Recently, revolutionary techniques have been described to avoid liver failure secondary to major hepatectomies.1 Robles et al.2 have described the associated liver tourniquet and right portal vein occlusion technique for staging hepatectomy (ALTPS) where portal ligation is performed and a tourniquet is placed over the parenchymal division line, accelerating hypertrophy and achieving effective regeneration in the first 7 days, with acceptable morbidity and mortality rates. The following is a case report of a complicated ALTPS with rotation of the left hepatic vein (HV). The patient is a 57-year-old woman with a history of laparoscopy-assisted sigmoidectomy in December 2014 for moderately differentiated adenocarcinoma of the sigmoid colon, pT3 N0. One year after surgery, elevated carcinoembryonic antigen (14.1ng/mL) was detected, which had previously been normal (0–3ng/mL). Computed tomography (CT) showed a solitary lung mass measuring 10mm in the left upper lobe and 2 hepatic lesions, one in segment IV (18mm) and another subcapsular in segment VII (8mm), suggestive of metastasis. She received 2 cycles of chemotherapy with leucovorin, oxaliplatin and fluorouracil (FOLFOX), after which a new CT with vascular reconstruction and volumetry and a PET showed an increase in the size of the lesion in segment IV to 22mm (no vena cava compromise) and stability of the hepatic lesion in segment VII and the lung lesion. The oncology committee discussed the case and decided to resect the liver lesions, followed by a second surgery to treat the lung lesion.
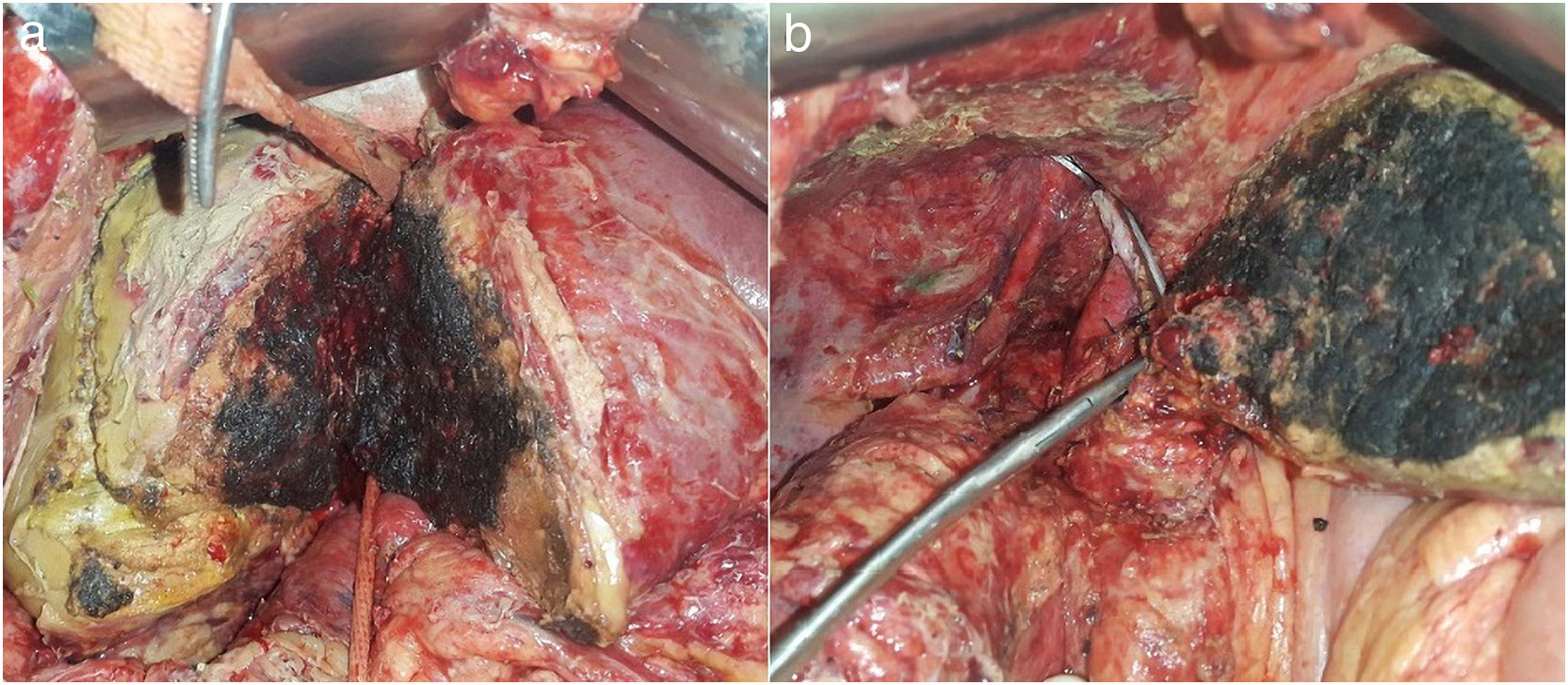
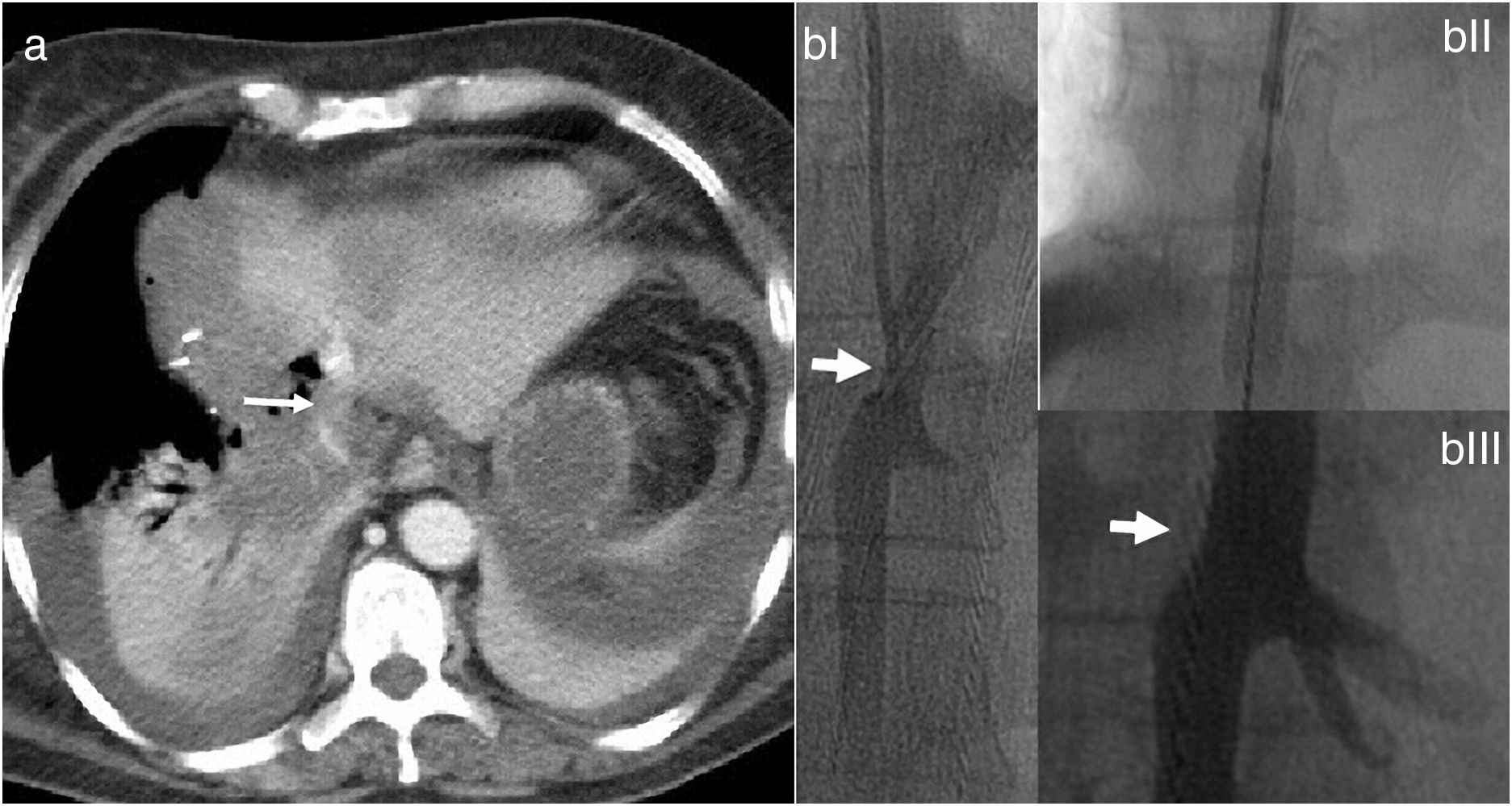
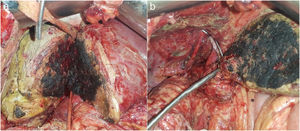
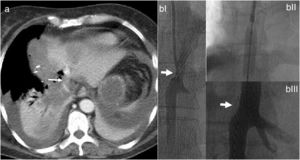
Intraoperative ultrasound confirmed the presence of the lesion located in segment VII; the lesion in segment IV was 4cm and in close contact with the right and middle hepatic veins. The left suprahepatic vein was tumor-free. The residual liver volume (RLV) of the left lobe was 231mL, representing 19% (total liver volume 1178mL) for a patient weighing 77kg. We decided to perform ALTPS following the volumetric criteria of Morales et al.3 due to the need for rapid hypertrophy for the second operation and the treatment of the lung disease in the shortest time possible. The immediate postoperative period transpired without complications. On the tenth day, CT confirmed an RLV of 463mL, which was 43%, and a second intervention was undertaken (Fig. 1a). A compromised vena cava was observed due to the segment IV lesion at the root of the right and middle hepatic veins. We then decided to resect the compromised vena cava segment using a Satinsky clamp, including the right and middle hepatic vein origins (Fig. 1b). The vena cava defect was closed with continuous Prolene® 3–0 suture, maintaining good caliber and flow. Twelve hours after surgery, the patient presented hemodynamic instability and the need for vasopressors, requiring intubation. The analytical parameters were compatible with acute liver failure. Doppler ultrasound showed evidence of altered flow in the left hepatic vein, and a CT scan revealed difficult venous drainage of the residual liver segment, compatible with torsion of the left hepatic vein (Fig. 2a).4 Venography detected stenosis of the vena cava and left hepatic vein. During the operation, balloon angioplasty of the inferior vena cava was conducted with a stent measuring 8.0×27mm in the HV (Fig. 2b).5
Postoperative patient progress was favorable. After an initial dose of acetylsalicylic acid (ASA) of 300mg during the procedure, treatment was started with 100mg 48h later. Likewise, subsequent Doppler ultrasound follow-up studies and analytical parameters showed trend toward normalization (10th postoperative day: GOT 50U/L, GPT 81U/L, GGT 220U/L, FAL 220U/L, total bilirubin 1.14mg/dL and TP 15.6s. Fifteen days later, the patient was discharged with double antiplatelet therapy (ASA and clopidogrel). The pathology study reported 2 lesions, one measuring 22×15×12mm with infiltration by moderately differentiated adenocarcinoma and notable necrosis, and another measuring 10×10×10mm with notable necrosis, no tumor remnant and free margins greater than 10mm. It is possible that tumor necrosis and peritumor inflammation are the cause of the difference in size between the pathology study and the intraoperative ultrasound. Two months later, tri-segmentectomy of the left upper lung lobe was conducted with video-assisted thoracoscopy, with no complications. The pathology report confirmed the metastatic origin of the lesion with free margins and disease-free lymph nodes. After one year and a half of follow-up, the patient is asymptomatic with no signs of recurrence or alterations in vascular flow.
In conclusion, we present a case of acute liver failure in the immediate postoperative period following a liver resection due to rotation of the left hepatic vein, which was diagnosed in a unit with experience in liver surgery and transplantation. This is a rare complication, most frequently observed in liver transplantation with a live donor than in large hepatectomies. In the review of the literature, 8 similar cases were found, suggesting that, after right hepatectomy, the remaining liver lobe tends to rotate spontaneously around the inferior vena cava, causing occlusion of the venous flow due to the rotation of the hepatic vein.4 The incidence of this complication after major hepatectomy is 0.1%,6 but in liver transplantation with a live donor it reaches 5%–13%7 and this increase is probably due to the hyperplasia and intimal fibrosis at the anastomotic sites, with compression and torsion of the anastomosis caused by the regeneration of the implant.8 We believe that, despite the absence of anastomosis, the growth of the lobe remaining after the second procedure9 can cause rotation given the presence of a large empty right subphrenic space and the lack of natural means of fixation that were divided during surgery. In addition, rapid hypertrophy with much earlier reoperation decreases the number of adhesions in collaboration with mobilization. The increasing use of new methods of liver resection may be associated with an increase in the diagnosis of this type of complications. To clarify, in this case it is possible that partial resection of the vena cava at the origin of the right and middle HV favors the development of this complication by partially reducing the caliber of the vena cava. Fixation of the falciform ligament should be considered in order to maintain correct anatomical position. Logically, the experience in this type of resection is generally limited, and time will tell which technical steps should be improved to minimize the number of negative outcomes.
The diagnosis and early treatment of a twisted suprahepatic vein is key and can be approached safely by hemodynamics. This complication should be kept in mind in patients with major resections and consequent contralateral hepatic hypertrophy.10
Please cite this article as: Giordano E, Alcaraz A, Signorini FJ, Marani M, Obeide LR. ALPPS torniquete complicado con giro de la vena suprahepática izquierda. Nuevas complicaciones en nuevas hepatectomías. Cir Esp. 2018;96:591–593.