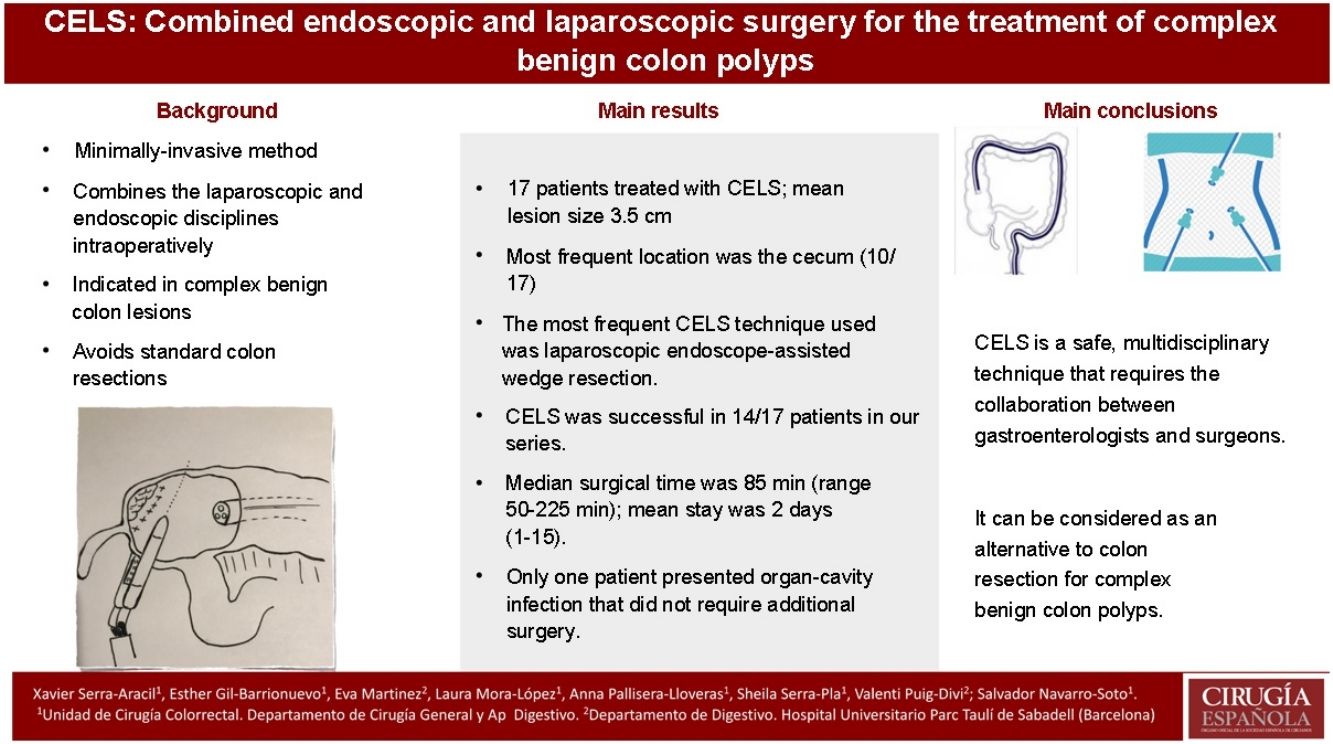
Combined endoscopic and laparoscopic surgery (CELS) has emerged as a promising method for managing complex benign lesions that would otherwise require major colonic resection. The aim of this study was to describe the different techniques and to evaluate the safety of CELS, assess its outcomes in a technique that is scarcely widespread in our environment.
MethodObservational retrospective study, short-term outcomes of patients undergoing CELS for benign colon polyps from October 2018 to June 2020 were evaluated. Postoperative outcomes, length of hospital stay and pathological findings were evaluated.
ResultsSeventeen consecutive patients underwent CELS during the study period. The median size of the lesion was 3.5 cm (range 2.5–6.5 cm), the most frequent location was the cecum (10 from 17). Most patients treated with CELS underwent an endoscopic-assisted laparoscopic wedge resection (11 from 17). In four patients this resection was combined with another CELS technique, and two patients underwent an endoscopic-assisted laparoscopic segment resection. The success rate of CELS in our series was in 14 from 17 (82.4%). The median operative time was 85 min (range 50−225 min). The median hospital stay was 2 days (range 1–15 days). One patient experienced an organ/space surgical site infection which did not require further intervention. Four lesions were shown to be malignant by postoperative pathology study.
ConclusionCELS is a safe and multidisciplinar technique that requires collaboration between gastroenterologists and surgeons. It can be considered as an alternative to colonic resection for complex benign colonic polyps.
La cirugía endoscópica y laparoscópica combinada (CELS) ha surgido como un método para el tratamiento de lesiones colónicas benignas complejas que, de otro modo, requerirían una resección quirúrgica. El objetivo de este estudio es describir las distintas técnicas CELS y evaluar su seguridad, en un procedimiento escasamente difundido en nuestro entorno.
MétodoEstudio observacional, retrospectivo, donde se evaluaron los resultados a corto plazo de pacientes diagnosticados de pólipos no resecables endoscópicamente sometidos a CELS entre octubre del 2018 a junio del 2020. Se valoraron los resultados postoperatorios, la estancia hospitalaria y los hallazgos patológicos.
ResultadosDiecisiete pacientes consecutivos fueron sometidos a CELS durante el período de estudio. El tamaño medio de la lesión fue de 3,5 cm (rango 2,5 a 6,5 cm), la localización más recurrente fue el ciego (10 de 17). La técnica CELS más frecuente aplicada fue la resección en cuña laparoscópica asistida por endoscopia (11 de 17). En cuatro pacientes, esta resección se combinó con otra técnica CELS. Dos casos se sometieron a una resección del segmento laparoscópico asistido por endoscopia. El éxito de CELS en nuestra serie fue en 14 de 17 (82,4%). La mediana del tiempo quirúrgico y estancia hospitalaria fue de 85 min (rango 50 a 225 min) y de dos días (rango uno a 15 días), respectivamente. Solo un paciente presentó infección del órgano-cavitaria que no requirió cirugía adicional.
ConclusionesCELS es una técnica segura multidisciplinar, que requiere la colaboración entre gastroenterólogos y cirujanos. Se puede considerar como una alternativa a la resección de colon para pólipos benignos complejos.
The usual treatment for the management of endoscopically unresectable benign polyps of the colon is segmental oncological colectomy. However, definitive pathology results show that only 3%–18% of these polyps harbor malignancy.1–4 This means that approximately 80% of these patients may be overtreated and undergo unnecessary bowel resection to remove a polyp that is likely to be benign.
Combined endoscopic laparoscopic surgery (CELS) emerged in the 1990s as an alternative treatment for these complex and benign-appearing polyps, which would normally be treated with surgical resection of the colon.5 The combined endoscopic and laparoscopic approach allows for laparoscopic mobilization of the colon to facilitate adequate exposure for endoscopic resection. In addition, during the same operation we are able to make evaluations, repair any possible injury to the colon wall, assess the resection margins, and ensure intestinal permeability.6 Using a combined approach, it is possible to perform different techniques and types of colonoscopy-assisted laparoscopic resection.
CELS techniques have been shown to be a feasible and safe alternative to colon resection, potentially avoiding the risks and complications associated with colectomy.2–4,7–12 However, despite the better short-term results, shorter hospital stay, and a cost-effectiveness analysis in favor of CELS,6 this minimally invasive technique is not widely used in our setting, where it requires the collaboration of endoscopists and expert laparoscopic surgeons.
The objectives of this are: (1) To describe this minimally invasive technique and promote multidisciplinary management; (2) To assess the safety and short-term results of CELS for the treatment of complex colon polyps at our hospital.
MethodsWe conducted a retrospective observational study, with prospective data collection. We evaluated the short-term results (30 postoperative days) of patients who underwent CELS with a diagnosis of complex benign colon polyps from October 2018 to June 2020.
- -
Inclusion criteria: patients who required surgical treatment for polyps that were not endoscopically resectable during this period. The indication of each lesion was evaluated by an expert endoscopist before referral for surgery. If the polyp was considered endoscopically unresectable, the patient was assessed by the multidisciplinary colorectal cancer committee for indication of CELS, after an extension study.
- -
Exclusion criteria: patients with preoperative malignant biopsy, endoscopic findings suggestive of malignancy or polyps considered unsuitable for endoscopic resection, for which the Narrow-band Imaging International Colorectal Endoscopic (NICE)13 classification and the PARIS macroscopic classification14 were used.
The study was approved by the institutional ethics committee of our center ([CEIC]: 2018/655). It met the Declaration of Helsinki criteria, and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies were followed.
TechniqueAll patients signed the informed consent form. The day before surgery, patients were given mechanical bowel preparation in accordance with the hospital protocol15 as well as oral antibiotic prophylaxis. During anesthetic induction, intravenous antibiotic prophylaxis was administered following our institutional protocol. The technical description was previously published by our group in video-vignette format.16
After laparoscopic inspection of the peritoneal cavity and identification of the marked area (if the polyp had been tattooed preoperatively), an intestinal clamp was placed on the terminal ileum to prevent air distention of the small intestine. Afterwards, the endoscopist performed a colonoscopy with carbon dioxide (CO2) insufflation and identified the intraluminal location of the polyp.17,18 Laparoscopic dissection and mobilization of the colon was performed when necessary.19
From among the different variations of the CELS technique,8,10 the following were carried out in this study:
Laparoscopy-assisted endoscopic resection/polypectomy (LEAR/LEAP):
Laparoscopic manipulation of the colon facilitates exposure of the lesion so the specialist may attempt a more aggressive endoscopic resection. Laparoscopic vision allows the surgeon to evaluate and repair possible full-thickness intestinal wall damage during endoscopic polypectomy.
Endoscopy-assisted laparoscopic wedge resection (EAWR):
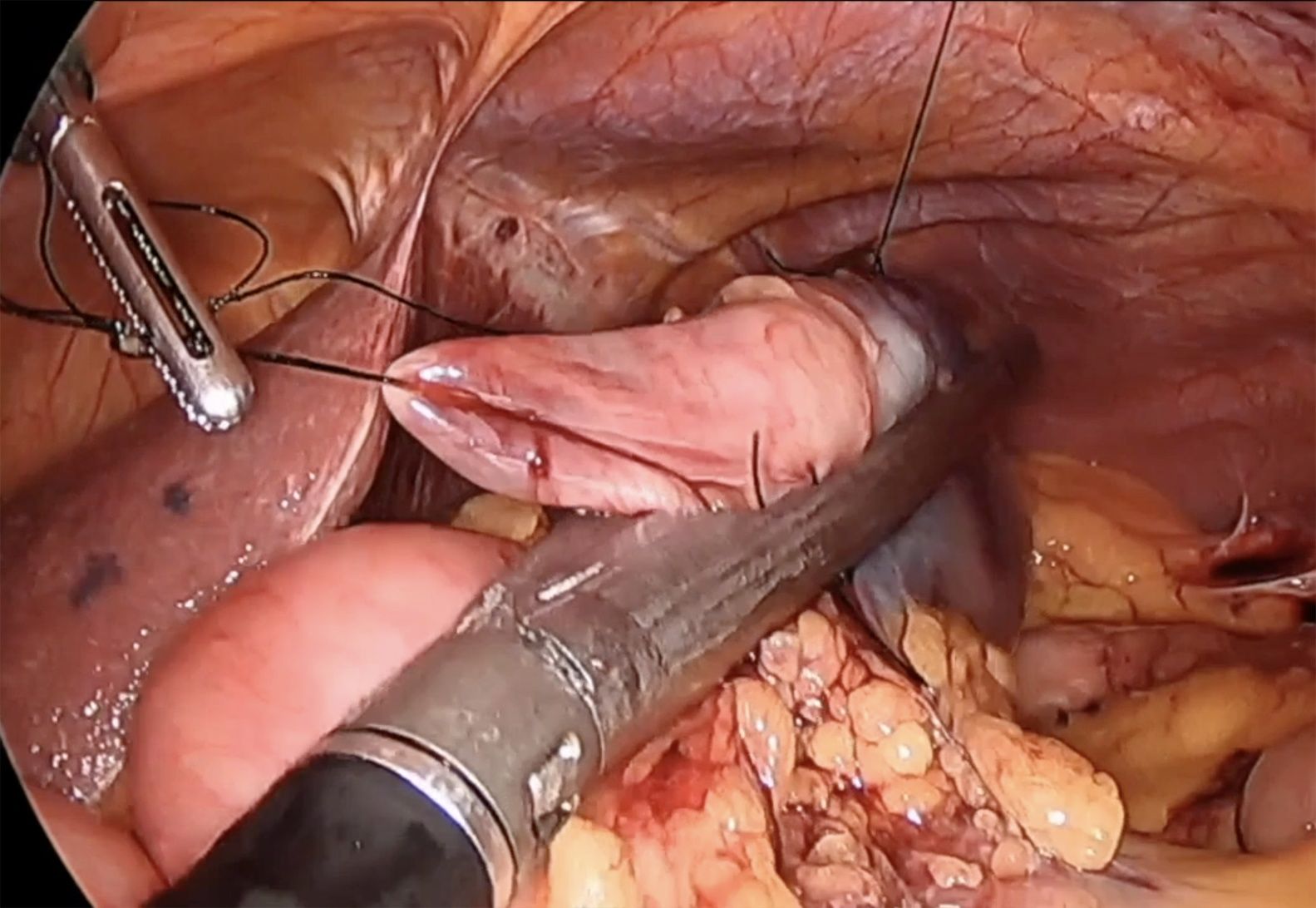
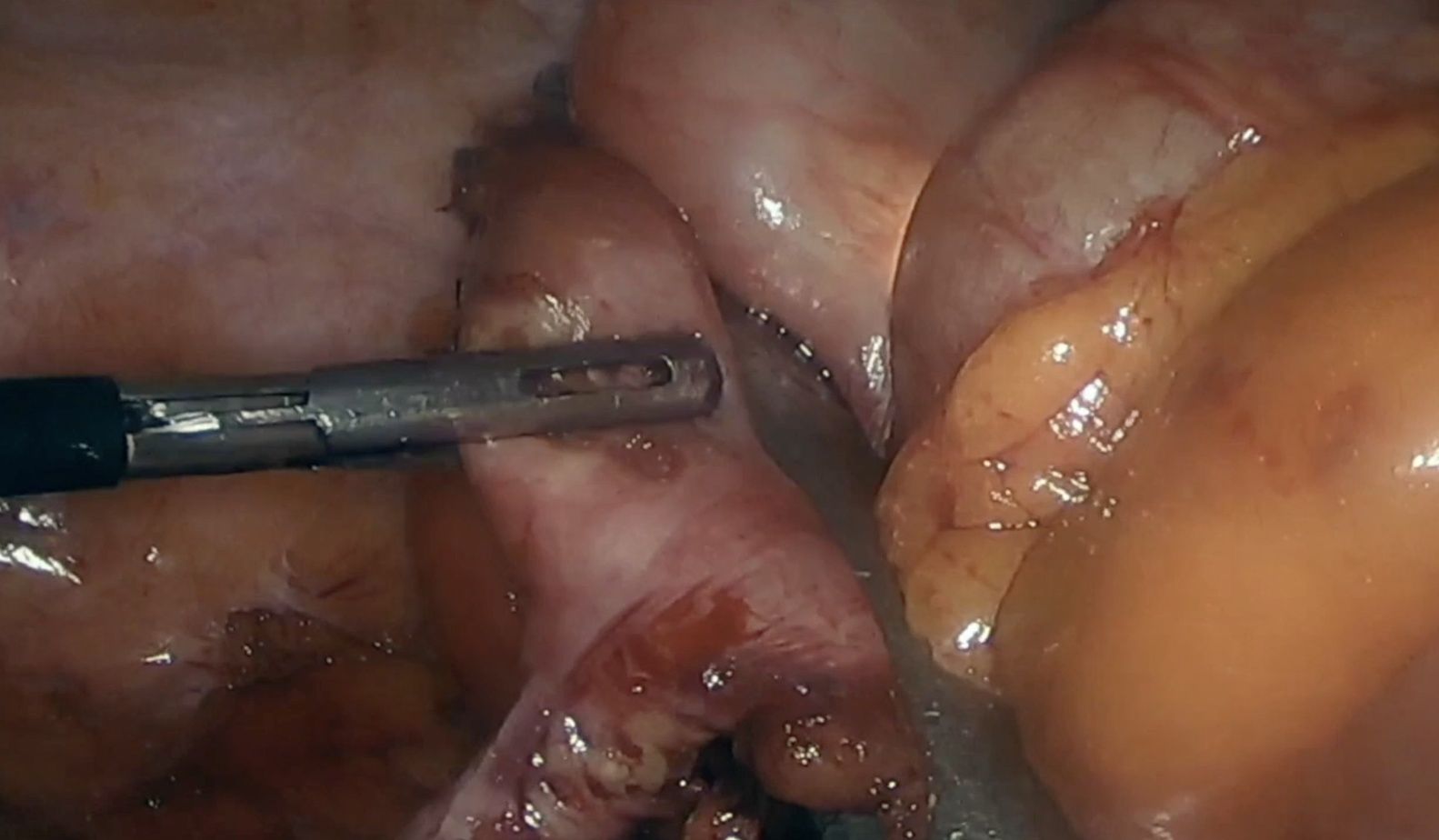
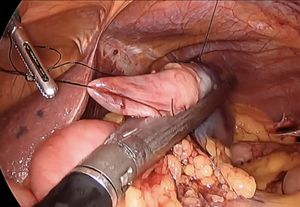
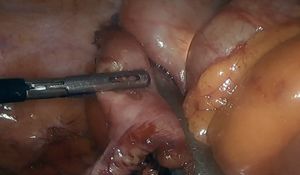
This technique is normally used to remove polyps in the antimesenteric area. The endoscopist indicates the exact location of the polyp by transillumination on the colon wall. The surgeon identifies and marks safe resection margins. Subsequently, an endostapler is placed to resect the polyp en bloc, using a linear mechanical suture to close the defect. The endoscopist confirms complete removal of the lesion and verifies the absence of stricture in the colon lumen. In certain instances, traction sutures are placed on the wall of the colon to facilitate the placement of the endostapler in the segment where the polyp is located (Fig. 1). In the case of cecal lesions (Fig. 2), the endoscopist can also ensure permeability of the ileocecal valve by inserting the colonoscope into the terminal ileum.
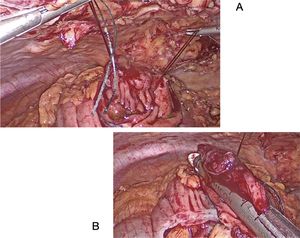
Endoscopy-assisted laparoscopic transluminal resection (EATR). Mesenteric injuries where placement of a linear stapler is not possible:
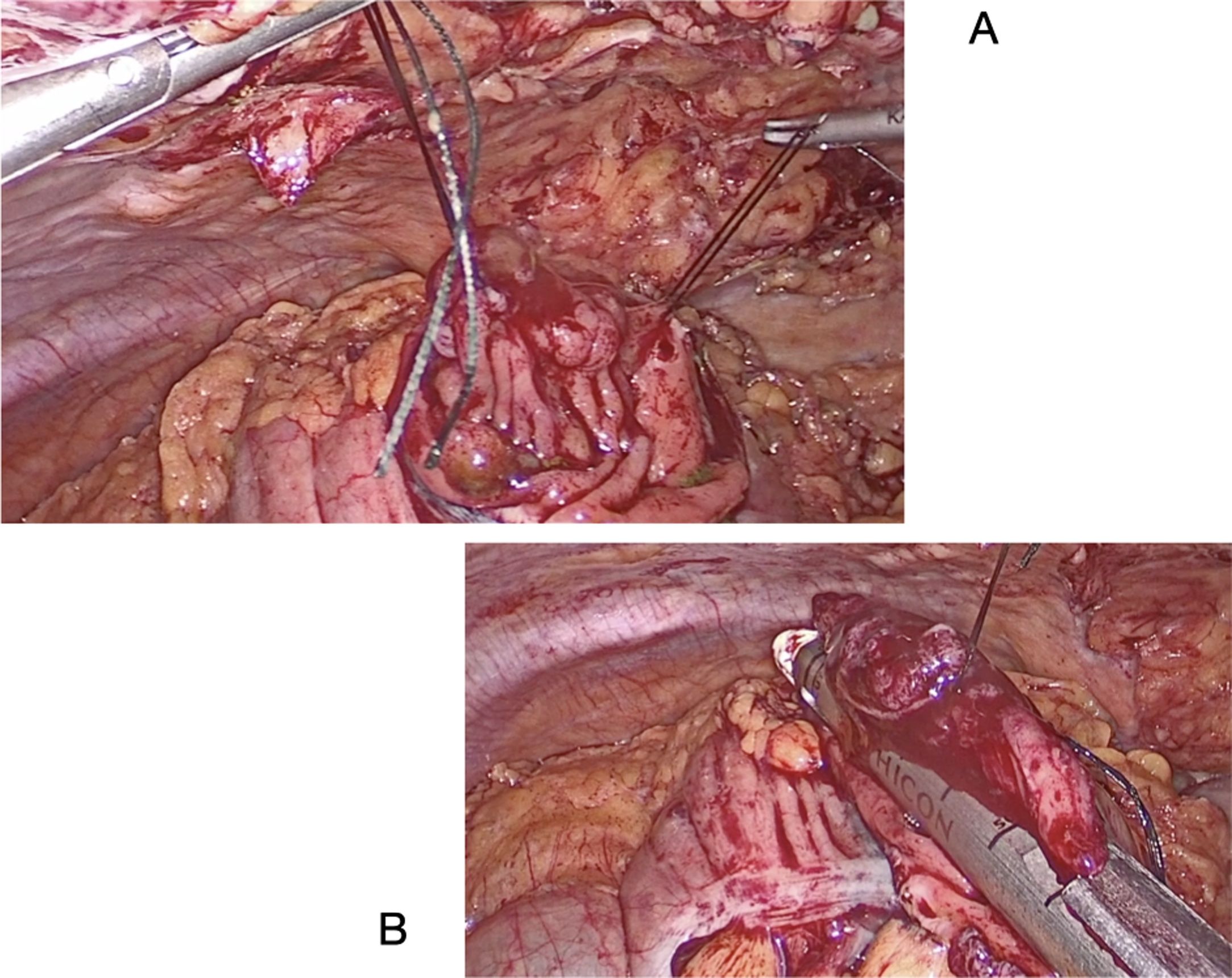
The polyp is resected through a colotomy in the antimesenteric part. Stitches are placed at the ends of the lesion, and the endostapler is applied (Fig. 3A and B). Subsequently, the defect is closed with a mechanical or manual suture.
Endoscopy-assisted laparoscopic colon segment resection (ECSR):
If CELS is ruled out intraoperatively due to the size or location of the lesion, a limited colon resection is performed, without the need for complete colon mobilization or lymph node dissection.
Patients are discharged within 24–48 h if there are no signs of medical or surgical complications. In cases of colon resection and anastomosis, these patients are managed and discharged according to the protocols of the Colorectal Surgery Unit of our hospital.
The follow-up visit is scheduled for 4 weeks after the procedure to assess recovery and the pathology results. Follow-up colonoscopy is done 6–12 months after surgery to rule out recurrence.
Variables and statistical analysisWe evaluated demographic data, patient comorbidities, lesion size and location, surgical technique, postoperative results, length of hospital stay, and pathologic findings. Categorical variables are reported as absolute numbers and percentages. Continuous variables are reported as medians and ranges.
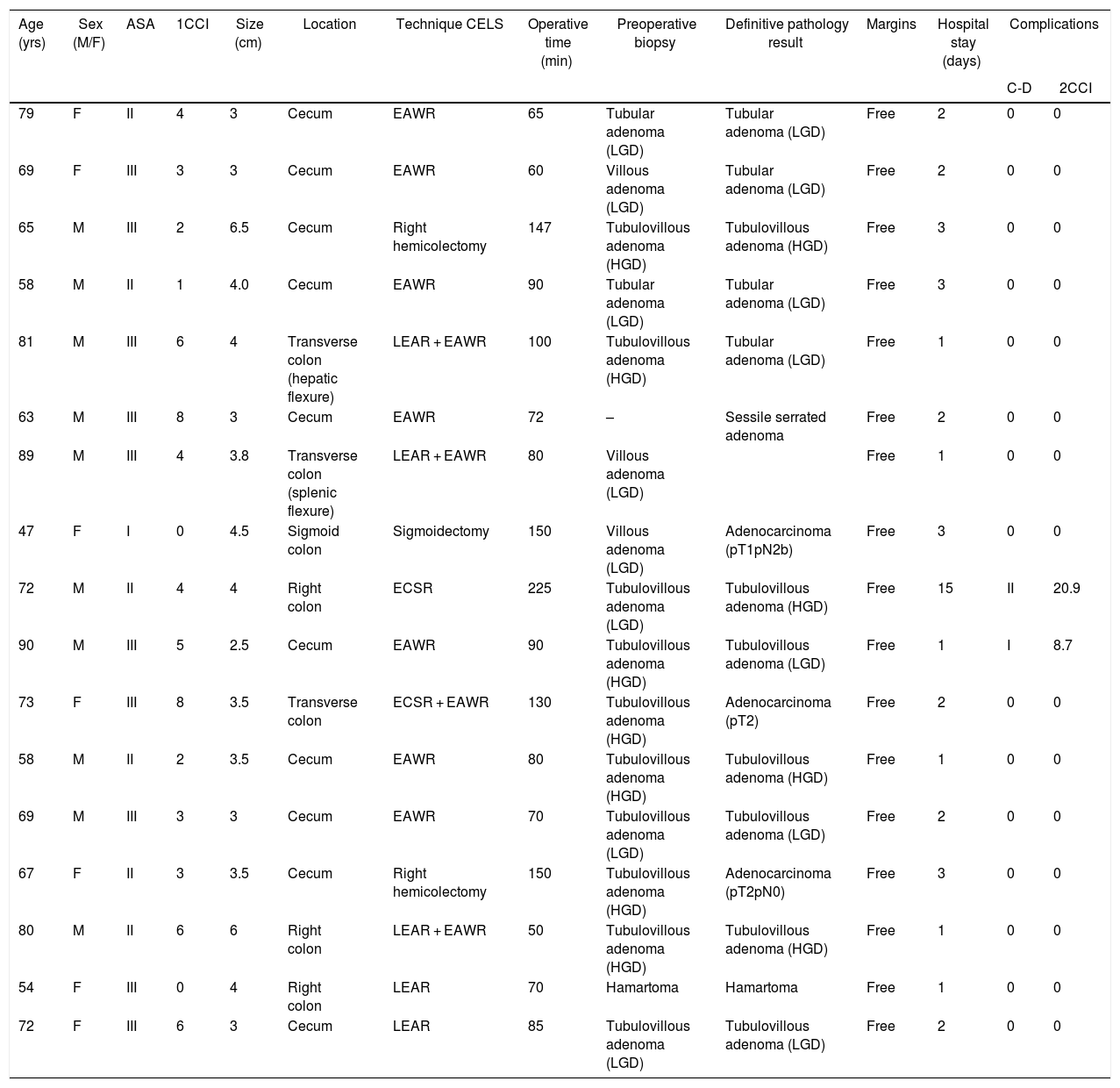
ResultsThe study included 17 patients with consecutive indications for CELS during the study period (Table 1). Median patient age was 69 years (range 47–90), with a higher proportion of men (10/17). According to the American Society of Anesthesiologists (ASA) classification, most were class II or III. The mean size of the resected polyps was 3.5 cm (range 2.5–6.5 cm). The most frequent location was the cecum (10/17). In the preoperative biopsy, fifteen lesions were adenomas, with a higher proportion of tubulovillous (10/17) and villous (3/17) types, and 7 were high-grade dysplasia. In one patient, the preoperative biopsy could not be obtained because the damage was located in the appendiceal orifice, although there were no morphological signs suggesting malignancy.
Characteristics of the lesions, techniques and patient results.
| Age (yrs) | Sex (M/F) | ASA | 1CCI | Size (cm) | Location | Technique CELS | Operative time (min) | Preoperative biopsy | Definitive pathology result | Margins | Hospital stay (days) | Complications | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-D | 2CCI | ||||||||||||
| 79 | F | II | 4 | 3 | Cecum | EAWR | 65 | Tubular adenoma (LGD) | Tubular adenoma (LGD) | Free | 2 | 0 | 0 |
| 69 | F | III | 3 | 3 | Cecum | EAWR | 60 | Villous adenoma (LGD) | Tubular adenoma (LGD) | Free | 2 | 0 | 0 |
| 65 | M | III | 2 | 6.5 | Cecum | Right hemicolectomy | 147 | Tubulovillous adenoma (HGD) | Tubulovillous adenoma (HGD) | Free | 3 | 0 | 0 |
| 58 | M | II | 1 | 4.0 | Cecum | EAWR | 90 | Tubular adenoma (LGD) | Tubular adenoma (LGD) | Free | 3 | 0 | 0 |
| 81 | M | III | 6 | 4 | Transverse colon (hepatic flexure) | LEAR + EAWR | 100 | Tubulovillous adenoma (HGD) | Tubular adenoma (LGD) | Free | 1 | 0 | 0 |
| 63 | M | III | 8 | 3 | Cecum | EAWR | 72 | – | Sessile serrated adenoma | Free | 2 | 0 | 0 |
| 89 | M | III | 4 | 3.8 | Transverse colon (splenic flexure) | LEAR + EAWR | 80 | Villous adenoma (LGD) | Free | 1 | 0 | 0 | |
| 47 | F | I | 0 | 4.5 | Sigmoid colon | Sigmoidectomy | 150 | Villous adenoma (LGD) | Adenocarcinoma (pT1pN2b) | Free | 3 | 0 | 0 |
| 72 | M | II | 4 | 4 | Right colon | ECSR | 225 | Tubulovillous adenoma (LGD) | Tubulovillous adenoma (HGD) | Free | 15 | II | 20.9 |
| 90 | M | III | 5 | 2.5 | Cecum | EAWR | 90 | Tubulovillous adenoma (HGD) | Tubulovillous adenoma (LGD) | Free | 1 | I | 8.7 |
| 73 | F | III | 8 | 3.5 | Transverse colon | ECSR + EAWR | 130 | Tubulovillous adenoma (HGD) | Adenocarcinoma (pT2) | Free | 2 | 0 | 0 |
| 58 | M | II | 2 | 3.5 | Cecum | EAWR | 80 | Tubulovillous adenoma (HGD) | Tubulovillous adenoma (HGD) | Free | 1 | 0 | 0 |
| 69 | M | III | 3 | 3 | Cecum | EAWR | 70 | Tubulovillous adenoma (LGD) | Tubulovillous adenoma (LGD) | Free | 2 | 0 | 0 |
| 67 | F | II | 3 | 3.5 | Cecum | Right hemicolectomy | 150 | Tubulovillous adenoma (HGD) | Adenocarcinoma (pT2pN0) | Free | 3 | 0 | 0 |
| 80 | M | II | 6 | 6 | Right colon | LEAR + EAWR | 50 | Tubulovillous adenoma (HGD) | Tubulovillous adenoma (HGD) | Free | 1 | 0 | 0 |
| 54 | F | III | 0 | 4 | Right colon | LEAR | 70 | Hamartoma | Hamartoma | Free | 1 | 0 | 0 |
| 72 | F | III | 6 | 3 | Cecum | LEAR | 85 | Tubulovillous adenoma (LGD) | Tubulovillous adenoma (LGD) | Free | 2 | 0 | 0 |
ASA: American Society of Anesthesiologists score; C-D: Clavien-Dindo classification; HGD: high-grade dysplasia; LGD: low-grade dysplasia; N: node-adenopathy; pT: pathologic tumor; EAWR: laparoscopic endoscopy-assisted wedge resection; LEAR: laparoscopic endoscopy-assisted resection; ECSR: laparoscopic endoscopy-assisted colon segment resection; 1CCI: Charlson Comorbidity index; 2CCI: Complication Comprehensive index.
The most used CELS technique was the EAWR (11/17). In 3 patients, EAWR was performed in combination with a previous LEAR to ensure complete removal of the polyp. One patient with a large polyp in the ascending colon underwent ECSR. In 3 cases (the two largest in our series, 6.5 cm and 4.5 cm, and a smaller lesion involving the ileocecal valve), CELS could not be performed. Due to the size and location of these two lesions, even ECSR was considered too extensive, and it was decided that conventional laparoscopic colon resection was indicated.
The median operative time was 90 min (range 50−225 min). Longer operative times were recorded when segmental resection and anastomosis were performed, either in the context of an ECSR or conventional colectomy. There were no intraoperative complications or conversions to open surgery. Two patients experienced postoperative difficulties One had a 4-cm polyp located in the ascending colon and presented an anastomotic leak on the 5th postoperative day after ECSR, requiring parenteral nutrition and antibiotic treatment for ten days, without the need for further intervention. Another patient who underwent EAWR to treat a cecal lesion was discharged 24 h after the procedure. He was re-admitted on the 7th postoperative day for abdominal pain, with no clinical signs of sepsis. Computed tomography showed postoperative pericecal changes with no conclusive signs of complication. He was treated with analgesia and discharged in 24 h with satisfactory evolution.
The median hospital stay was two days (range 1–15 days). This wide range was due to the long stay experienced by the patient with an anastomotic leak, who was discharged 15 days after surgery (Table 1).
Four lesions initially described as adenomas (2 with low-grade dysplasia and 2 with high-grade dysplasia) were found to be malignant in the final pathology report. One of these was in a 47-year-old woman with a large polyp in the sigmoid colon. As mentioned above, the lesion measured 4.5 cm, and the endoscopic or wedge resection technique was not considered feasible; therefore, laparoscopic oncologic sigmoidectomy was performed. The second lesion was in an 89-year-old male patient, located in the hepatic flexure of the colon. EAWR was performed on this patient and, although he was considered for a second oncological intervention, it was not carried out due to his elevated comorbidities. The third, a lesion in the transverse colon in a 73-year-old female patient, was reported in the definitive pathological anatomy report as a pT2 (pT: pathologic tumor), and a radical extended right hemicolectomy was performed 5 weeks later. The last adenocarcinoma was in a 67-year-old woman with a lesion that affected the ileocecal valve. It was not possible to carry out a local CELS technique, so a right hemicolectomy was performed with a definitive anatomical-pathological result of the specimen of pT2, N0.
DiscussionVarious CELS techniques have been described in the literature, including individual variations performed at different institutions.8,10 Despite this heterogeneity, procedures have evolved from endoscopic techniques in which laparoscopy was performed as a supportive measure2,12,19–23 to combined procedures in which laparoscopy and endoscopy are used in conjunction.3,9,11,24–28 This study describes the most commonly performed CELS techniques with short-term (30-day) postoperative results.
In our study, the success rate was 14 out of 17 (82.4%), which is in line with figures of 73%–91% published in larger series.2,3,9 Fukunaga24 described that polyps whose diameter is greater than half the caliber of the intestine have a risk of colonic stenosis after closing the wall, so CELS would not be indicated in these cases. Although there is no maximum size limit for the indication of CELS, we believe that the feasibility of the technique and the risk of postoperative intestinal stricture should be carefully evaluated during the procedure. The best technical approach must be chosen individually.
Despite the lack of comparative studies between CELS and colon resection, there is evidence that the complication rates associated with the former are lower.10 In our series, we recorded only two (11.8%) postoperative complications, one after ECSR and the other after EAWR. These were not life-threatening and did not result in long-term sequelae or require reoperation. Despite the size of the sample, these results are similar to previous studies that have observed complication rates after CELS between 0% and 18.1%.10 There were no conversions to laparotomy in our series, although conversion rates of up to 5% have been reported by larger series.3,9,10
There is evidence of a shorter operative time and hospital stay with CELS compared to laparoscopic colon resection.8,10 Studies with larger series describe a median stay of 1.1 days2 and 1.5 days.12 Lee et al. reported a median hospital stay of one day for CELS compared to 5 days for patients who required colon resection.9 The median hospital stay of our patients was 2 days. In fact, we believe that CELS can be performed in an outpatient setting; given the favorable short-term results described.
We consider CELS to be a technique that can be adapted to each situation, since it allows for each lesion to be evaluated intraoperatively, while also being able to assess any morphological sign of malignancy that would later make oncological resection mandatory. However, a careful preoperative diagnosis should be made, and polyps with suspected invasive cancer should be excluded. The perioperative biopsy of suspicious lesions has been proposed; however, this technique raises possible discrepancies with regard to the final pathological result. Due to these possible divergences, we prefer trying to make a correct preoperative diagnostic evaluation and not perform intraoperative biopsies.
Malignancy was found in 23.5% of resected polyps (4/17 patients), which is at the higher end of what is described in the literature.1–4 However, one of the patients was 89 years old with many morbidities, so it was considered definitive treatment. We also believe that it is a consequence of the learning curve in the selection of cases, and that a function of the oncology committee is to more precisely determine the indication of CELS, thereby avoiding double surgeries.
Since the first description of CELS in 1993,5 several studies have been published with the main objective of avoiding colon resection for lesions that are probably benign. Despite the benefits in terms of complications, operative time, hospital stay and favorable cost analysis published by Jayaram,6 the technique has not been widely disseminated, especially in Spain. The reasons for the lack of acceptance of CELS may be: the limited number of large series in the literature, heterogeneity between hospitals in terms of technical capabilities, difficult collaborative association between surgeons and gastroenterologists in certain hospitals, and the lack of prospective studies comparing procedures with advanced endoscopic techniques and intestinal resection.
The limitations of the study are the relatively small number of patients included. Since the CELS program was introduced at our hospital in October 2018, it has not yet been possible to assess the recurrence and long-term outcomes in our series. However, our data do corroborate previous studies in the literature on safety and good short-term clinical results. Furthermore, they show that this minimally invasive approach is feasible and can improve the quality of patient care by avoiding unnecessary major colon resections in cases of complex benign polyps.
ConclusionsThese results support the use of CELS as a minimally invasive procedure. In selected patients, it is a safe technique that provides favorable postoperative results for the treatment of complex benign polyps of the colon, while requiring collaboration between gastroenterologists and surgeons.
FundingThis study has received no funding of any kind.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank all the members of the Coloproctology Unit of the general Surgery Department, Digestive Endoscopy Service and all the members of the Multidisciplinary Colorectal Cancer Team for their help in applying the study protocol. Thanks also go to surgical nurses Marta Arizu and Merche Muñoz for their collaboration.
Please cite this article as: Serra-Aracil X, Gil-Barrionuevo E, Martinez E, Mora-López L, Pallisera-Lloveras A, Serra-Pla S, et al. Cirugía endoscópica y laparoscópica combinada para el tratamiento de pólipos de colon benignos complejos (CELS): estudio observacional. Cir Esp. 2022;100:215–222.