The use of biological valves in the aortic position has become more liberal in recent years due to improvements in prostheses and the possibility of performing valve-in-valve procedures, thus avoiding anticoagulation.
MethodsWe retrospectively evaluated 246 adults in whom the Crown PRTTM biological valve was used in the aortic position, including elective and emergency cases, isolated and combined surgeries (CS). We also evaluated mortality at 1, 3, and 5 years of follow-up.
ResultsIn this study, CS involved 94 patients (38%), while 39 patients (16%) underwent urgent or emergency procedures, which included cases of aortic dissection and endocarditis. Approximately 69% of the patients received a valve more significant than 21mm. A minimally invasive surgical approach was employed in 42 patients (17%). The in-hospital mortality for the entire patient population was 3.6% (n=9), with isolated aortic valve replacement (AVR) accounting for 3.3% (n=5) and CS for another 4.3% (n=4). The mortality for isolated AVR and CS in elective situations was n=2 (1.3%) and n=1 (1.1%), respectively. During the follow-up period, only seven patients required reoperation, with two patients (0.8%) experiencing structural valve deterioration and five other patients (2.1%) requiring reoperation due to prosthetic valve endocarditis.
ConclusionThe use of the Crown valve in the aortic position appears to be safe regarding postoperative morbidity and mortality. Further studies are necessary to assess its applicability in younger patients and predict its performance in the event of a valve-in-valve procedure.
El uso liberal de válvulas biológicas en posición aórtica en los últimos años se debe a la posibilidad de evitar la anticoagulación, a mejoras en las prótesis y la posibilidad de realizar procedimientos de ViV, por sus siglas en inglés, ante futuras reintervenciones.
MétodosEvaluamos retrospectivamente a 246 adultos en quienes se utilizó la válvula biológica Crown PRTTM en la posición aórtica, incluyendo casos electivos y de emergencia, así como cirugías aisladas y combinadas (CC). También evaluamos la mortalidad a 1, 3 y 5 años de seguimiento.
ResultadosNoventa y cuatro pacientes (38%) recibieron CC, mientras que 39 pacientes (16%) se sometieron a procedimientos urgentes o de emergencia, que incluyeron casos de disección aórtica y endocarditis. En el 69% se implantó una válvula de más de 21mm. Se empleó cirugía mínimamente invasiva en 42 pacientes (17%). La mortalidad hospitalaria total fue del 3,6% (n=9), en RVA aislado fue del 3,3% (n=5) y en CC del 4,3% (n=4). La mortalidad por RVA aislado y CC en situaciones electivas fue de n=2 (1,3%) y n=1 (1,1%), respectivamente. Durante el período de seguimiento, siete pacientes requirieron reoperación, 2 pacientes (0,8%) con deterioro estructural de la válvula y cinco pacientes adicionales (2,1%) requiriendo reoperación debido a endocarditis protésica.
ConclusiónEl uso de la válvula Crown en la posición aórtica parece ser seguro en cuanto a morbilidad y mortalidad postoperatorias. Se necesitan estudios adicionales para evaluar su aplicabilidad en los pacientes más jóvenes y predecir su rendimiento en caso de un procedimiento de ViV.
The use of biological prostheses in aortic position has had a more liberal use in recent years. The possibility of percutaneous valve replacement has generated this type of valve become more eligible for doctors as well as patients, even in the youngest patients, because of the possibility of Valve in Valve 1(ViV) procedures in the future and avoid of anticoagulation. This last is added to previous well-known indications as patients with contraindications to anticoagulants, pregnant, and patients over 65 years 2 old.
In Latin America, the adoption of valve prostheses has been limited primarily due to their high cost. Consequently, this limitation has created the opportunity to obtain multiple options within each healthcare facility, allowing the selection of the most suitable choice for each patient based on established effectiveness and the clinical judgment of the 3surgeon.
The Crown PRTTM prosthesis (Corcym, United Kindom) has been used during the last years. However, its performance at mid-term follow-up has not been well proved yet.
This article aims to demonstrate the safety and efficacy of Crown prosthesis in aortic position after five years of follow-up in one single institution in Chile.
Materials and methodsStudy populationThis study is a single-center, single-arm observational design to assess the safety of the Crown PRTTM prosthesis (Corcym, United Kindom) when used in the aortic position. Baseline characteristics, perioperative details, and in-hospital outcomes were systematically recorded in the computerized cardiac surgical database at Las Higueras Hospital in Talcahuano, Chile. The study encompassed all patients aged 18 years and older (n=275) who underwent isolated or combined surgeries involving aortic valve replacement (AVR) utilizing the biological Crown PRTTM valve between January 2017 and June 2022. Exclusion criteria included AVR procedures prostheses other than the Crown PRTTM and patients under 18 years of age. Out of these, 29 patients were excluded due to loss of follow-up, leaving 246 patients for analysis.
Throughout the study period, 570 AVR procedures were performed at our center, excluding Transcatheter Aortic Valve Implantation (TAVI). The study protocol received approval from the Institutional Review Board of Las Higueras Hospital (approval number: 5442; approval date: August 29, 2022), and the board waived the requirement for obtaining individual patient consent.
Study device and surgical proceduresThe device consisted of a bovine pericardial valve mounted outside the stent, which theoretically optimizes the valve performance maximizing the flow area. This tri-leaflet bioprosthetic valve is also the evolution of the Mitroflow pericardial valve, which has 25 years of follow-up.
Usually, elective, isolated AVR was performed under cardiopulmonary bypass (CPB) using normothermia with aortic cross clamping. Only six patients (2.4%) had moderate hypothermia at 26°C during hemi-arch or arch replacement following an acute type A aortic dissection surgery. In all the cases, an intraoperative transesophageal echocardiography was used to exclude paravalvular leaks and intraoperative valve complications. Regarding the surgical approach, a mini upper sternotomy to the four intercostal spaces was used in 42 patients (17%) and a full sternotomy in 204 patients (83%). Central cannulation was used in all elective surgeries. However, when a hemi-arch or arch replacement was required, a second arterial cannula was placed in the innominate artery or right axillary artery according to surgeon preference and case presentation.
The decision to implant a current valve was based on the indication for surgical AVR, appropriate risk profile, and surgeon criterion after patient consenting. Regrettably, not all patients underwent postoperative trans-thoracic echocardiography follow-up, restricting the current article to clinical outcomes. We will reserve this evaluation for its second publication at the 10-year follow-up, where it can attain statistical significance to recommend or dismiss its usage in scenarios with a high reoperation rate. All patients received 100mg of Aspirin for life after their surgery. Also, 180 patients (74%) received anticoagulation therapy following the American College of Cardiology/ American Heart Association Guidelines 2016, as well as surgeon criteria.
Study endpoints and follow-upTo describe the results of a real-world patient population using Crown prosthesis in the aortic position, this article was analyzed as only isolated AVR, combined surgery (CS) using the named aortic valve, and the entire cohort, which also comprise elective, urgency, and emergency cases.
Endpoints were evaluated during the in-hospital and after 1, 3, and 5 years of follow-up. Primary endpoints were in hospital, early and late mortality after AVR in patients using Crown PRTTM (Corcym, United Kindom). Secondary endpoints were in hospital and late postoperative complications associated with the procedure and valve-related complications (structural valve dysfunction that require reoperation and endocarditis). All events that could have influenced in early or late mortality were also analyzed, included all-cause mortality, major bleeding that required reoperation, major paravalvular leak (PVL), valve thrombosis, endocarditis, valve explant, non-structural valve dysfunction, and structural valve dysfunction (SVD). Major PVL was defined as a PVL of any grade resulting in intervention or considered a serious adverse event. The definition of SVD included dysfunction or deterioration involving the operated valve (exclusive of infection or thrombosis), as determined by reoperation.
Statistical analysisWe divided the patient's data into three categories: perioperative, intraoperative, and postoperative variables. Continuous variables were described as mean±standard deviation or median with interquartile range, while categorical variables were presented as proportions, frequency (n, %), and Odds Ratio (OR). To estimate the freedom from all-cause mortality at 1, 3, and 5 years, we used Kaplan Meier analysis. We compared the type of surgery (AVR or CS), type of intervention (Elective or Urgency), and prosthesis size (≤21, >21) variables for each Kaplan-Meier test to assess their contribution to population mortality. Statistical significance was considered when the p-value was <0.05. We conducted all analyses using IBM SPSS Statistics for Windows, version 26.0, Armonk, NY: IBM Corp.
ResultsBaseline characteristics and in-hospital outcomes of the entire cohortBaseline characteristics are shown in Table 1. Of the whole cohort, 161 patients (65%) were female, with a mean age of 66±11 years (26-85), BMI of 28±5 (18- 43), CS comprised 94 patients (38%), urgency/emergency 39 patients (16%) counting aortic dissection and endocarditis. The mean EUROscore II was 3.2±3.6 (1-27).
Preoperative baseline characteristics of the entire cohort. Isolated aortic valve replacement and combine surgery are also detail.
| AVR | CS | Total | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Gender (male) | 94 (62%) | 67 (71%) | 161 (65%) |
| Age (years) | 66 (±12) | 66 (±11) | 66 (±11) |
| Weight (Kg) | 74 (±12) | 77 (±16) | 75 (±14) |
| Height (cm) | 162 (±9) | 164 (±10) | 163 (±9) |
| BMI_kg/m2 | 28 (±4,5) | 29 (±4,5) | 28 (±5) |
| Creatinine (mg/dL) | 1,1 (±0,6) | 1,1 (±0,7) | 1,1 (±0,6) |
| Euroscore_II | 2,4 (±3,2) | 4,4 (±3,9) | 3,2 (±3,6) |
| >50% | 133 (88%) | 61 (65%) | 194 (79%) |
| LVEF. ≤50%->30% | 13 (8,6%) | 21 (22%) | 34 (14%) |
| ≤30% | 6 (3,9%) | 12 (13%) | 18 (7,3%) |
| Dyslipidemia | 119 (78%) | 56 (60%) | 175 (71%) |
| Diabetes | 25 (16%) | 29 (31%) | 54 (22%) |
| Hypertension | 8 (5,3%) | 9 (9,6%) | 17 (6,9%) |
| COPD | 36 (24%) | 24 (26%) | 60 (24%) |
| PAD | 48 (32%) | 40 (43%) | 88 (36%) |
| AF | 113 (74%) | 85 (90%) | 198 (80%) |
| Pulmonary hypertension | 14 (9,2%) | 8 (8,5%) | 22 (8,9%) |
| Dialysis | 1 (0,7%) | 2 (2,1%) | 3 (1,2%) |
| Aortic dissection | 4 (2,6%) | 11 (12%) | 15 (6,1%) |
| Endocarditis | 5 (3,3%) | 2 (2,1%) | 7 (2,8%) |
| Stroke | 3 (2%) | 3 (3,2%) | 6 (2,4%) |
| Myocardial infarction | 0 (0%) | 6 (6,4%) | 6 (2,4%) |
AF: Atrial fibrillation; AVR: Aortic valve replacement; BMI: Body mass index; COPD: chronic obstructive pulmonary disease; CS: Combined surgery; LVEF: Left ventricle ejection fraction; PAD: Peripheral artery disease
Intra-operative characteristics and in-hospital outcomes for the entire population are shown in Table 2. The 69% (n=170) of the patients received a valve bigger than 21mm. A minimally invasive surgery was done in 42 patients (17%). As expected, CPB time and cross-clamp time were significatively less in isolated AVR versus CS [73±24 (40 – 268) and 59±17 (32 – 186) versus 117±42 (61-256) and 93±28 (51 – 178)] respectively.
Intra operative characteristics of the entire cohort. Isolated aortic valve replacement and combine surgery are detail separately.
| AVR | CS | Total | |
|---|---|---|---|
| N 152 (%) | N 94 (%) | N 246 (%) | |
| Type of intervention | |||
| Elective | 134 (88%) | 73 (78%) | 207 (84%) |
| Urgency | 18 (11,8%) | 21 (22%) | 39 (16%) |
| Type of surgery | |||
| Sternotomy | 112 (74%) | 92 (98%) | 204 (83%) |
| Mini-Sternotomy | 40 (26%) | 2 (2,1%) | 42 (17%) |
| Cross-Clamp time (min) | |||
| <60 | 96 (63%) | 9 (10%) | 105 (43%) |
| ≥60 | 56 (37%) | 85 (90%) | 141 (57%) |
| CPB) time (min) | |||
| ≤120 | 147 (97%) | 59 (63%) | 206 (84%) |
| >120 | 5 (3,3%) | 35 (37%) | 40 (16%) |
| Prothesis Size | |||
| ≤21 | 57 (38%) | 19 (20%) | 76 (31%) |
| >21 | 95 (63%) | 75 (80%) | 170 (69%) |
AVR: Aortic valve replacement; CPB: Cardiopulmonary bypass; CS: Combined surgery
In-hospital postoperative adverse events are shown in Table 3; only 5 patients (2%) needed ECMO assistance and dialysis after the surgery. Central bleeding account for 14 patients (5,7%) and pacemaker implantation 18 patients (7,3%). Postoperative events in isolated AVR and elective cases are also depicted in the same table.
In-hospital mortality and adverse events of the entire cohort. Isolated aortic valve replacement and combine surgery are detail separately.
| AVR | CS | Total | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Oral anticoagulants | 108 (71%) | 72 (77%) | 180 (73%) |
| Bleeding with reintervention | 8 (5,3%) | 6 (6,4%) | 14 (5,7%) |
| Pacemaker | 8 (5,3%) | 10 (11%) | 18 (7,3%) |
| ECMO | 2 (1,3%) | 3 (3,2%) | 5 (2%) |
| renal insufficiency | 8 (5,3%) | 5 (5,3%) | 13 (5,3%) |
| Dialysis | 3 (2%) | 2 (2,1%) | 5 (2%) |
| Mediastinitis | 0 (0%) | 1 (1,1%) | 1 (0,4%) |
| Sepsis | 3 (2%) | 3 (3,2%) | 6 (2,4%) |
| Cardiogenic Shock | 1 (0,7%) | 6 (6,4%) | 7 (2,8%) |
| Stroke | 3 (2%) | 5 (5,3%) | 8 (3,3%) |
| AF | 29 (19%) | 24 (26%) | 53 (22%) |
| Mechanical respiratory support (>8h) | 44 (29%) | 43 (46%) | 87 (35%) |
| In hospital mortality (Elective) | 2 (1,3%) | 1 (1,1%) | 3 (1,2%) |
| In hospital mortality (Total) | 5 (3,3%) | 4 (4,3%) | 9 (3,7%) |
AF: atrial fibrillation; AVR: Aortic valve replacement; ECMO Extracorporeal membrane oxygenation; CS: Combined surgery
In-hospital mortality for the entire population was 3.6% (n=9/246); of them, isolated AVR 3.3% (n=5/181) and CS was 4.3% (n=4/94). Mortality for isolated AVR and CS in elective situations was n=2 (1.3%) and n=1 (1.1%).
Patients operated in urgency, those with acute aortic dissection, prior myocardial infarction, and primary brain stroke show more risk of death until 1 year of follow-up. CPB time more than 120min and CS was also associated with increased mortality. On other hand, postoperative variables related to mortality are oral anticoagulants use, dialysis, mechanical ventilatory support more than 8hours and reintervention for bleeding. Table 4.
Risk factors associated with early and medium-term mortality.
| PREOPERATIVE VARIABLES | Period | OR | p | Risk |
|---|---|---|---|---|
| Aortic dissection | IH (<30d) | 13 | 0.01 | > 13 |
| Stroke | IH (<30d) | 4.9 | 0.04 | > 4,9 |
| Myocardial infarction | IH (<30d) | 5.7 | 0.02 | > 5,7 |
| 1 year | 3.3 | 0.04 | > 3,3 |
| Intraoperative Variables | Period | OR | p | Risk |
|---|---|---|---|---|
| Type of intervention (Urgency) | IH (<30d) | 7.3 | 0.00 | > 7,3 |
| 1 year | 3.6 | 0.02 | > 3,6 | |
| 3 years | 3.0 | 0.06 | > 3 | |
| 5 years | 2.6 | 0.02 | > 2,6 | |
| Type of surgery (Combined) | 1 year | 2.4 | 0.03 | > 2,4 |
| CPB time (>120 min) | IH (<30d) | 4.8 | 0.007 | > 4,8 |
| 1 year | 2.9 | 0.02 | > 2,9 | |
| Prothesys type (≥21) | 5 years | 0.38 | 0.01 | <62% |
| Postoperative variables | Period | OR | p | Risk |
|---|---|---|---|---|
| Oral anticoagulants | IH (<30d) | 0.22 | 0.01 | <72% |
| 1 year | 0.39 | 0.02 | <61% | |
| 3 years | 0.39 | 0.01 | <61% | |
| 5 years | 0.45 | 0.03 | <55% | |
| Bleeding with reintervention | IH (<30d) | 15 | 0.00 | > 15 |
| 1 year | 7.5 | 0.00 | > 7,5 | |
| 3 years | 5.4 | 0.00 | > 5,5 | |
| 5 years | 4.8 | 0.00 | > 4,8 | |
| Dialysis | IH (<30d) | 156 | 0.00 | > 156 |
| 1 year | 38 | 0.00 | > 38 | |
| 3 years | 28 | 0.00 | > 28 | |
| 5 years | 25 | 0.00 | > 25 | |
| Sepsis | 3 years | 6.7 | 0.01 | > 6,7 |
| 5 years | 6.0 | 0.02 | > 6 | |
| Cardiogenic Shock | 1 year | 25 | 0.00 | > 25 |
| 3 years | 18 | 0.00 | > 18 | |
| 5 years | 16 | 0.00 | > 16 | |
| Mechanical respiratory support (>8h) | IH (<30d) | 4.5 | 0.02 | > 4,5 |
| 1 year | 5.2 | 0.00 | > 5,2 | |
| 3 years | 3.1 | 0.00 | > 3,1 | |
| 5 years | 2.8 | 0.00 | > 2,8 |
CPB: Cardiopulmonary bypass; OR: odds ratio
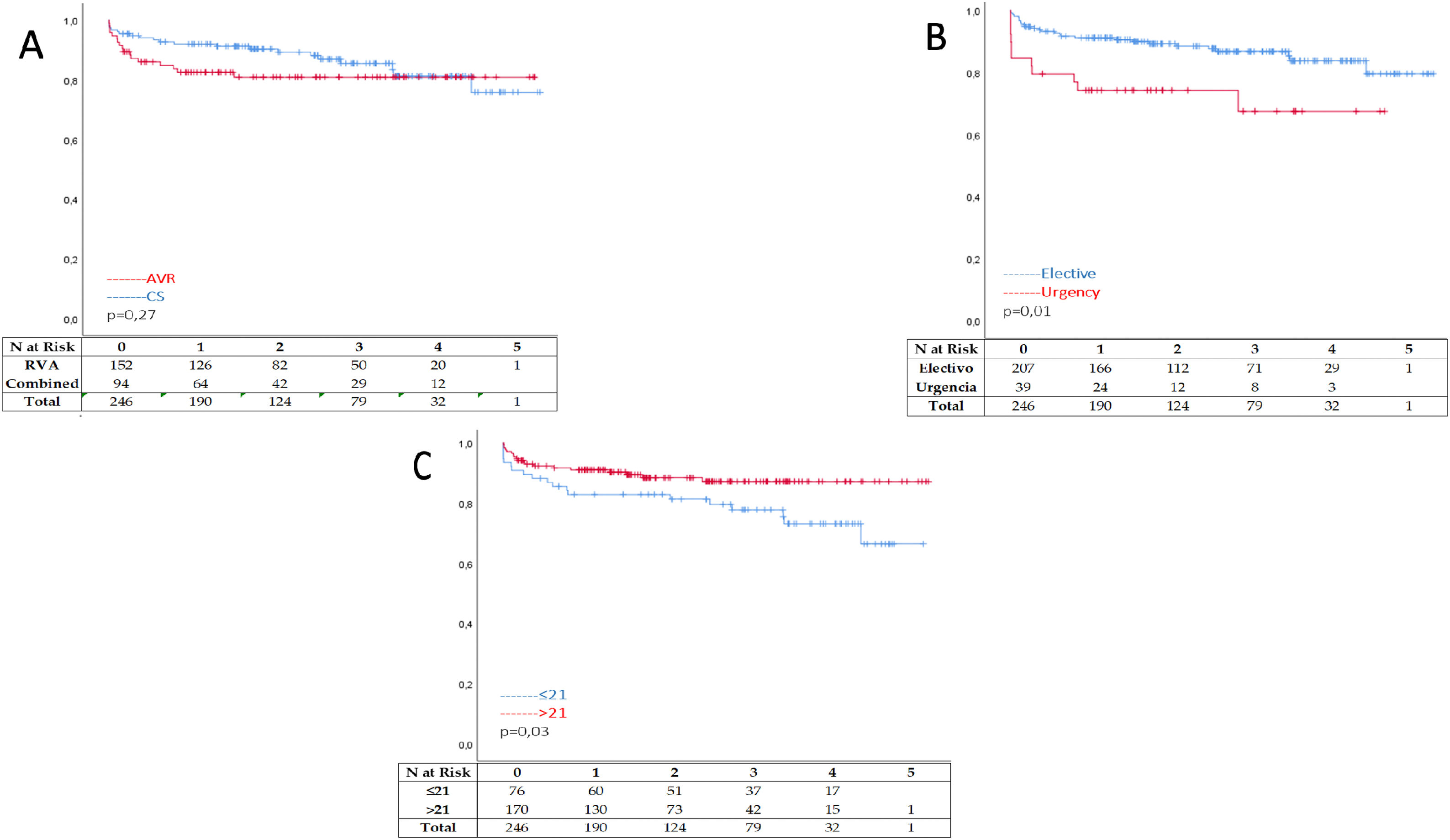
Freedom from all-cause mortality after one year of follow-up shows better results after an isolated AVR when compared with CS p=0.03 Figure 1 A. Also, elective surgery shows significantly less mortality after one year than patients operated in urgency p< 0.05 Figure 1 B. Nevertheless, patients with valves smaller than 21mm had no statistical difference p=0.07 over prostheses bigger than 21mm after one year of follow-up. Figure 1C. At 3 and 5 years of follow-up, difference are less impressive when comparing isolated RVA with CS p=0.12 and p=0.27 Figure 2 A. Curves grew apart in favor of elective surgery when this is compared with urgency cases p<0.05 and p=0.01 Figure 2 B. Regarding valve size, valves smaller than 21mm had significantly worse outcomes at 5 years compared with valves bigger than 21mm p=0.09 and p=0.03 Figure 2C respectively. During follow-up, only seven patients needed reoperation, two patients (0. 8%) because SVD happened, and 5 other patients (2.1%) after un prosthetic valve endocarditis was diagnosed.
The significant finding of this article underscores the safety of the Crown PRTTM prosthesis (Corcym, United Kindom), a biological bovine tissue valve, when used in the aortic position during mid-term follow-up. To our knowledge, this is the initial study presenting results spanning five years of follow-up for the Crown prosthesis within a real-world patient population. Notably, this population includes both urgent and combined surgical cases, which adds credibility that may be lacking in articles influenced by corporate interests.
The surgical replacement of diseased heart valves is based on the assumption that the prosthesis chosen will improve or prevent further deterioration of heart function, relieve symptoms, improve functional status, and prolong overall survival.4 Thus, it is a trade-off between the disease's natural course, the chances of surgery, and the recognized complications of prosthetic heart valves. None of the available valve substitutes is closest to being ‘ideal’.5,6 Each valve substitute has suitable inherent advantages and disadvantages for all patient subgroups.
Issues and potential complications related to mechanical valves have been described and discussed on previous occasions.3,7 We only refer to two common limitations frequently encountered in our population: the cost constraints to their use and the failure to conduct follow-up assessments for patients far from healthcare facilities. These limitations can lead to vocational losses of anticoagulation therapy.8
Over the last few years, bioprosthetic valves have steadily increased due to their enhanced hemodynamics, improvements in longevity, and reduced complication rates.9 However, it is crucial to address this context by determining if the outstanding durability observed in the latest generation bioprostheses among older age groups can be confidently extended to younger surgical candidates. This information is important when deliberating potential treatment strategies, including elective bioprosthetic aortic valve replacement for younger candidates and the possible future use of ViV technique.10
Particularly for patients over 60 years of age; the event-free life expectancy is better with a bioprothesis than a mechanical prosthesis. On the other hand, these valves are susceptible to SVD, especially in younger patients requiring multiple reoperations in their life. Patient-related risk factors for SVD have been previously described 11 and should be considered before selecting the most suitable prosthesis for each patient. One of the prominent mechanisms by which SVD occurs with tissue valves is believed to be due to calcification over time; several methods of processing leaflet tissue have been developed to try to reduce tissue calcification, bovine pericardial as the valve we analyze are associated with less leaflet calcifications.12
Another essential factor to consider when choosing the best valve is the patient's life expectancy. If the patient's life expectancy is shorter than the anticipated lifespan of the prosthetic valve, then that valve would be a suitable choice for that patient.
A French study 13 assessed the performance of biological heart valves in a cohort of patients under 60 (n=416), with 55% of these patients falling within the 50-60 age range. The study reported an overall mortality rate of 3.6%, which included 34.9% of cases involving CS. The 15-year freedom of need for reoperation stood at 55.4%, and the 15-year stretch from deaths directly related to the valve was 97.1%. Although there is a significant risk of SVD in patients over 60 years old at the time of surgery, the durability of bioprosthetic valves remains consistent during the first decade, showing no signs of accelerated SVD. Notably, there were no differences in long-term freedom from SVD across different age subgroups, and the long-term risk of thromboembolic and hemorrhagic events remained exceptionally low. Similar to the findings reported earlier in Chile.14 All the above aligns with our results as well. Out of the total number of patients analyzed, 59 (21.45%) were under 60, and 9 (3.27%) were under 40 years old, for whom a biological valve was primarily chosen due to limited access to postoperative healthcare follow-ups. However, only seven patients required surgical reintervention, with only two cases being due to SVD. All of this contradicts what was published by Etnel et al., where they found that the use of bioprostheses in the aortic position in patients under the age of 55 carries a higher risk of structural deterioration and reoperation. However, it is associated with a lower risk of bleeding and thromboembolism.5
Even Becker et al., in an analysis of 142 patients with biological aortic valves, including the Crown prosthesis, reported an operative mortality of 2.8% and a 5-year survival rate of 86%.3 It is essential to highlight the excellent outcomes observed in our series. Despite the 3.6% overall mortality rate, including emergency cases, elective AVR's mortality is just 1.3%. Furthermore, in CS elective cases, the mortality rate was as low as 1.1%.
Also, Bartus et al. 15 evaluated patients (n=133) with an average age of 65.3 years and a 5-year follow-up after a Resilia Valve was used in the aortic position. They reported a 30-day mortality of 2.3% and that 12% of the patients underwent surgery through a mini-sternotomy approach.
Choosing the best valve for each patient, or in other words, recommending the “king” of biological valves in the aortic position, continues to be a topic of debate due to the lack of information in the literature.16 A new model, with different tissue preservation and an anti-calcification method but otherwise similar construction, was introduced with the Crown PRTTM. Long-term results for the Crown model are lacking.17
A study in Sweden evaluated 16,983 patients with bioprosthetic aortic valves, including Mitroflow/ Crown prostheses, with an average age of 76.3 years. Of these patients, 3% required valve replacement through conventional surgery or transcatheter procedures. They found that the incidence of reoperation was higher in patients with Mitroflow or Crown valves compared to the Perimount Magna valves after ten years of follow-up, with rates of 12.2% versus 3.6%. The 5-year survival rate for these valves was 77%. The study concluded that Mitroflow /Crown valves were associated with a higher risk of rehospitalization due to heart failure, reoperation, and mortality at the 10-year.17
In 2021, Montero Cruces et al 4 published hemodynamic results comparing the Crown, Trifecta, and Magna Easy valves in a one-year study (n=154). The median age was 76.5 years, with 14.9% of cases performed using a mini-sternotomy and 38% using a 21mm valve. After one year, there were no statistically significant differences among the three valves regarding postoperative events or survival.
From Latino America, the BYPASS study in Brazil 18,19 reported its results on 920 patients from 17 centers over 4 years, with a mean age of 56.7 years. The 30-day mortality rate was 7.3%, including CS, while the isolated AVR mortality was 5.1%, and only 1.6% of their cases were performed through minimally invasive surgery.
Persson et al. evaluated 1643 Mitroflow/Crown valves without differentiation between these two lasts. The mean age was 74.8 years. 36.4% of the population had a valve smaller than 21mm. They founded a 5-year survival rate of 77%, and emergency cases account for 1.7% of their patients. Reintervention at five years was 3,5%. We found an 85% survival rate at five years only with crown prosthesis counting 16% of emergency cases. For isolated AVR at five years freedom from all-cause mortality was 91%. At five years our reoperation rate for all causes was 2.8%. Its performance in patients with small aortic annuli has also been evaluated in 55 patients.20 The study confirmed excellent, effective orifice area (EOA) for 21mm and 23mm valve sizes, demonstrating its suitability for use in patients with small annuli. Our 31% of valves sized 21mm or smaller coincides with the general literature on patients with small annuli. However, in our series, especially at 3 and 5 years of follow-up patients using valves smaller than 21mm died more than those with larger valves. It's important to note that our population in Chile comprises individuals of shorter stature, resulting in even smaller annuli, some as small as 17mm. Therefore, our percentage of small valves is relatively low. While the precise definition of small annuli remains somewhat unclear and should be evaluated based on each patient's body surface area, we concur with avoiding mismatch and selecting valves that provide gradients suitable for the patient's specific needs.
The performance of the Mitroflow valve has been assessed multiple times,21,22 even after 25 years of follow-up, showing a perioperative mortality of 2.5%. Rates of 20-year actuarial freedom from valve-related complications were as follows: SVD was 84.8% (actual rate was 96.6%) in patients aged 70 years or older.
Finally, while the possibility of performing ViV procedures is becoming increasingly feasible, there are several crucial points to consider when choosing a prosthetic valve with future ViV treatment in mind. Many of these patients have low coronary implantation, which requires a higher level of expertise during implantation. Additionally, 30% of prostheses sized 21mm or smaller may contraindicate the possibility of ViV in some cases due to the risk of severe mismatch. In South America, it is important to consider the limited availability and cost of prostheses when contemplating ViV procedures.23,24
ConclusionThe utilization of the Crown prosthesis in the aortic position appears to be safe regarding postoperative morbidity and mortality. Research with extended follow-up periods and data on gradients is required to ascertain its efficacy in comparison to alternative devices, as well as its suitability for younger patients.
LimitationsThe primary limitation of this study lies in its retrospective nature. We are aware of the absence of echocardiographic data regarding postoperative gradients and the value it would add to the topic. As a result, we were unable to investigate the relationship between the valve model and structural valve disease based on contemporary definitions, apart from its clinical presentation involving reintervention and mortality. Nevertheless, we believe that the clinical evaluation, encompassing complications and, most importantly, long-term mortality in a series of real-world cases without discriminating elective status or risk, holds the utmost significance when deciding whether or not use a particular prosthesis.
Institutional Review Board Approval Number5442; date of approval: August 29, 2022.
FundingNone.
Conflict of InterestThe authors have nothing to disclose.
We would like to express our gratitude for the excellent work of Yosselin Vargas and Abigail Valle during data storage and collection.