In recent years, advances in molecular biology have resulted in innovations in breast cancer diagnostics and therapeutics. The development of genomics has revolutionised our understanding of this disease. MammaPrint® was developed as a diagnostic tool to predict risk of breast cancer recurrence using the expression of 70 genes altering the clinicopathologic paradigm of selection of patients for adjuvant cytotoxic chemotherapy.
Materials and methodsA study of stages I and II breast cancer patients on whom the MammaPrint® genomic assay was performed.
ResultsThe use of the MammaPrint® assay was a decisive factor for the recommendation of adjuvant treatment with chemotherapy and/or hormone therapy in patients with high risk for relapse. In our group, the patients with low-risk have not presented local or systemic recurrences.
DiscussionThe determination of the genetic characteristics and its alterations in breast cancer, is fundamentally important for a better identification of risk, as well as a better selection of cancer therapy.
ConclusionMammaPrint® is an effective study to determine risk of recurrence of in early stage breast cancer.
En los últimos años, los avances en biología molecular han puesto en marcha innovaciones en el diagnóstico y la terapéutica del cáncer de mama. El advenimiento de la genómica ha revolucionado nuestra compresión en esta patología. MammaPrint® fue desarrollado como una herramienta de diagnóstico para predecir el riego de recurrencia de cáncer de mama mediante la expresión de 70 genes. La era de la genómica ha cambiado el paradigma clínico-patológico de selección de paciente para quimioterapia citotóxica adyuvante.
Material y métodosEl estudio incluyó a pacientes con diagnóstico de cáncer de mama en etapa clínica I-II que se hayan sometido al estudio genómico MammaPrint®.
ResultadosEl uso de MammaPrint® fue un factor decisivo para la recomendación de tratamiento adyuvante con quimioterapia, en pacientes con riesgo elevado para recaída. En nuestro grupo de pacientes con bajo riesgo no han presentado recidiva.
DiscusiónLa determinación de las características genéticas y sus alteraciones en el cáncer de mama es de fundamental importancia para una mejor identificación del riesgo, así como una mejor selección de los tratamientos.
ConclusiónMammaPrint® es un estudio eficaz para determinar riesgo/recurrencia de cáncer de mama en etapa temprana.
Breast cancer is a public health challenge due to the increase in cases worldwide. In accordance with the World Health Organisation report, the incidence of this malignant neoplasm may increase up to 50% in 2020, the year in which there would be 15 million new cases worldwide and this would increase to 20,000 cases in Mexico.1
Gene expression profiles are a relatively new technology which identify gene activity to be used as molecular signature to predict prognosis and serve as a guide to treatment.2 Three main genomic tests are available commercially to use in early stage breast cancer: Oncotype DX, MammaPrint® and PAM50.3
The three tests provide an evaluation of the total risk of breast cancer recurrence, although there are major differences between them. In one sense, these 3 genomic tests are biomarkers of prognosis, in virtue of the fact they offer an estimated risk of recurrence and independent prognosis information from that obtained with clinical factors and standard pathological findings.4,5
The terms “prognostic” and “predictive” with frequencies are used indistinctively but there are major differences. In general, a predictive biomarker identifies the patients who may benefit from a specific intervention. A prognostic biomarker sends information about the probable outcome of the disease regardless of the treatment.6,7
The MammaPrint®, unlike the Oncotype DX, which only evaluates 21 genes and may report an uncertain result, is an expression profile of 70 genes initially developed from arrangements in the whole genome of samples from patients with breast cancer.7,8 It is an independent prognostic marker for clinical factors and conventional pathologies, such as tumour size, status of hormonal receptors and status of epidermal growth factor 2 (HER2). This test was submitted to scrutiny and validation by the Food and Drug Administration and was authorised in 2007, as the only genomic test for breast cancer approved to date by this organisation.9,10 It is worth noting that the Ecotype DX was developed on file samples whilst the Mamma Print® was made on fresh tumour tissue, and could therefore imply a benefit in the creation of this test.11
Biological functions of the 70 genes of the MammaPrint® firm are related to the essential necessary steps for tumour progression and metastasis. It reflects the malignant characteristics acquired by a cancerous cell together with the tumour growth process and the biological activities of migration and systemic implantation.12,13 One of the most important characteristics of the study is that it was designed on the basis of total levels of gene expression to divide patients into low and high risk groups, which correspond to survival rates at 10 years free from metastasis at a distance greater than 90% or lower than 90%, respectively.13,14
With analysis of gene expression they may develop other profiles and be read in the tissue submitted for original classification. For example, the tissue submitted for analysis in MammaPrint® may also be used to determine other profiles such as BluePrint®, which establishes molecular subtypes through ARN messenger (ARNm) of 80 genes, classifying them into 4 different subtypes (baseline, luminal A, luminal B and HER2), providing information on sensitivity to chemotherapy and TargetPrint®, which is a microarrangement study of genetic expression that provides a quantitative evaluation of the levels of oestrogen receptors, progesterone receptors and epidermal 2 growth factor (HER2) in the ARNm. TargetPrint® provides a numerical result on a continuous scale between −1.0 and +1.0, with a final positive or negative result.15,16 Combining MammaPrint® with BluePrint® and TargetPrint® means stratifying patients into the following subgroups: low risk luminal type by MammaPrint® (similar to luminal A), high risk luminal type by MammaPrint® (similar to luminal B), epidermal growth factor 2 (HER2) and baseline type.17,18
Although there is no one standard test and none of the technologies were uniformly accepted, many clinics have adopted multiple genes because they are an effective instrument for taking therapeutic decisions in patients with early stage breast cancer.19
The aim of this study was to assess efficacy, understood as the incidence of patients with early stage breast cancer, with high clinical risks but low genomic risk of recurrence, who had not received chemotherapy, with a greater metastasis-free survival, compared with those who had received chemotherapy (46% of women with breast cancer who have a high clinical risk may not require chemotherapy20) and the clinical use of the genomic MammaPrint® test as a prognosis of breast cancer in our sample compared with conventional indications of a clinical and pathological nature.
Material and methodsAn observational, longitudinal, analytical, descriptive and prospective study was carried out. Data collection was made from the clinical file of the Centro de Estudios Mastológicos S.A. of C.V. Mastológica, Lomas9 during the period from August 2011 to November 2015. The universe of the sample was all patients diagnosed with early stage breast cancer (I and II) submitted to the MammaPrint® genomic test.
Patients diagnosed with early stages I and II breast cancer who had undergone the genomic MammaPrint® test were included. Patients whose results were pending at the time of research were not included.
Dependent variables of this study were: clinical breast cancer stage, financial solvency for undertaking the study, histological type of breast cancer, immunohistochemical markers, the presence of affected lymph nodes and tumour size. Independent variables were age and occupation.
The SPSS software programme version 15.0 for MAC (SPSS; Chicago, IL, USA) was used for Statistical analysis.
ResultsThe data collection period included patients who participated in the study from August 2011 to November 2015, for whom follow-up will be given for 10 years, with review intervals every 5 years (minimum period for evaluation of disease-free survival).27 records were located and collated, with 28 cases (one bilateral); only one patient was rejected due to the number of lymph nodes they presented (6); the others presented with the requisites mentioned in the variables, i.e. diagnosed with early stage breast cancer (I or II), tumour size under 5cm and no involvement of lymph nodes from clinical features.
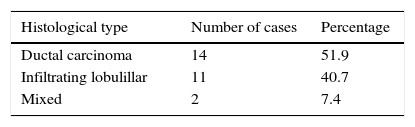
Ages ranged from 38 to 78, with a median age of 52. With regard to histological type, 3 infiltrating ductal variants were found: lobulillar infiltrating and mixed (lobulillar 70%, ductal 30%) (Table 1).
Four biological subtypes were found: luminal A, luminal B, Baseline and HER2, with luminal A being the most common (Table 2).
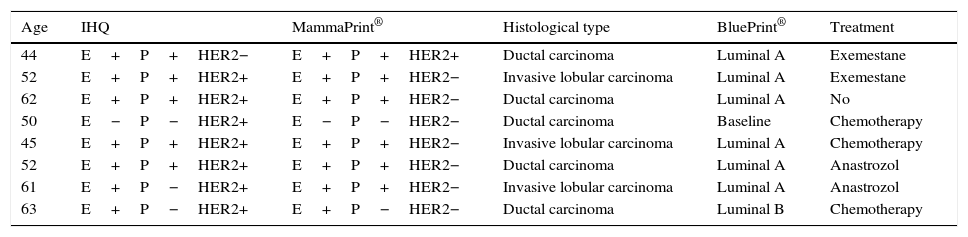
Discrepancies were present in the results of the HER2 markers obtained by immunohistochemistry in comparison with those obtained with MammaPrint® in 8 records; in these cases we have to bear in mind that the MammaPrint® study includes a study called TargetPrint® where the HER2/neu at ARn level is assessed and when negative it was considered that these patients were negative (probably due to an error in reading the immunohistochemistry against the realisation of the inefficient technique of the same), and it was therefore decided not to offer treatment with trastuzumab or carry out complementary studies (Table 3).
Discrepancy of outcome found between immunohistochemical studies and MammaPrint®.
| Age | IHQ | MammaPrint® | Histological type | BluePrint® | Treatment |
|---|---|---|---|---|---|
| 44 | E+P+HER2− | E+P+HER2+ | Ductal carcinoma | Luminal A | Exemestane |
| 52 | E+P+HER2+ | E+P+HER2− | Invasive lobular carcinoma | Luminal A | Exemestane |
| 62 | E+P+HER2+ | E+P+HER2− | Ductal carcinoma | Luminal A | No |
| 50 | E−P−HER2+ | E−P−HER2− | Ductal carcinoma | Baseline | Chemotherapy |
| 45 | E+P+HER2+ | E+P+HER2− | Invasive lobular carcinoma | Luminal A | Chemotherapy |
| 52 | E+P+HER2+ | E+P+HER2− | Ductal carcinoma | Luminal A | Anastrozol |
| 61 | E+P−HER2+ | E+P+HER2− | Invasive lobular carcinoma | Luminal A | Anastrozol |
| 63 | E+P−HER2+ | E+P−HER2− | Ductal carcinoma | Luminal B | Chemotherapy |
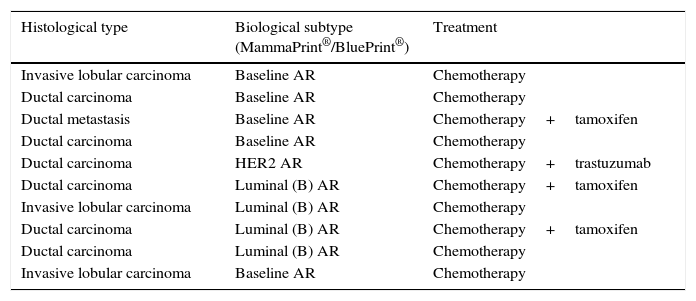
10 cases were identified (37%) considered to be high risk, of subtype luminal, Baseline and HER2, with the most common being the baseline type (Table 4).
High risk cases.
| Histological type | Biological subtype (MammaPrint®/BluePrint®) | Treatment |
|---|---|---|
| Invasive lobular carcinoma | Baseline AR | Chemotherapy |
| Ductal carcinoma | Baseline AR | Chemotherapy |
| Ductal metastasis | Baseline AR | Chemotherapy+tamoxifen |
| Ductal carcinoma | Baseline AR | Chemotherapy |
| Ductal carcinoma | HER2 AR | Chemotherapy+trastuzumab |
| Ductal carcinoma | Luminal (B) AR | Chemotherapy+tamoxifen |
| Invasive lobular carcinoma | Luminal (B) AR | Chemotherapy |
| Ductal carcinoma | Luminal (B) AR | Chemotherapy+tamoxifen |
| Ductal carcinoma | Luminal (B) AR | Chemotherapy |
| Invasive lobular carcinoma | Baseline AR | Chemotherapy |
It is worth mentioning that the biological subtype obtained by the combination of MammaPrint®/BluePrint® was an essential factor for the introduction of adjuvant treatment with chemotherapy, which was implemented separately from its biological type and on the basis of the recommendations provided by the genomic study evaluating the risk of recurrence with and without adjuvant treatment. Up until now, out of the total patients in the study no relapse data have been found for an interval of 5 years and this remains the same for patients with low and high risk.
DiscussionDespite the number of cases being relatively small, this study represents a study conducted in the Mexican population which is an enriching experience, since it is pioneer in Mexico. As it was a high cost study, although major costs were covered by the majority of medical insurances there was a financial limit which some patients who were suitable candidates for treatment and who could have benefited from it, were unable to access. In this group of women with early stage breast cancer, who lacked resources to pay for it, traditional criteria continued being used, for the decision to receive or not receive chemotherapy. Many of them would not have to have this therapy and some kind of strategy should therefore be applied for its incorporation into the national health systems.
The determination of genetic characteristics and its changes in breast cancer are of fundamental importance for a better identification of risk, and a better treatment selection. It is of particular interest in accordance with traditional criteria of favourable prognosis, such as the presence of positive hormonal receptors (oestrogen and progesterone) negative lymph nodes and tumour size. These patients would not have received benefits with chemotherapy treatment. However, if the result of the MammaPrint® classifies them within the high risk group they should receive chemotherapy due to the possibility of recurrence (the pattern of chemotherapy is individually designed in accordance with the mammary panel study obtained from the tumour tissue immunohistochemical test which includes oestrogen receptors, progesterone receptors, HER2neu, Ki-67, p. 53), and this is the main benefit in the use of this genomic signature. Patients with low risk present with positive oestrogen and/or progesterone receptors, and will receive hormonal therapy (tamoxifen in premenopausal women or aromatase in post menopausal women).
Taking into account that this was a prospective study, which required more follow-up time to reach final conclusions, it demonstrated the use of the genomic study for the detection of high risk subtypes and subsequent introduction of personalised adjuvant treatment, in addition to the calculation of recurrence in patients with a diagnosis of early stage breast cancer. Up until now no cases of recurrence have presented in the patients under study.
ConclusionMammaPrint® is an effective study for assessing the risk of recurrence of early stage breast cancer, and providing essential information about the specific type and subtype of each case. It is therefore considered to be a tool of vital importance for taking adjutant chemotherapy administration decisions for a specific patient, avoiding unnecessary treatments, improving quality of life, and saving costs, or in contrast, in patients with high risks, providing benefits of anti-neoplasic therapy to reduce the probability of failure in the mid and long term. This all forms part of what nowadays is considered to be personalised healthcare, and has a direct impact on the reduction of the mortality rate from this disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez-Forgach ER, Carpinteyro-Espín U, Alemán-Áviles JA, Sánchez-Basurto C. Validación y aplicación clínica de MammaPrint® en pacientes con cáncer de mama. Cir Cir. 2017;85:320–324.