Intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery facilitates the identification of anatomical structures in cervical endocrine surgery reducing the frequency of vocal cord paralysis.
ObjectiveTo study the normal electrophysiological values of the vague and recurrent laryngeal nerves before and after thyroid surgery. To compare rates of injury of recurrent nerve before and after the introduction of the intraoperative neuromonitoring in thyroid surgery.
Material and methodsAn observational, descriptive and prospective study in which a total of 490 patients were included. Between 2003 and 2010, surgery was performed on 411 patients (703 nerves at risk) with systematic identification of recurrent laryngeal nerves. Between 2010 and 2011 neuromonitorisation was also systematically performed on 79 patients.
ResultsBefore the introduction of intraoperative neuromonitoring of 704 nerves at risk, there were 14 recurrent laryngeal nerve injuries. Since 2010, after the introduction of the intraoperative neuromonitoring in thyroid surgery, there has been no nerve injury in 135 nerves at risk.
ConclusionsWe consider the systematic identification of the recurrent laryngeal nerve is the ‘gold standard’ in thyroid surgery and the intraoperative neuromonitoring of nerves can never replace surgery but can complement it.
La neuromonitorización intraoperatoria del nervio laríngeo recurrente facilita la identificación de las estructuras anatómicas en cirugía endocrina cervical, disminuyendo así la frecuencia de parálisis de las cuerdas vocales.
ObjetivoEstudiar los valores electrofisiológicos normales de los nervios vagos y recurrentes, antes y después de la cirugía tiroidea. Comparar la tasa de lesiones de los nervios recurrentes antes y después de la introducción de la neuromonitorización en la cirugía tiroidea.
Material y métodosEstudio observacional, descriptivo, y prospectivo en el que se incluyeron un total de 490 pacientes. Entre los años 2003 y 2010, se intervienen 411 pacientes (703 nervios en riesgo), con identificación sistemática de nervios laríngeos recurrentes. Entre 2010-2011, además realizamos la neuromonitorización sistemáticamente de los mismos en 79 pacientes.
ResultadosAntes de la neuromonitorización, de 704 nervios en riesgo, hubo 14 lesiones de nervio laríngeo recurrente. Desde 2010, de 135 nervios en riesgo no se ha identificado ninguna lesión nerviosa.
ConclusionesConsideramos que el «estándar de oro» en la cirugía tiroidea debe ser la identificación sistemática del nervio laríngeo recurrente, y la neuromonitorización de los mismos nunca debe sustituirla, sino complementarla.
Despite many advances in surgical techniques in the last few decades, the risk of recurrent laryngeal nerve injury in thyroid surgery and parathyroid surgery has decreased, but not altogether disappeared. Lahey and Hoover1, and later Ridell,2 described a technique for thyroidectomy with which they attempted to identify the recurrent laryngeal nerve in all cases, instead of continuing the traditional focus of avoiding identification. The superiority of this focus was reported by Hermann et al.,3 who reviewed thyroidectomies for benign diseases from 1979 to 1990, where the nerves were not identified (n=15,865), and from 1991 to 1998, when the recurrent laryngeal nerve was viewed and this was standard practice (n=10,548). These authors show that the risk of permanent injury of the laryngeal nerve in the first group was 1.1%, whilst in the last group, where the recurrent laryngeal nerve was visualised as standard, risk dropped to 0.4%.
The majority of surgeons try to identify the recurrent laryngeal nerve, thereby minimising the risk of damaging it. Despite this technique, several circumstances have been described which may increase the risk of injury. Thomusch et al.4 carried out a multivaried analysis where risk factors were analysed for patients who underwent thyroidectomy for benign disease and they found that a broad degree of resection and recurrent goitre were the independent variables which would contribute to the increase in the probability of recurrent laryngeal nerve injury. Furthermore, Dralle et al.5 identified changed anatomy, major thyroid disease and surgical inexperience as additional risk factors to recurrent laryngeal nerve injury. Routine identification of the laryngeal nerve has been adopted by the majority of surgeons and corresponds to a permanent risk of injury below 2%. Despite this, this complication continues being a problem for patients and surgeons. Permanent dysphonia may result and this has been documented.6 It is not unusual for the nerve injuries to continue being a source of frequent complaints of medical malpractice against the surgeon.7 Nerve monitoring is an attempt to reduce nerve injury risk during thyroid and parathyroid surgery and this has gained in interest in the last few years, although it was also of note in previous decades. At the beginning of the 1960s8 several doctors explored the use of electrical stimulation of the recurrent laryngeal nerve, as a means of its identification and preservation.9 However, it has only been in recent years that this has been used on a regular basis and is available, and for this reason, monitoring has become established. These changes have led to many studies in the last decade which describe intraoperative monitoring of the nerve during thyroid surgery.
The foundations of intraoperative monitoring technology involves 2 components: a method of stimulating the recurrent laryngeal nerve intraoperatively and a method for evaluating the response of the vocal cords to stimulation. Nerve stimulation is performed by low voltage stimulating of the tissues close to the recurrent laryngeal nerve or indirectly through stimulation of the vagus nerve. Monitoring of response to nerve stimulation included different techniques. Several groups described the digital palpation of the criocoid arytenoid muscle during nerve stimulation, as a method of demonstration that the nerve remains intact.10 Riddell11 and later Eltzschig et al.12 described the monitoring of the nerve function through observation of the vocal cord by direct and optic fibre laryngoscopy. This monitoring technique is effective, but requires skill and experience in correct placement of the electrodes. Although less common, the use of electrodes in the postcriocoid area have been described and appear to be effective. The most widely used method of neuromonitoring in recent years has been the use of electrodes in the surface of the endotracheal tube, in part thanks to easy management and commercial availability. No special skill or experience is required for correct placement of the electrode. However, with fibre optic intubation, appropriate placement of the electrodes in contact with the vocal cords is usually more complex.
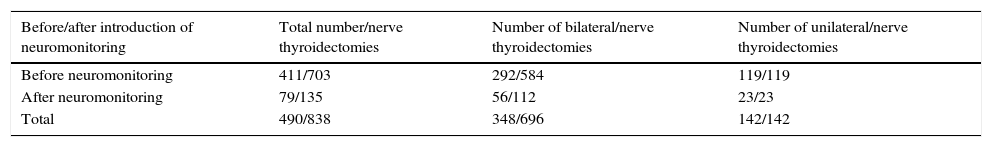
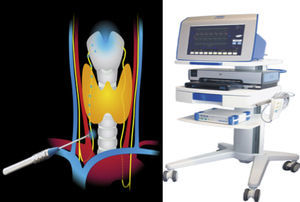
The 3 most used systems of neuromonitoring for recurrent laryngeal nerve are nerve integrity monitor (NIM), a system manufactured by Medtronic Xomed® (Minneapolis, Minnesota, United States), the Nervean system manufactured by Neurovision Médica® (Ventura, California, United States) and the Avalanche® XT Monitor system manufactured by Dr. Langer Medical (Waldkirch, Germany).
The Avalanche® system uses hand probes for stimulation which maybe monopolar or bipolar, and take signals using different electrodes. The stimulation probe transmits very small currents to the tissues which generate potential action in the vocal muscle, when the nerve has been identified and is intact. The surgeon then perceives the potential of muscle action through a visual sign and single acoustic. The muscle action potential is guided by special electrodes through the endotracheal tube used for anaesthesia or through a fine needle electrode transligamentary in the corresponding vocal muscle.
In the monopolar probe, the electric current flows from the tip of the probe through the tissue towards the electrode which is placed in the muscles surrounding the neck. In the bipolar probe, when it touches the tissue, the orbicular electric current flows from one pole to the other through the tissues. In comparison with the monopolar probes, the bipolar probes stimulation field is relatively small with localisation of the nerve in absolute form.
When neuromonitoring is carried out using a type of electromiographic sensor or when laryngeal palpation is used, the neuromuscular block is avoided during induction of general endotracheal anaesthesia. If neuromuscular blocking is required during anaesthetic induction, a short action agent is generally recommended. Marusch et al.13 reported that the monitoring of the recurrent laryngeal nerve may even be performed in the presence of neuromuscular blockers, at least when electrodes are necessarily used in vocal muscles for monitoring the potentials of action. However, and despite this report, neuromuscular blocking must be avoided when electrodes are used in the surface area of the endotracheal tube.
ObjectiveThe objectives of our study were:
- 1.
To study the normal electrophysiological values of the vagus and recurrent nerves before and after thyroid surgery.
- 2.
To compare the rate of injury of recurrent nerves before and after the introduction of neuromonitoring in thyroid surgery.
An observational, descriptive and prospective study was carried out where the findings from intraoperative neuromonitoring of the vagus and recurrent laryngeal nerves were analysed, applied to thyroid or parathyroid pathology, during the period between 1st January 2003 and 31st December 2011. All the patients were attended at the outpatients department by one of the members of the endocrine unit of general surgery service and were included in the study. The patients were incorporated into the study consecutively, and were evaluated by the anaesthesiology service.
In all cases operations were performed by the same team of surgeons. In all patients systematic identification was made of both recurrent nerves, throughout their length.
Furthermore, in those cases where neurostimulation was made (IONM), an electrode was placed on the surface area of the endotracheal tube which was Mallinckrodt® calibre 5.5mm and throughout the whole Avalanche® TX Monitor system by Dr. Langer Medical (Waldkirch, Germany).
All the patients underwent intubation and balanced general anaesthesia performed by the same team of 2 anaesthetists.
In patients with neuromonitoring, 0.5mg/kg i.v. rocuronium was used as a standard muscle relaxant in all of them. This drug competes with the nicotinic cholinergic receptors of the muscle end-plate, with intermediate action and the beginning of fast action.
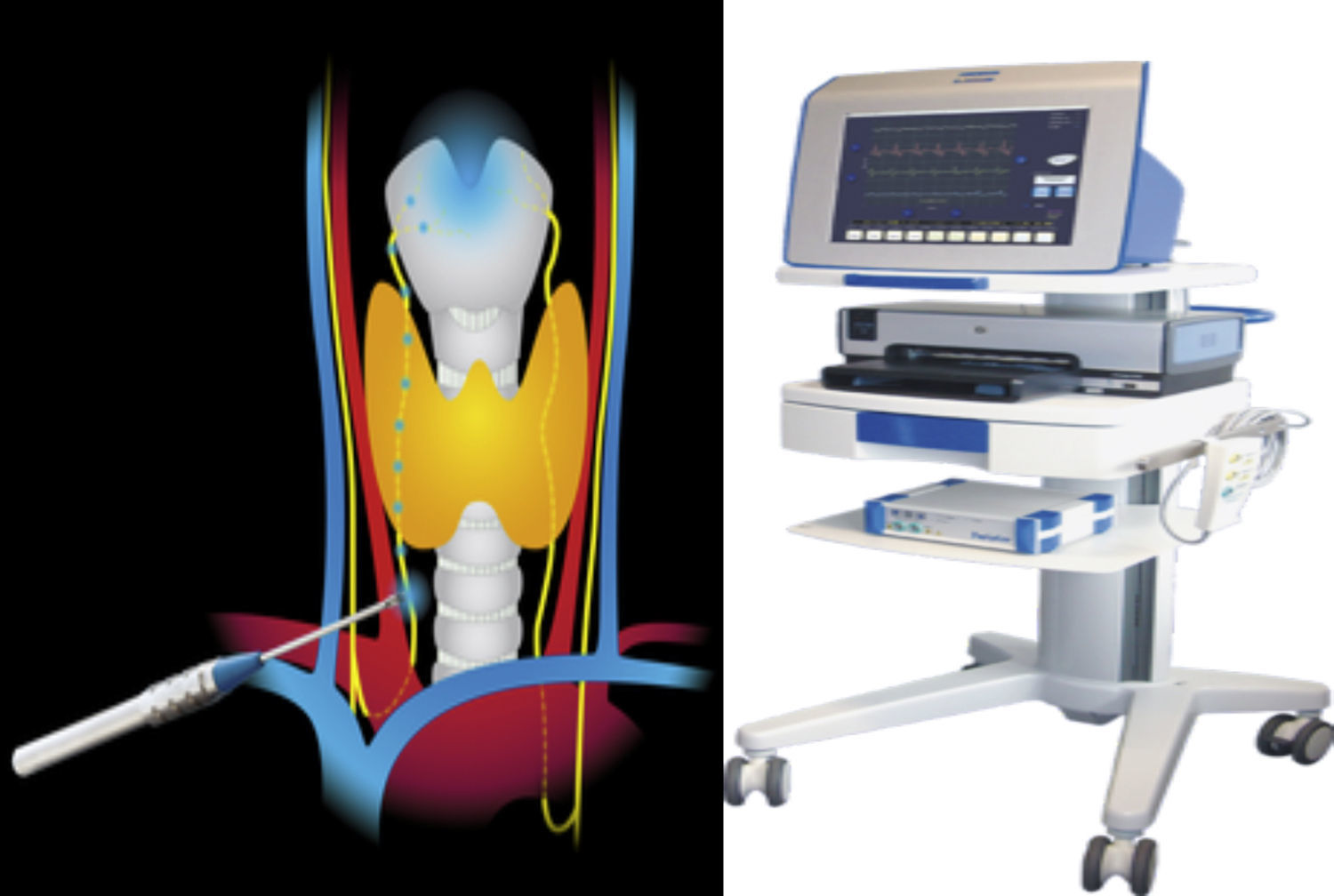
The Avalanche® system uses hand probes for stimulation, which may be monpolar or bipolar, and signals using different electrodes. The stimulation probe transmits very small currents to the tissue which generate potential action in the vocal muscle, when the nerve has been identified and is intact. The surgeon then perceives the potential of muscle action through a visual sign and single acoustic. The muscle action potential is guided by special electrodes through the endotracheal tube used for anaesthesia. We use the bipolar probe which, on maintaining contact with the tissue, the electric orbicular current flows frow one pole to the other through the tissue. Biploar probes have a relatively small stimulation field enabling location of the nerve in absolute form (Fig. 1).
Neuromonitoring technique14The patients was placed in a decubitus supine position, with moderate cervical hyper extension. A Kocher incision was made between the internal edges of both sternocleidomastoid muscles; opening was performed by planes and miocutaneous flaps were prepared. We opened the musculature midline and dissected the said musculature of the thyroid capsule. We dissected the vessels and nerves (comprising the carotid artery from inside, the internal jugular vein from outside and the vagus nerve from behind) prior to dissecting the thyroid gland.
First step. Stimulation of the vagus nerve at the beginning of surgery, which we will call vagus 1 (V1). Dissection of approximately 1–3cm between the carotid vein and the jugular vein, prior to any surgery and to identify the recurrent laryngeal nerve, for the purpose of locating the vagus nerve. Its stimulation with 0.5mA, directly touching it with the bipolar probe. If we do not receive a signal, we increase it to 0.8mA. We would consider equipment has failed if we do not receive a signal after stimulating it to 1.0mA and after checking placement of the endotracheal electrode and the connections. We will call this measurement V1. On the one hand it validates the correct functioning of the system and on the other the integrity of the nerve prior to surgery. It thus serves as control of the nerve over itself.
Second step. Stimulation of the recurrent nerve at the start of surgery, which we will call recurrent 1 (R1). Dissection and identification of the recurrent laryngeal nerve in the tracheal oesophagus groove or the cross-over with the inferior-thyroid artery. It is directly stimulated with the bipolar probe at 0.5mA.
Third step. Stimulation of the recurrent nerve at the end of surgery, which we will call recurrent 2 (R2). Dissection and identification of the recurrent laryngeal nerve in the Berry ligament. It is directly stimulated with the bipolar probe at 0.5mA.
Fourth step. Stimulation of the vagus nerve at the end of surgery, which we will call vagus 2 (V2). Finally, we will stimulate the vagus nerve again, after surgery.
We consensually consider a negative outcome of the test when: 1) there was no electrical response of any nerve following examination at 0.5, 0.8 and 1mA, successively, and on confirming the electrode and the connections and 2) when after a register is obtained, the stimulation of V2 has no electrical transmission.
After surgery we confirmed that said negative corresponded or did not correspond with clinical repercussions in the form of dysphonia or swallowing disorders. In cases where there were clinical features compatible with recurrent injury, laryngoscopy was performed after one month.
Definitive injury was considered to be present when there was paralysis of any cords through laryngoscopy, beyond 6 months after surgery.
All patients had check-ups 30 days and 6 months after surgery.
Statistical analysisFor statistical analysis the SPSS programme for Windows version 17 was used. Quantitative variables were described using the mean, typical deviation and range. For quantitative variables absolute and relative frequencies were obtained, expressed in percentages.
For comparison of injury prevalence before and after neuromonitoring, the exact Fisher test was used. The differences between V1 and V2 were measured, using the Student's t-test for related samples. For all analysis the statistical significance level was fixed at 5%, with a value of α=0.05.
ResultsTotal bilateral or unilateral thyroidectomy was the surgical technique used, with systematic identification of the recurrent laryngeal nerves.
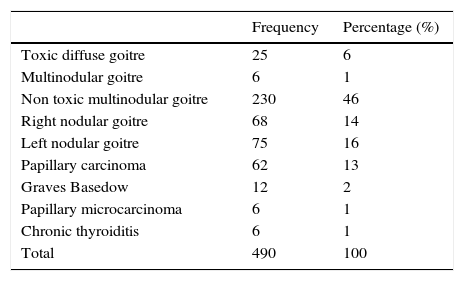
Between 2003 and 2010 a total of 411 patients underwent surgery. 292 total bilateral thyroidectomies were performed (584 nerves at risk) and 119 unilateral thyroidectomies (119 nerves at risk) in this group, with a total of 703 nerves at risk included in the study.
Since September 2010, for systematic identification of recurrent laryngeal nerves, we systematically carried out neuromonitoring of them. During this period, between 2010 and 2011, a total of 79 patients underwent surgery. 56 bilateral thyroidectomise were performed (112 nerves at risk) and 23 total unilateral thyroidectomise s (23 nerves at risk) in this group, with a total of 135 nerves at risk included in the study. The total nerves at risk was therefore 838 (Table 1).
Total thyroidectomies/risk nerves included.
| Before/after introduction of neuromonitoring | Total number/nerve thyroidectomies | Number of bilateral/nerve thyroidectomies | Number of unilateral/nerve thyroidectomies |
|---|---|---|---|
| Before neuromonitoring | 411/703 | 292/584 | 119/119 |
| After neuromonitoring | 79/135 | 56/112 | 23/23 |
| Total | 490/838 | 348/696 | 142/142 |
Of the 490 patients included in the study, 88.6% were women, whilst only 11.4% were men.
We observed that the proportion of patients who had undergone surgery were classified by pathologies before and after the introduction of neuromonitoring and similar percentages were maintained (Table 2).
Total thyroid pathologies operated on between 2003 and 2011.
| Frequency | Percentage (%) | |
|---|---|---|
| Toxic diffuse goitre | 25 | 6 |
| Multinodular goitre | 6 | 1 |
| Non toxic multinodular goitre | 230 | 46 |
| Right nodular goitre | 68 | 14 |
| Left nodular goitre | 75 | 16 |
| Papillary carcinoma | 62 | 13 |
| Graves Basedow | 12 | 2 |
| Papillary microcarcinoma | 6 | 1 |
| Chronic thyroiditis | 6 | 1 |
| Total | 490 | 100 |
With regard to age, the mean age was 44.71 (95% CI: 41.77–47.65) and the median age was 46. The response mean and latency of vagus and recurrent nerves after stimulation are presented in Table 3.
Mean response and latency of the vagus and recurrent nervesy after neuromonitoring.
| Mean response/latencya | Right vagus N. V1 (at beginning | Right recurrent N. R1 (at beginning) | Right recurrent N R2 (end) | Right vagus N. V2 (end) | Left vagus N. V1 (at beginning) | Left recurrent N. R1 (at beginning) | Left recurrent N. R2 (end) | Left vagus N. V2 (end) |
|---|---|---|---|---|---|---|---|---|
| Response | 425.57μV (median: 381) | 557.54μV (median: 519) | 525.59μV (median: 448) | 424.6μV (median: 409) | 365μV (median: 350) | 447.28μV (median: 391.5) | 479.94μV (median: 427) | 382.56μV (median: 329.50) |
| Latency | 8.46±4.7ms (median: 3.8) | 1.9±0.908ms (median: 1.8) | 1.82±0.0849ms (median 1.700) | 3.736±0.1416ms (median: 3.700) | 6.091±0.1490ms (median: 6.000) | 1.99±0.081ms (median: 1.90) | 1.80±0.0569ms (median: 1.800) | 6.144±0.1131ms (median: 6.100) |
After comparing the vagus nerve means at the beginning and end of surgical intervention regarding response and latency, we observed that there were no statistically significant differences in the means (Table 4).
Comparison of vagus nerve means at the beginning and end of surgical intervention with regards to response and latency.
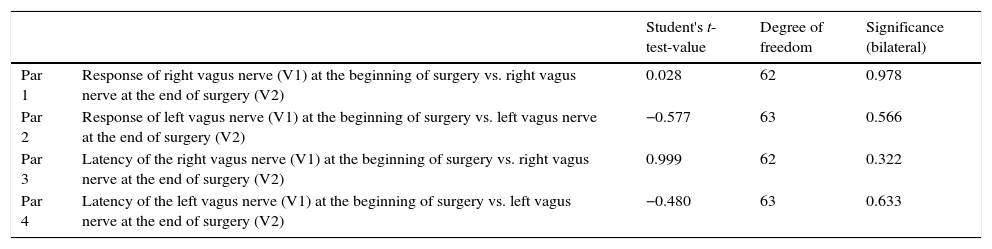
| Student's t-test-value | Degree of freedom | Significance (bilateral) | ||
|---|---|---|---|---|
| Par 1 | Response of right vagus nerve (V1) at the beginning of surgery vs. right vagus nerve at the end of surgery (V2) | 0.028 | 62 | 0.978 |
| Par 2 | Response of left vagus nerve (V1) at the beginning of surgery vs. left vagus nerve at the end of surgery (V2) | −0.577 | 63 | 0.566 |
| Par 3 | Latency of the right vagus nerve (V1) at the beginning of surgery vs. right vagus nerve at the end of surgery (V2) | 0.999 | 62 | 0.322 |
| Par 4 | Latency of the left vagus nerve (V1) at the beginning of surgery vs. left vagus nerve at the end of surgery (V2) | −0.480 | 63 | 0.633 |
We see that before neuromonitoring, of the 704 nerves at risk, there were 14 injuries of the recurrent laryngeal nerve.
With the Avalanche® system, out of the 135 nerves at risk, no nerve injury occurred.
In total, since 2003, out of 839 nerves at risk, there were only 14 nerve injuries (1.7%) and all of them occurred before neuromonitoring.
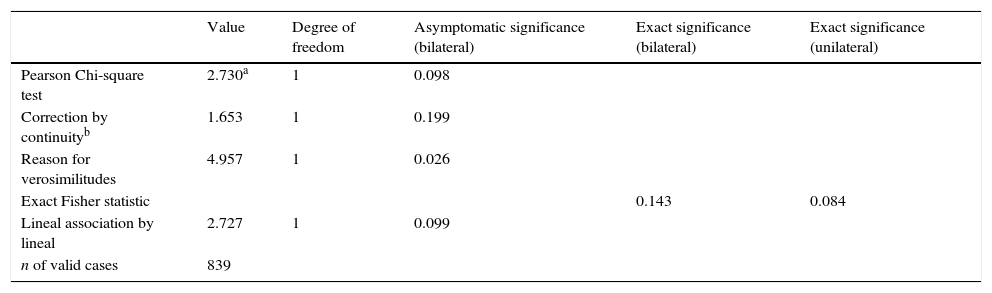
According to the exact Fisher statistic, there are no significant differences before and after neuromonitoring, with a p=0.084, although with a larger sample it would definitively be possible to defect differences (Table 5).
Chi-square tests. Comparison before and after the introduction of neuromonitoring.
| Value | Degree of freedom | Asymptomatic significance (bilateral) | Exact significance (bilateral) | Exact significance (unilateral) | |
|---|---|---|---|---|---|
| Pearson Chi-square test | 2.730a | 1 | 0.098 | ||
| Correction by continuityb | 1.653 | 1 | 0.199 | ||
| Reason for verosimilitudes | 4.957 | 1 | 0.026 | ||
| Exact Fisher statistic | 0.143 | 0.084 | |||
| Lineal association by lineal | 2.727 | 1 | 0.099 | ||
| n of valid cases | 839 |
Neuromonitoring of recurrent and vagus nerves during thyroid surgery has become another useful tool, to anatomically locate the recurrent nerve. The greatest risk of injury to the recurrent laryngeal nerve is produced in: substernal goitre, hyperthyroidism, reinterventions and malignant tumours.14
Neuromonitoring has not been universalised because of initial cost, but it has become an essential technical aid in centres specialising in thyroid surgery. Indirect stimulation of the recurrent laryngeal nerve through the vagus nerve after thyroidectomy is more precise than direct stimulation. Indirect stimulation is more sensitive to high risk than in the low risk patients.15 In the work of Thomusch et al.16 it was concluded that indirect stimulation of the vagus nerve is a better predictor of postoperative nerve malfunction than direct stimulation of the recurrent laryngeal nerve.
For less experienced surgeons, neuromonitoring reduces permanent paralysis of the recurrent laryngeal nerve. However, for experts in thyroid surgery, obtaining rates under 1% in benign first-stage thyroid surgery leaves little margin for useful neuromonitoring.
Stimulation in the nearby areas of the nerve may help with dissection, because several anatomical variants present greater risk of injury. A bifurcated recurrent laryngeal nerve is particularly liable to injure close to the inferior thyroid artery or the Berry ligament. The sensitivity of vagus nerve stimulation during surgery was 63%, which results in the prevention of 2 out of every 3 paralyses during surgery.
The high rate of false negatives (no acoustic or electromiographic response in the system without real injury) is due to a greater number of technical errors (such as displacement of electrodes, errors in connection or lack of knowledge about how they work), and that the rate of pareses of the recurrent laryngeal nerve is low. It also depends on when the first postoperative laryngoscopy is performed. (the sooner it is, the easier it is for pareses to present).
Changes in latency and extent of its potential after thyroid resection compared with preoperative values may be more important than the acoustic signal. A reduction in breadth over 50% or a latency time of over 20% predicts dysfunction of the recurrent laryngeal nerve.
In our study, we cannot conclude that monitoring is useful for getting to know preoperative function since none of the 79 patients had any nerve injury. It would be a good idea, in future works, to correlate the differences in these parameters in the nerve before and after thyroidectomy, with functioning (and clinical repercussions) of the nerve. For this, there would obviously have to be some type of injury. It is interesting to comment that, since intraoperative locating of the nerve was always performed, when there were differences they had to be attributable to the introduction of the neuromonitoring system.
Regarding positive and negative predictive values, we were unable to reach any conclusions because no injuries were produced since the Avalanche®. Neuromonitoring system was introduced. In this study, since there were no nerve injuries, the rate of true negatives and false positive was 0% in both cases.
Neuromonitoring was not a reliable predictor of postoperative evolution, despite its use in identifying the recurrent laryngeal nerve, in aberrant anatomical situations. An anatomically intact nerve may show alteration of the function after operation, due to neural stretching during goitre retraction.
In one study,17 the use of neuromonitoring was unable to significantly reduce the risk of laryngeal nerve paralysis and there was no influence of neuromonitoring in other surgical complications. The application of nerve neuromonitoring is a useful tool, but does not replace intraoperative identification of the recurrent laryngeal nerve.
Arguments in favour of using neuromonitoring systematically: 1) helps to locate the recurrent laryngeal nerve; 2) differentiates the anterior and posterior branch of the recurrent laryngeal nerve in case of bifurcations; 3) helps to take decision in cases of difficult surgery; 4) is also useful in cases of non recurrent inferior laryngeal nerve; 5) helps orientation in the case of complex surgery: reinterventions, complex goitre and medistinic spread, and 6) neuromonitoring may detect that the nerves do not work, although they do not visually seem to be impaired.
Arguments against neuromonitoring: 1) the use of neuromonitoring may lengthen surgical intervention; 2) costs are high; 3) locating the vagus nerve may damage the cervical sympatric nerve; 4) there is no unanimity in the literature regarding a significant reduction in temporary or definitive paralysis of the recurrent laryngeal nerve; 5) it appears that for surgeons with a great deal of experience and rates under 1%, the said rates do not improve greatly and 6) the lack of a standard protocol may lead to an increase in the rate of recurrent paralysis.
In our study, from September 2010 the Avalanche® neuromonitoring system was applied to all patients who had undergone thyroid surgery.
We systematically used the combination of routine visual identification of the recurrent laryngeal nerve with neuromonitoring. Several results coincide with previous studies,18 in which it was concluded that neuromonitoring did not reduce the rate of recurrential paralysis compared with a group with no neuromonitoring, showing that visualisation of the nerve was the gold standard, as in our study. One study19 concluded that it is necessary to standardise neuromonitoring procedures to avoid injury and the rate of paralysis is significantly reduced with the use of neuromonitoring. Another study17 revealed that the use of neuromonitoring could not significantly reduce the risk of injury of the recurrent laryngeal nerve and that the application of neuromonitoring is a useful tool in thyroid surgery, but does not replace intraoperative identification of the recurrent laryngeal nerve.
ConclusionsOur results, with regards to nerve injury findings, before and after the introduction of neuromonitoring, were not statistically significant and we may conclude that since its implementation no recurrential nerve injury has been recorded. A stratified randomised, prospective study would need to be conducted with a sample that was larger than ours, for findings to be truly valuable mid-term.
In this study, we could not correlate the results with the clinical features, because since the introduction of neuromonitoring in 2010 no injury took place and therefore all value obtained were normal.
However, we consider that the gold standard in thyroid surgery is the systematic identification of the recurrent laryngeal nerve and neuromonitoring of it and this should never be replaced, only enhanced.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Motos-Micó JJ, Felices-Montes M, Abad-Aguilar T. Neuromonitorización intraoperatoria en cirugía tiroidea. Cir Cir. 2017;85:312–319.