 1266 (673–2541) | 0.02 |
| CK-MB (U/L) | 42.7 (27.9–79.2) | 50.4 (30.5–89) | 57.5 (29.8–85) | 41.3 (31.2–81.7) | 39.1 (27.2–54.9) | 0.25 |
| Troponin I (ng/L) | 782 (527–1468) | 1039 (563–1985) | 749 (568–1297) | 869 (551–1675) | 810 (359–1251) | 0.26 |
| LDH (UL) | 496 (396–638) | 528 (445–694) | 510 (406–681) | 486 (418–655) | 453 (377–636) | 0.22 |
| AST (U/L) | 112 (73.7–160) | 120 (88.6–214) | 115 (77.4–164) | 117 (77.2–154) | 93.6 (72.4–148) | 0.08 |
| CPR (mg/dL) | 43.1 (27.2–68.9) | 42.8 (19.5–67.7) | 42.9 (25–61.1) | 33 (18.4–63) | 42.5 (32.6–81.2) | 0.94 |
| Leucocytes 109L | 12.8 (10.7–15.8) | 14.5 (11–16.7) | 14.1 (11.3–17.9) | 14.3 (11.5–17.6) | 12.2 (10.2–15) | 0.29 |
| 24h | ||||||
| CK (U/L) | 1129 (618–2429) | 1186 (790–3276) | 1501 (780–2659) | 1519 (832–2872) | 1014 (684–2202) | 0.65 |
| CK-MB (U/L) | 20.1 (11.2–34.6) | 21.8 (15.3–32.4) | 22.4 (11.7–36.9) | 21.2 (18.5–47.5) | 19.3 (12–26.1) | 0.87 |
| Troponin I (ng/L) | 529 (309–1026) | 656 (350–1436) | 685 (349–1014) | 800 (404–1191) | 386 (261–534) | 0.38 |
| LDH (UL) | 455 (363–627) | 536 (400–827) | 471 (377–644) | 492 (427–562) | 401 (322–598) | 0.02 |
| AST (U/L) | 97.1 (60.7–160) | 110 (63.7–243) | 114 (69.2–191) | 104 (66–170) | 78.2 (53.7–159) | 0.15 |
| CPR (mg/dL) | 96.1 (73.6–129) | 99.3 (83.7–130) | 89.7 (72.1–116) | 107 (82.4–126) | 88.7 (63.8–130) | 0.29 |
| Leukocytes 109L | 16.1 (13.3–19.5) | 16.5 (14.6–20.6) | 16.2 (13.6–18) | 21 (15.2–24.1) | 15.9 (13.9–19.5) | 0.25 |
CK: creatine kinase, LDH: lactic dehydrogenase, AST: aspartate transaminase, CPR: C-reactive protein.
The most frequent postsurgical syndrome was hypovolemia (40.7%), followed by mediastinal bleeding (11.8%) and low cardiac output syndrome (9.6%). The most frequent adverse outcome was acute kidney injury (37.5%), followed by liver injury (20.1%), delirium (12.2%), and hospital-acquired pneumonia (10.9%). In-hospital mortality was 6.7%. The logistic regression model for outcomes showed a higher probability of developing low cardiac output syndrome in patients with elevated CK level at 12h (OR 3.16, CI 95% 1.23–8.09; P value: 0.01). Also, those patients with elevation of the LDH at 24hours had a higher probability to develop in-hospital mortality (OR 3.32, CI 95% 1.07–10.29, P value: 0.03), delirium (OR 2.55, CI 95% 1.14–5.7, P value: 0.02), need of transfusion (OR 1.74, CI 95% 1.08–2.82, P value: 0.02), acute kidney injury (OR 1.8, CI 95% 1.09–2.96, P value: 0.02), renal replacement therapy (OR 4.82, CI 95% 1.37–16.96, P value: 0.01), liver failure (OR 2, CI 95% 1.07–3.71, P value: 0.02), and low cardiac output syndrome (OR 7.57, CI 95% 2.22–25.78, P value: <0.01) (Supplemental Table 2).
Postsurgical syndromes and outcomes.
| Variable | Totaln=280 |
|---|---|
| Post-surgical syndromes | |
| Hypovolemian (%) | 114 (40.7) |
| Mediastinal bleedingn (%) | 33 (11.8) |
| Low cardiac output syndromen (%) | 27 (9.6) |
| Vasoplegic syndromen (%) | 19 (6.8) |
| Outcomes | |
| Transfusionn (%) | 128 (46) |
| Acute kidney injuryn (%) | 105 (37.5) |
| Liver injuryn (%) | 56 (20.1) |
| Deliriumn (%) | 34 (12.2) |
| In-hospital pneumonian (%) | 30 (10.9) |
| Renal replacement therapyn (%) | 19 (6.8) |
| In-hospital mortalityn (%) | 18 (6.7) |
| Mediastinitisn (%) | 12 (4.3) |
| Cerebrovascular diseasen (%) | 8 (2.9) |
| Days in intensive care unitMedian (IQR) | 3 (2–4) |
| Days with mechanical ventilationMedian (IQR) | 1 (1–1) |
| Days of hospitalizationMedian (IQR) | 9 (7–14) |
| SOFA score at 24hMedian (IQR) | 4 (3–6) |
| SOFA score at 72hMedian (IQR) | 2 (2–4) |
SOFA: Sequential Organ Failure Assessment.
Skeletal muscle is highly dependent on energy availability; 95% contributes to mitochondrial metabolic activity. Intracellular energy is present in the form of ATP, adenosine diphosphate (ADP), adenosine monophosphate (AMP), and CP. The anaerobic production of ATP can occur through two pathways in skeletal muscle: CP degradation and glycogen metabolism. Skeletal muscle contains large stores of CP that can donate a high-energy phosphate to an ADP molecule, converting it to ATP, a reaction catalyzed by the enzyme CK. Skeletal muscle also contains large reserves of glycogen and cytoplasmic enzymes capable of producing ATP through glycolysis. Glycogen metabolism leads to the production of pyruvate. To maintain this pathway, pyruvate is converted to lactate by LDH with the release of a hydrogen ion.
During skeletal muscle ischemia, lactate production is continuous, and its accumulation acidifies the intracellular environment and inhibits glycolysis. The low ATP concentration induces an increase in cytosolic Na concentration. The Na–Ca2+ antiporters attempt to restore cytosolic Na concentration, which leads to cytosolic Ca2+ accumulation. This accumulation of Ca2+ can cause irreversible damage to cellular integrity by degrading cellular enzymes such as phospholipases, lysozymes, proteases and nucleases, which contribute to inflammation and cell death through necrosis and apoptosis.8,13
In states of shock or in the presence of circulatory instability, muscle ischemia secondary to the diversion of flow toward more vital areas stimulates the aerobic pathways for the generation of ATP through the consumption of reserves of PC and glycogen, which leads to an increase in CK and LDH enzymes activity. Therefore, the elevation of these enzymes in an appropriate clinical context could be a marker of muscle damage or ischemia and may reflect an overall imbalance in the VO2–DO2 relationship. Given the short half-life of these enzymes, restoration of their normal levels may constitute a goal for hemodynamic resuscitation.
Of note, elevation of LD at 24h was an important marker of adverse outcomes, including increased mortality. This could be secondary to the depletion of muscle glycogen stores converted to pyruvate and subsequently lactate, in low flow states of skeletal muscle. However, LD can also be hyperactivated by the hyperdynamic and hyperadrenergic state of cardiac surgery (with increased glycolytic activity and “aerobic” lactate production),14 and also secondary to small intestinal ischemia in low-flow states.15 However, it definitely constitutes a marker of severity, coinciding with a profile of low macro and microcirculatory flow.
It is important to mention that these elevations were not related to differences in the inflammatory response, as shown by leukocyte count and C-reactive protein level, nor of more cardiac-specific muscle proteins, such as AST and troponin I. This finding suggests that the elevations observed were secondary to the effects of a state of low systemic flow on skeletal muscle.
Postcardiotomy cardiogenic shock represents the maximum expression of low cardiac output syndrome after cardiac surgery. It refers to the hemodynamic situation in which cardiac output is unable to satisfy tissue metabolic demands. Postcardiotomy cardiogenic shock is defined by a decreased cardiac output that leads to hypotension and hypoperfusion, cardiac index <2.0L/min/m2, systolic blood pressure <90mmHg (or the need for vasopressors to achieve systolic blood pressure ≥90mmHg), pulmonary capillary wedge pressure ≥16mmHg, and oliguria.16 Alterations in tissue perfusion and oxygenation due to damage to microcirculation contribute to the development of organ dysfunction as well as poor clinical outcomes.17
In our study, the presence of elevated CK at 12h was associated with the development of postcardiotomy low cardiac output syndrome. Although not necessarily indicative of a causal relationship, this association may provide an indicator of the presence or severity of the state of low circulatory flow.
Study limitationsThis study was conducted at a single medical center and, therefore, should be replicated at other centers to assess the reproducibility of the results. The results of the study should be interpreted with caution given the small sample size and the fact that it focused on a specific subpopulation of patients during the postoperative period after cardiac surgery.
ConclusionIn the first 24h after cardiac surgery, the presence of a state of low macrocirculatory flow with the consequent deficit flow at the capillary–cell interface in the presence of anaerobic metabolism was associated with clinically and statistically significant elevation of the levels of CK at 12h and LDH at 24h. These may be markers of skeletal muscle ischemia and may provide an additional tool in the monitoring and resuscitation of these critically ill patients.
Authors’ contributionsDMS: Original idea, methodology, analysis and writing the original draft, review and editing, RGN: analysis and writing the original draft, review, JOSD: methodology, data collection, RES: writing the original draft, RSG: writing the original draft, JLES: data collection, GMJR: analysis, review, GRV: review.
Ethical considerationsThe local institutional research and ethics committees waived approval for this study.
Patient consent statementThe patient or a legally authorized representative provided written informed consent for patient information and images to be published.
Data and material availabilityThe data that support the findings of this study are available on request from the corresponding author [DMS].
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that there are no conflicts of interest to disclose.
To all the staff of the Cardiovascular Critical Care Unit of the Instituto Nacional de Cardiología Ignacio Chávez.