Aortic valve-sparing operations were developed at Toronto General Hospital in early 90's in an aim to preserve normal native aortic valve in the setting of root disease. This procedure has been mastered over the last 30 years. In this manuscript, we present our clinical and long-term outcomes using the implantation technique for both tricuspid and bicuspid valves, along with a detailed step-by-step explanation of the surgical technique, based on our experience.
ResultsThe reimplantation technique can be performed in many elective and urgent situations, but not all valves are suitable for optimal repair and an extensive valve morphology assessment using echocardiogram or intraoperative direct assessment, has been the key to achieve excellent long-term results.
Our group showed 1% postoperative death with low pacemaker implantation (1.9%) and stroke rates (0.6%). All patients left the operating room with mild or less aortic regurgitation. Excellent perioperative results are achieved with a perfect and careful surgical technique. A detailed step-by-step description of the reimplantation technique is provided in this manuscript. Important technical considerations are included. Our series showed 84% 15-year survival and less than 10% recurrence of more than mild aortic regurgitation.
ConclusionsThe valve-sparing root replacement procedure provides excellent long-term clinical outcomes in terms of survival, freedom from reoperation and valve-related complications, along with stable aortic valve function, for both tricuspid and bicuspid aortic valve with favorable cusp morphology.
La operación de preservación de la válvula aórtica fue desarrollada en el Hospital General de Toronto a inicios de los años noventa con el objetivo de preservar las válvulas aórticas nativas normales en el contexto de la enfermedad de la raíz. Este procedimiento se ha ido perfeccionado en los últimos 30 años. En este manuscrito presentamos nuestros resultados clínicos perioperatorios y a largo plazo utilizando la técnica de implantación aórtica tanto para válvulas tricúspides como bicúspides, junto con una explicación detallada paso a paso de la técnica quirúrgica, basada en nuestra experiencia.
ResultadosLa técnica de reimplantación aórtica puede emplearse en situaciones electivas y urgentes, pero no todas las válvulas son aptas para una reparación óptima, haciendo necesaria una evaluación exhaustiva de la morfología valvular mediante ecocardiograma y mediciones directas intraoperatorias, siendo la clave para lograr excelentes resultados a largo plazo.
Nuestro grupo presentó el 1% de muerte postoperatoria, con una baja tasa de implantación de marcapasos (1,9%) y de accidentes cerebrovasculares (0,6%). Todos los pacientes abandonaron el quirófano con regurgitación aórtica ligera o menor. Para unos resultados postoperatorios excelentes es necesario completar una técnica quirúrgica pulida y cuidadosa. En este manuscrito se ofrece una descripción detallada paso a paso de la técnica de reimplantación, incluyendo consideraciones técnicas importantes. Nuestra serie mostró una supervivencia a los 15 años del 84% y menos del 10% de recidiva de insuficiencia aórtica mayor a ligera.
ConclusionesLa operación de preservación de la válvula aórtica proporciona excelentes resultados clínicos a largo plazo en términos de supervivencia, tasa de reintervención y complicaciones relacionadas con la válvula, junto con una función estable de la válvula aórtica, tanto para válvulas aórticas tricúspides como para las bicúspides, con morfología de cúspide favorable.
Thirty years ago, patients with aortic root disease, either with concomitant aortic stenosis or regurgitation, were treated with mechanical or biological valved conduits along with reimplantation of the coronary ostia. After many years of understanding the aortic root anatomy in the animal laboratory and in humans, developing the Toronto Stentless Porcine (SPV) aortic valve prosthesis, and performing isolated sinus of Valsalva aneurysm replacements, we realized that a structurally normal aortic valve may exist despite the presence of a dilated root. These particular cases appeared to be an opportunity to preserve the native valve while removing all diseased aortic tissue. The first aortic valve sparing operation was attempted in 1989 and an initial series of 10 patients was then published.1
The initial technique mimicked a Bentall procedure, preserving the patient's native aortic valve. First, the aneurysmal portion of the ascending aorta and the aortic sinuses of Valsalva were excised, leaving a rim of aortic wall attached to the left ventricular outflow tract. The valve was implanted within a tubular Dacron graft secured to the aortic annulus. The aortic rim remnant was used as a hemostatic layer when sutured inside the graft. The coronary ostia were reimplanted in the graft, and the distal end of the graft was connected to the native aorta.
Although the series was small, the results were encouraging; only one valve failed to be preserved, all patients were discharged in good condition, and after 30 months of follow-up, all were symptom-free with competent native aortic valves, and none were on anticoagulants. This new procedure also avoided the potential problems associated with prosthetic valves including prosthetic valve endocarditis.
Simultaneously with our publication, another technique aimed at preserving the aortic valve was developed, the remodeling procedure by Yacoub, which was published in 1993.2 In this technique, each valve commissure was sutured to a graft cut longitudinally to form three separate sinuses, thus recreating neo-sinuses of Valsalva, in contrast to the reimplantation technique. A major difference between the two techniques was that the original remodeling technique did not include external annuloplasty and thus did not stabilize the aortic annulus. This led, in some patients, particularly those with genetic aortic disorders to develop annular dilatation and late aortic insufficiency. Therefore, the reimplantation has become the procedure of choice for patients with inherited connective tissue disorders of the aortic root, as it was shown to provide optimal long-term annular stabilization and freedom from aortic insufficiency and reintervention.3 This technique has become known as the David procedure in recognition of its primary inventor.
Over the past 30 years, the technique has been perfected and well described, making it reproducible worldwide. This manuscript summarizes the experience with the aortic valve reimplantation technique at the Peter Munk Cardiac Centre, its pioneer center.
IndicationsAortic valve-sparing (AVS) surgery was originally designed to preserve the “morphologically normal” native cusps of tricuspid aortic valves. The reported experience after two decades4 showed that this technique was mainly used in these patients, as only 11% had bicuspid aortic valves. Nevertheless, the results after 10 years of follow-up were excellent, with a survival rate of 93.1±4.4% and 97.8±5.3% free of reoperations, with 92.9±6.5% free from more than mild aortic insufficiency. These outcomes, together with the improvement and familiarization with the surgical technique, have extended its indications to include patients with severe aortic insufficiency due to cusp prolapse, regurgitant bicuspid aortic valves (BAV) and acute aortic dissections.
Although many bicuspid aortic valves may also benefit from AVS, we suspect that some morphologies (i.e. very asymmetrical valves) will be more prone to failure over time.5,6 However, the number of cusps is less important than the quality of tissue. The principal criterion to accept a patient for AVS is normal or near normal aortic cusps tissue. Small fenestrations are not a contraindication. Any calcification beyond the raphe of BAV is a contraindication. We avoid dysmorphic, calcified or restricted BAV leaflets, keeping the repair of these leaflets simple using plication or free-edge reinforcement, with minimal raphe excision. The results of this conservative approach have been recently reported by our group, through a propensity-matched analysis comparing outcomes after AVS in patients with bicuspid vs. tricuspid valves. This showed no significant difference in the likelihood of developing aortic insufficiency over time between the groups when patients were carefully selected.7
Even though most patients treated with this technique are elective cases, patients with acute aortic syndromes (AAS) may also benefit from AVS surgery in selected cases. However, AAS themselves are associated with increased operative morbidity and mortality, and AVS in this setting adds additional operative complexity and myocardial ischemic time. Therefore, AVS in the setting of AAS should be performed only by experienced surgeons.8,9
Technical principles of reimplantationWe will provide a detailed step-by-step description of how our group performs the AVS procedure, focusing on certain important considerations.10 First, the aortic root should be systematically evaluated with transesophageal echocardiography to predict the possibility of preserving the valve as well as any additional techniques that may be required for an effective and durable repair. The size of the aortic annulus, the geometry and morphology of the cusps, and the orientation of the commissures are all necessary to consider.11 Despite echo evaluation, these parameters can be difficult to fully obtain prior to surgery. However, once the aortic valve is exposed the entire root morphology including the size of the aortic annulus, geometric height of the cusps and detailed anatomy can be assessed and also confirmed using the commercially available caliper (MSS-1, Fehling Instruments, Karlstein, Germany).
Aortic root preparationOur first goal is to prepare the aortic root. Distal aortic arch cannulation is routine, which allows for maximal resection of the diseased tubular ascending aorta and reduces the risk of dissection and thromboembolism during cannulation. After applying the cross clamp, we transect the mid-portion of the ascending aorta and arrest the heart with root or ostial cardioplegia delivery depending on the degree of aortic insufficiency. The aortic root is dissected from surrounding structures; it is necessary to reach at least 5mm below the aorto-ventricular junction to properly stabilize the aortic annulus. Deep circumferential dissection helps maintain repair durability in the dilated annulus.12 The aortic sinuses are excised and the coronary buttons are mobilized, leaving a 3–5mm aortic remnant around the aortic annulus and coronary artery orifices.
Sizing the Dacron graftThe size of the Dacron graft can be estimated by using the height of the cusps, the height of the commissure between the left and non-coronary cusps, and the idealized diameter of the sinotubular junction. As the aortic annulus itself is often pathologically dilated, we do not use it to estimate graft size. The estimated “normal” STJ diameter is obtained by pulling the three commissures upward and approximating them until all the cusps touch. We believe that the cusp height is the most important measurement for determining graft size and this can be estimated using the original formula,1 which is average heights of the aortic cusps multiplied by two-thirds. The size of the graft is then increased 6–8mm to accommodate the added thickness of the left ventricular outflow tract just beneath the aorto-ventricular junction. The most commonly used graft sizes have ranged from 26 to 32mm for the tricuspid aortic valve and from 30 to 34mm for the bicuspid aortic valve.
Left ventricular outflow tract suture placementA circumferential annuloplasty at the level of the basal ring is performed with 9–12 horizontal mattress 2-0 polyester sutures with Teflon pledgets, which are used to minimize the risk of anterior mitral valve leaflet or membranous ventricular septum perforation. Mattress sutures are placed from the inside of the left ventricular outflow tract, along a single horizonal plane as much as possible. The pledgets must not touch the belly of the aortic cusps. The suture corresponding to the sub-commissural triangle of the right and non-coronary cusps is placed slightly higher or skipped to avoid the bundle of His. These circumferential sutures are necessary for annular stabilization, especially in patients with hereditary thoracic aortic disease and annulo-aortic ectasia.
The aortic cusps are examined and the Dacron graft is divided according to the native intercommissural distance. Often the left aortic cusp is smaller and its intercommissural distance in the graft should be proportionally smaller. Mattress sutures must be passed through the graft with the same degree of scalloping as they were placed in the left ventricular outflow tract and distributed correctly in space. Once completed, the entire aorto-ventricular junction must be within the graft. Otherwise, the rate of early failure of this procedure will increase.
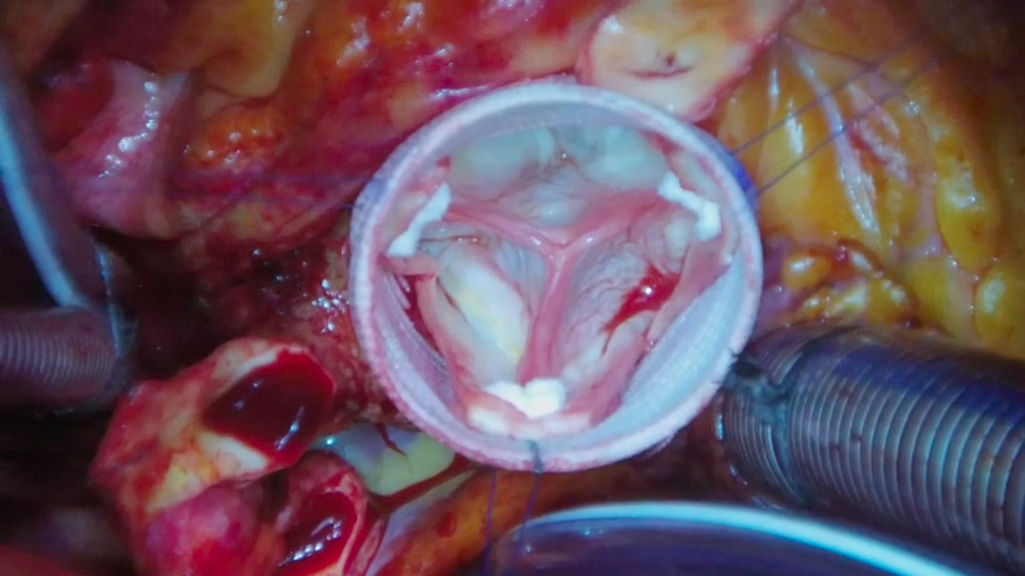
Commissural reimplantation and hemostatic suture lineCareful commissural positioning inside the graft is required to ensure proper leaflet coaptation. To do this, the graft is mildly stretched and each commissure is pulled upward inside, aligned with the sub-commissural triangles and fixed with a 4-0 polypropylene pledgeted suture. Cusps must meet each other in a central position at the middle of the Dacron graft and above the aortic annulus (Fig. 1). This is probably the most critical part of the entire operation. If the three cusps are not properly aligned in both a horizontal and vertical plane the surgeon should make appropriate adjustments until the cusps are correctly aligned. Fixing misaligned cusps after the fact, while possible, is not ideal. Once satisfied with the placement of the commissures, these sutures are tied and each arm is used to attach the remnants of the aortic sinuses to the Dacron graft starting at the commissure and ending at the nadir of the aortic sinus.
Cusp assessment and repairThe free margin of each leaflet must lay 9–10mm above the nadir (effective height) which can be easily assessed using a caliper.13 If the effective height is less than 9mm, leaflet prolapse should be corrected by plication of the central cusp portion (nodule of Arantius). If the leaflets are excessively thinned or contain fenestration, the free margins can be reinforced with a double layer of a fine Gore-Tex suture by weaving it in and out the free margin from commissure to commissure.10
At this point the surgeon should fill the aortic root with saline to see how much, if any, fluid leaks through the valve into the left ventricle. Minimal or no leaking at this stage is predictive of a successful repair. If the leak is very noticeable then the leaflet alignment should be carefully reassessed and adjusted as necessary before proceeding with the remainder of the operation. Once satisfied with the leaflet coaptation, the coronary buttons are reimplanted into their respective sinuses in typical fashion.
Pressure test and image evaluation of the valvePrior to the completion of the distal anastomosis, the valve can be tested by clamping the graft and injecting high-pressure root cardioplegia. The root Dacron must be under pressure and the unvented left ventricle must remain empty. Once the reconstruction of the root is completed, root and valve functioning must be confirmed with transesophageal echo after weaning from cardiopulmonary bypass. We do not tolerate more than mild aortic regurgitation (AR), eccentric jets or a coaptation height less than 8mm or coaptation length less than 4mm as they are all predictors of AR recurrence.
Early and long-term resultsThe results after 30 years of experience in our center have been published14 showing excellent results for the AVS procedure in all patients, including tricuspid (85.1%) and bicuspid (14.4%) valves, patients with connective tissue disorders (38%) and urgent cases of acute aortic dissection (7.1%).
A successful aortic valve reimplantation was achieved in all patients, although the second patient on this series (0.2%) required a Bentall procedure on second postoperative day due to recurrence of severe AR. See Table 1 for details of the operations performed. Intraoperative mortality was 1% with a low postoperative risk profile with a reoperation rate of 8% (mainly due to postoperative bleeding, 7.3%), low stroke rate (0.6%) and pacemaker implantation (1.9%), and a median hospital stay of 6 days.
Operative data.
| Reimplantation of the aortic valve, n (%) | 465 (100) |
| Size of graft, mm, median (IQR) | 30 (28–32) |
| Aortic cusp plication, n (%) | 178 (38.2) |
| Free margin reinforcement with Gore-Tex, n (%) | 111 (23.8) |
| Creation of neo-aortic sinuses, n (%) | 153 (32.9) |
| Mitral valve repair, n (%) | 38 (8.1) |
| Mitral valve replacement, n (%) | 1 (0.2) |
| Tricuspid annuloplasty, n (%) | 2 (0.4) |
| Coronary artery bypass, n (%) | 46 (9.9) |
| Replacement of aortic arch/hemiarch, n (%) | 52 (11.2) |
| Closure of atrial septal defect, n (%) | 25 (5.3) |
| Closure of ventricular septal defect, n (%) | 3 (0.6) |
| Maze procedure, n (%) | 7 (1.5) |
| Repair of abdominal aortic aneurysm, n (%) | 1 (0.3) |
| Cardiopulmonary bypass time, min (IQR) | 137 (118–163) |
| Aortic clamping time, min (IQR) | 113 (98–135) |
The 20-year survival was 73% with a low rate of developing aortic regurgitation greater than mild (11.8% at 20 years). The cumulative proportion of aortic valve reoperations at 20 years was 7.0% (95% CI, 4–12.2%). The indication for reoperation was recurrence of moderate or severe AR (84% of the patients) and infective endocarditis (16%). Among the patients who experienced AR recurrence and required reoperation, most underwent valve replacement although re-repair is attempted if the mechanism of the aortic insufficiency is identifiable and amenable to repair. In a multivariable analysis performed we could not identify any variable associated with AR recurrence. Table 2 shows the cumulative incidence of adverse events during long-term follow-up after AVS in our center.
Long-term results after AVS in our center.
| 1 year | 10 years | 20 years | |
|---|---|---|---|
| Death from any cause | 2.5 (1.3–4.6) | 9.6 (6.9–13.3) | 26.7 (20.6–34.2) |
| Moderate/severe AR | 1.2 (0.5–3.0) | 8.7 (6.2–12.3) | 11.8 (8.5–16.5) |
| Aortic valve reintervention | 0.7 (0.2–2.3) | 1.9 (0.9–4.1) | 7.0 (4.0–12.2) |
| Endocarditis | 0.0 | 0.5 (0.1–2.2) | 1.6 (0.4–6.0) |
| Thromboembolism | 1.5 (0.7–3.3) | 6.3 (4.2–9.4) | 9.5 (6.4–14.2) |
| Pacemaker implantation | 2.2 (1.2–4.3) | 5.4 (3.5–8.3) | 7.2 (4.6–11.3) |
| New distal aortic dissection | 1.0 (0.4–2.6) | 3.4 (1.9–5.9) | 13.2 (8.9–19.6) |
Values in percentages (95% CI). AVS: valve-sparing root replacement; AR: aortic regurgitation.
Aortic valve-sparing surgery, over the last three decades, has shown excellent results and has proven to preserve the native aortic valve anatomy and function. This technique was developed to remove the entire diseased aortic root while preserving structurally normal tricuspid aortic valves to avoid the potential complications associated with prosthetic valves, including thromboembolism and endocarditis. However, its indications have been extended to include bicuspid valves and acute aortic syndromes.
Knowledge of the anatomy is essential to safely dissect the aortic root and its elements in order to reduce the potential intraoperative complications. Once a surgeon has mastered the operative technique, long-term outcomes will for the most part depend only the quality of the aortic cusps.
Despite its complexity compared to standard aortic valve replacement, this surgical technique is reproducible and teachable. In this manuscript, we described the different steps of this operation in detail with tips and tricks to avoid pitfalls. Should the reader wish more detail, additional information along with illustrations is available.10
We carefully select our patients in a conservative approach that does not preserve complex valve morphologies that require multiple cusp repairs, as they are more likely to fail over time. We aim to not repair thickened, sclerotic, and/or calcified cusps. We advocate a simple repair with plication, free-edge reinforcement, and/or minimal raphe excision. The results of this conservative approach, either with bicuspid or tricuspid valves, have been reported by our group using a propensity-matched analysis and show excellent valve function over time.7
In our series, only 7% of the patients required aortic valve reoperation after 20 years of follow-up. The Achilles heel of AVS surgery is the recurrence of AR. However, the reoperation rate for valve failure is not comparable to the recurrence of AR rate, given that not all failed valves are re-operated upon. We are well aware that the recurrence of AR after valve-sparing surgery may be underestimated in other centers given the variability in echocardiographic follow-up. We follow-up of all our patients with annual echocardiogram in our center, despite it, we failed to detect any variable associated with valve failure using multivariate analysis.15
ConclusionsValve-sparing root replacement has proven to be a very useful technique with excellent perioperative and long-term clinical outcomes in terms of survival, freedom from reoperation, and valve-related complications associated with artificial valves. Careful selection of patients with favorable cusp morphology increases the success of the technique, which can be used for both tricuspid and bicuspid valves. Careful surgery, with particular attention to the technical details, is the key to achieve an optimal and long-lasting results.
Ethical considerationsThe authors assure that this is an original work and that ethical approval is not required as this study retrieves and synthesizes data from previously published studies.
Conflict of interestThe authors declare that they have no conflict of interest.