Video-assisted thoracic surgery (VATS) has significantly developed over the last decade. However, a VATS approach for thymoma remains controversial. The aim of this study was to evaluate the feasibility of VATS thymectomy for the treatment of early-stage thymoma and to compare the outcomes with open resection.
MethodsA comparative study of 59 patients who underwent surgical resection for early stage thymoma (VATS: 44 and open resection: 15) between 1993 and 2011 was performed. Data of patient characteristics, morbidity, mortality, length of hospital stay, the relationship between miasthenia gravis-thymoma, recurrence, and survival were collected for statistical analysis.
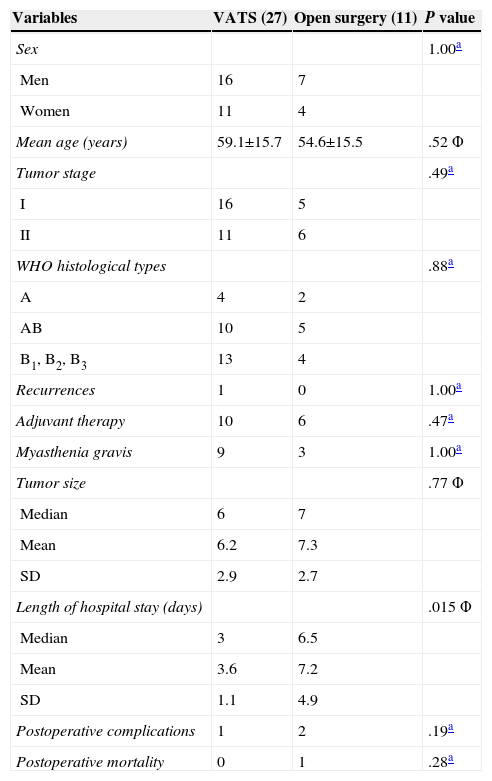
ResultsThymomas were classified according to Masaoka staging system: 38 in stage I (VATS group: 29 and open group: 9) and 21 in stage II (VATS group: 15 and open group: 6). The mean tumor size in the open group was 7.6cm (13–4cm) and in the VATS group 6.9cm (12–2.5cm). The average length of stay was shorter in the VATS group than in the open group (P<.001). No significant differences were found in the estimated recurrence-free and overall 5-year survival rates (96% vs 100%) between the 2 groups.
ConclusionsVATS thymectomy for early-stage thymoma is technically feasible and is associated with a shorter hospital stay. The 5-year oncological outcomes were similar in the open and VATS groups.
La cirugía torácica video-asistida (VATS) es una técnica que ha evolucionado en las últimas décadas. A pesar de sus ventajas, este abordaje continúa siendo discutido para el tratamiento de los timomas. El objetivo de este estudio fue evaluar los resultados obtenidos por el abordaje convencional y la VATS para el tratamiento de timomas en estadio i-ii.
MétodosEstudio comparativo en 59 pacientes a los que se les realizó una timectomía por timoma en estadio i-ii (VATS: 44 y cirugía convencional: 15) entre los años 1993 y 2011. Se analizaron las siguientes variables: características de los pacientes en ambos grupos, morbilidad, mortalidad, estancia hospitalaria, la relación miastenia gravis-timoma, recidiva y supervivencia a los 5 años.
ResultadosLos timomas se clasificaron según la clasificación de Masaoka: 38 en la etapa i (grupo VATS: 29 y grupo convencional: 9) y 21 en la etapa ii (grupo VATS: 15 y grupo convencional: 6). El tamaño medio del tumor en el grupo convencional fue de 7,6cm (13-4cm) y en el grupo VATS 6,9cm (12-2,5cm). La duración media de la estancia hospitalaria fue más corta en el grupo VATS que en el grupo de cirugía convencional (p<0,001). No se encontraron diferencias significativas entre los 2 grupos, en las recidivas ni en la supervivencia a los 5 años (96% vs 100%).
ConclusiónLa timectomía mediante VATS es una técnica factible y segura en el tratamiento de timomas estadio I-II. Se asocia a una menor estancia hospitalaria y a unos resultados oncológicos a los 5 años similares a los de la cirugía convencional. Los resultados oncológicos con un seguimiento de 5 años fueron similares a los obtenidos por la cirugía convencional.
Thymoma is the most common tumor of the anterior mediastinum, although it is a relatively rare disease. Recent studies have estimated an incidence in the United States of 0.15 per 100000 person-years.1 Thymomas are characterized as slow-growing tumors that spread by local extension. Local recurrence (pleura, diaphragm, pericardium) and metastases are highly uncommon, but have been reported in all stages and all histological subtypes of the disease.2,3 Surgical resection is the best available treatment and has been considered the most important determining factor for long-term survival.4,5
A review of the literature provides the description of a number of surgical approaches for thymectomy to treat thymoma. Although total thymectomy via median sternotomy is the standard approach, a number of reports support the feasibility of using video-assisted thoracic surgery (VATS) to perform thymectomy for the treatment of early-stage thymoma (stages I and II). Unfortunately, in the literature, there are no published series comparing the long-term results of a laparoscopic approach with those of open surgery. As a consequence, the approach to thymoma using VATS continues to be controversial. The objectives of the present study were to determine the feasibility of VATS thymectomy for the treatment of early-stage thymoma and compare the long-term results of a minimally invasive intervention (VATS) with those obtained after resection with open surgery.
MethodsBetween January 1993 and December 2011, a total of 59 patients with a diagnosis of stage I–II thymoma was treated surgically: 44 by VATS thymectomy and 15 by open surgical resection. We retrospectively reviewed our experience with thymectomy for the treatment of stage I–II thymoma up to January 2007. Thus, all the patients studied had a minimum follow-up period of 5 years. We identified a total of 38 patients who had undergone thymectomy for stage I–II thymoma during this time period.
Patient CharacteristicsOf the 38 patients, 21 had stage I thymoma and 17stage II; there were 15 women and 23 men. The mean age was 53.5±15.5 years in the open surgery group and 59.1±15.7 years in the VATS group. The median sizes were 6cm and 7cm, respectively. Twelve patients had myasthenia gravis (MG).
Surgical Technique: Video-assisted Thoracic Surgery ApproachUnder general anesthesia with selective intubation, the patient was placed in left lateral semidecubitus position (30°). At the start of the intervention, transitory carbon dioxide insufflation at pressures of 5–8mmHg was used to facilitate rapid and complete lung collapse. In the first 3 procedures, a left transthoracic approach was utilized. However, the presence of the pericardium and the difficulty involved in gaining access to the brachiocephalic trunk (which is easily approached from the right side by following it from Pirogoff's triangle) made this approach problematic.
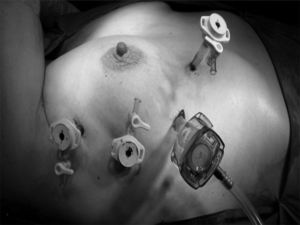
Three access ports, located in the 3rd and 6th intercostal spaces in the right posterior axillary, midaxillary, and anterior axillary lines, were generally employed. In some cases, when dissection was difficult or traction was required, other access ports in the midclavicular line were utilized (Fig. 1). The camera was introduced through a trocar inserted in the inferior access port in the 6th intercostal space, although the superior access ports could also be used to facilitate the dissection of the inferior poles.
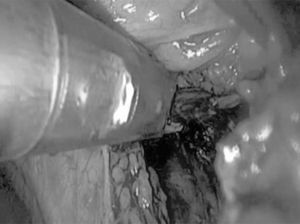
Once the thymus had been located, the mediastinal pleura was opened with an incision medial to the phrenic nerve to avoid damage and beneath the sternum to free the anterior part of the gland. The dissection was begun in the normal thymus, far from the mass, with minimal manipulation of the tumor to avoid any rupture of the capsule. Traction was then applied to the right superior horn of the thymus, which was dissected to separate it first from the pericardium and then from the aortic arch, toward the cervical vertebrae. The right horn of the thymus was dissected (in some cases with difficulty) to the point at which the superior pole ended over the brachiocephalic trunk. Once this had been performed, the horn was lifted up and 1 or 2 thymic veins (that drained into left brachiocephalic vein) were dissected; as the dissection progressed toward the left, bleeding was controlled until hemostasis was achieved. In our last interventions, we used a harmonic scalpel, which ensured perfect hemostasis, whereas in the preceding cases, we had used surgical clips (Fig. 2). Subsequently, the inferior horn was dissected until the cardiophrenic angle was reached. Then, while keeping close track of the contralateral phrenic nerve, the same steps were repeated to free the left superior horn; however, this intervention was somewhat more difficult given the position of the superior horn which passes over the left brachiocephalic vein.
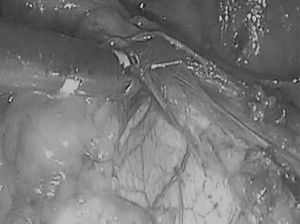
Nevertheless, we were always able to remove the thymus in one piece. Once the gland was withdrawn, the next step consisted in resecting all the perithymic fat in the pretracheal space (the area surrounding the internal mammary vessels and the pericardiophrenic angles [Fig. 3]), since, in many cases, these areas contain ectopic thymic tissue. After a careful examination of the surgical field and verification of the hemostasis, we placed a chest tube in the inferior access port (camera port). Finally, we visually verified lung reexpansion. The patients were transferred to the intensive care unit and, 24h later, to the ward.
For postoperative follow-up, all patients underwent chest computed tomography at 6-month intervals for the first 2 years, which is to be repeated yearly for the rest of their lives, to detect possible tumor recurrence.
Statistical AnalysisThe characteristics of the patients in the open resection group and the VATS group were compared using Fisher's exact test for the categorical variables and Wilcoxon test for continuous variables. The overall survival rate was calculated according to the Kaplan–Meier method, and the survival curves corresponding to the 2 groups were compared by a log-rank test. A P value <.05 was considered to indicate statistical significance.
ResultsOf the 38 patients, 11 underwent thymectomy with open surgery and 27, VATS resection. The tumor stages, World Health Organization (WHO) histological types and mean tumor size were comparable in the 2 surgical groups. There were no significant differences in the number of patients with MG or in the number that received adjuvant therapy in the 2 groups. The results of these analyses are summarized in Table 1.
| Variables | VATS (27) | Open surgery (11) | P value |
|---|---|---|---|
| Sex | 1.00a | ||
| Men | 16 | 7 | |
| Women | 11 | 4 | |
| Mean age (years) | 59.1±15.7 | 54.6±15.5 | .52 Φ |
| Tumor stage | .49a | ||
| I | 16 | 5 | |
| II | 11 | 6 | |
| WHO histological types | .88a | ||
| A | 4 | 2 | |
| AB | 10 | 5 | |
| B1, B2, B3 | 13 | 4 | |
| Recurrences | 1 | 0 | 1.00a |
| Adjuvant therapy | 10 | 6 | .47a |
| Myasthenia gravis | 9 | 3 | 1.00a |
| Tumor size | .77 Φ | ||
| Median | 6 | 7 | |
| Mean | 6.2 | 7.3 | |
| SD | 2.9 | 2.7 | |
| Length of hospital stay (days) | .015 Φ | ||
| Median | 3 | 6.5 | |
| Mean | 3.6 | 7.2 | |
| SD | 1.1 | 4.9 | |
| Postoperative complications | 1 | 2 | .19a |
| Postoperative mortality | 0 | 1 | .28a |
SD: standard deviation; WHO: World Health Organization.
Of the 11 patients who underwent thymectomy with open surgery, one died of septic shock after the operation. The operative mortality rate was 0% in the VATS group. No significant differences were observed (P=.28). With respect to postoperative morbidity, one patient in the VATS group developed left phrenic nerve paralysis and, in the open surgery group, there was one case of poststernotomy mediastinitis and another of pulmonary atelectasis. There were no statistically significant differences (P=.19). We should point out that fewer analgesics were needed after thoracoscopic surgery, in comparison with those patients who had undergone sternotomy (the dose was up to one third lower in 14 patients and was reduced by half in 13), as oral medication was sufficient and there was no need for epidural catheters. This decrease in postoperative pain led to a more rapid recovery than we had expected and an early return to normal activities. Only 3 patients reported mild to moderate pain (treated with oral analgesics) that persisted up to 5 months. The median length of the hospital stay was shorter in the VATS group than in the open surgery group (P=.015) (Table 1).
Myasthenia Gravis and ThymomaOf the 38 patients, 12 had MG, 9 from the VATS group and 3 from the open surgery group. The clinical stages of the patients, according to the modified Osserman classification, are shown in Table 2. The postoperative changes in medical treatment (anticholinergics, corticosteroids, etc.) are also indicated in Table 2. No differences were observed between the 2 surgical approaches, and there was an improvement in the medical treatment.
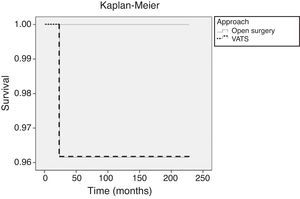
Overall SurvivalThe minimum follow-up in the remaining patients was 5 years and the maximum, 19 years (median follow-up: 147 months for the open surgery group and 107 months for the VATS group). During this period, 1 patient from the VATS group with a stage II thymoma (6.7cm) who refused adjuvant therapy died as a result of cerebral metastases one year after the surgical intervention. Two patients died of other causes: lung cancer (4 years later) and colon cancer (3 years later). On comparing the surgical approaches, the estimated 5-year overall survival probability was 96% for the VATS group and 100% for the open surgery group (Fig. 4). There were no significant differences in overall survival (Mantel–Cox log-rank test; P=.556).
RecurrencesAll the patients, except those with stage I thymoma, received postoperative adjuvant radiotherapy. There was 1 case of a pleural recurrence, 5 years after surgery, in a 36-year-old woman in the VATS group who had a 5.6-cm stage II thymoma. The pleural implants were resected and the patient is alive and remains disease-free. There were no significant differences between the 2 approaches in terms of the recurrence rate (P=1.00).
DiscussionThe treatment of thymoma by transsternal thymectomy, which is the classical approach, was long considered the most effective strategy, despite the severe postoperative pain and the prolonged recovery period due to the degree of functional deterioration and the greater need for postoperative assisted ventilation in patients with symptoms of respiratory distress and in those with long-standing MG.6,7 Although the operative field obtained with a transsternal approach is excellent, VATS provides superb visualization of the mediastinal compartment, with a reduction of postoperative lung function deterioration, which is translated into faster recovery and fewer complications.8
In the 1990s, Landreneau et al. reported the thoracoscopic resection of a stage I thymoma with a satisfactory outcome.9 Since then, a number of studies have demonstrated the feasibility and safety of the thoracoscopic approach. Cheng et al. pointed out that thoracoscopic surgery for Masaoka stage II thymoma could achieve results comparable to those obtained with interventions involving open median sternotomy.10 Pennathur et al. published the largest series to date, in which they compared the results of minimally invasive thoracoscopic resection of thymoma with those of an open surgical technique, and reported that thymus resection with VATS for the treatment of early-stage thymoma was associated with a shorter hospital stay and a lower morbidity rate.11 In our series, there were no significant differences between the two approaches with respect to the morbidity rate, although the hospital stay was shorter with the thoracoscopic approach (P=.015).
However, these advantages of VATS would be secondary if the oncological principles were not maintained. In this respect, Pennathur et al. reached the conclusion that the oncological outcomes also appear to be equivalent in the open surgery and VATS groups during medium-term follow-up (median follow-up, 36 months).11 However, thymoma is an indolent disease, and early-stage recurrences can develop even decades after the initial operation. Thus, longer follow-up is necessary for the evaluation of the oncological outcome of thoracoscopic thymectomy.12,13 In our series, we analyzed 38 patients who had undergone resection of a stage I-II thymoma with a minimum follow-up of 5 years, and the mean follow-up was 147 months for the open surgery group and 107 months for the VATS group. During this period (5 years after the initial operation), there was 1 regional recurrence in a patient in the VATS group with a large thymoma (stage II). Some authors have expressed concern about the use of minimally invasive techniques to perform thymectomy to treat thymoma, especially with respect to rupture of the capsule, with the corresponding risk of pleural dissemination. In our case, the thymoma was a large, stage II lesion, but was well encapsulated. Although the manipulation of the tumor had been difficult, on examination of the surgical specimen, it appeared to be intact. Since then, we have always attempted to apply traction to mobilize the normal thymus and create a plane before we have clearly reached the site of the mass and before attempting to mobilize it. Thus, the risk of pleural dissemination is low. On the other hand, there are reports in the literature of recurrences in the pleura and the chest wall in patients who have undergone transsternal thymectomy to treat stage II thymoma. Sakamoto et al. reviewed the medical records of 145 patients with early-stage thymoma who had undergone extended thymectomy via median sternotomy from 1976 to 2009.14 These authors observed 4 regional and distant recurrences (the latter in lung, liver, pleura and chest wall), with a median time between surgery and recurrence of 63 months (range: 5–134 months).
Transcervical and robotic thymectomy are other minimally invasive approaches for thymus resection. Most of the thymomas excised using the transcervical approach were small or corresponded to incidental findings during thymectomy to treat MG.15 In our series, a large thymoma, measuring more than 5cm, was resected using VATS with good results. Deeb et al. reported a series of 9 patients with small thymomas (<4cm) that were successfully treated by transcervical thymectomy. Although the results were promising, the authors extended the incision to perform sternotomy to remove 36% of the pathologically confirmed thymomas. With respect to robotic thymectomy, several groups have reported good results with this intervention in MG and in early-stage thymomas. Boedner et al. described complete extended thymectomy in 10 patients with mediastinal masses16: early-stage thymoma (n=6), thymic cyst (n=1), and neurogenic tumor (n=3). In their series, there were no postoperative complications and the chest tubes were removed on the second postoperative day. Nevertheless, in the literature there are few reports of series involving minimally invasive techniques, the number of patients is small and only short-term follow-up is described.
To the best of our knowledge, the present series is the largest published in which the results of thoracoscopic thymoma resection are compared with those of sternotomy, with a mean follow-up of 107 months. However, thymoma is an indolent disease, and longer-term follow-up is necessary to evaluate and compare the oncological outcomes, as there are reports of recurrences detected 10 years after the initial surgery. Although the limitations of our study include those common to nonrandomized, retrospective studies and the small number of cases, the preliminary results of our series indicate that thoracoscopic thymectomy to treat Masaoka stage I or II thymoma is technically feasible and safe, and is less invasive for the patient.
Conflicts of InterestAll of the authors of this original research article declare that they have no conflicts of interest.
Please cite this article as: Triviño A, Congregado M, Loscertales J, Cozar F, Pinos N, Carmona P, et al. Análisis comparativo del abordaje para el tratamiento del timoma estadio i-ii: VATS versus abordaje convencional. Cir Esp. 2015. http://dx.doi.org/10.1016/j.ciresp.2014.02.021
This manuscript was presented at the 20th European Conference on General Thoracic Surgery, Essen, Germany, 11 June 2012.