Colon cancer is a highly prevalent disease whose incidence has increased in recent years.1 The current treatment of locally advanced colon cancer (stages II [T3-4/N0/M0] and III [T1-4/N+/M0]) is based on surgical oncology. Adjuvant chemotherapy is recommended in stages III and II, which, in the absence of microsatellite instability, associate factors for poor prognosis – T4 tumors, undifferentiated, with perineural or lymphovascular invasion, intestinal obstruction or perforation, lymphadenectomy of less than 12 nodes, affected margins after the intervention or a CEA value at diagnosis greater than 5–10ng/dL.2–5
With this therapeutic combination, 5-year survival rate range widely, from 66% in stage IIA to 28% in stage IIIC.6 These results, which are far from optimal, represent a partial failure in the local and remote control of the disease.
In this scenario, it seems reasonable to seek alternative treatments to improve patient prognosis. One is neoadjuvant chemotherapy, with which there has been extensive experience in other gastrointestinal tumors.7 This modification of the therapeutic sequence has a series of theoretical advantages: reduced preoperative tumor volume, less risk of cell dissemination of the tumor during surgery, in vivo study of the effectiveness of chemotherapy and its early administration, and increased completion rate as it is not affected by surgical complications. In contrast, there is the risk of overtreatment – secondary to radiological overstaging –, fear of tumor progression during the administration period of neoadjuvant chemotherapy and the theoretical risk of higher postoperative morbidity.
This preoperative therapeutic approach is currently under study, and several papers have been published with preliminary results that have shown it to be a safe practice in terms of surgical complications and oncological toxicity, providing an adequate clinical and pathological tumor response with little risk of progression and encouraging survival results.8–10 There are presently several clinical trials in the active patient recruitment phase in various countries.
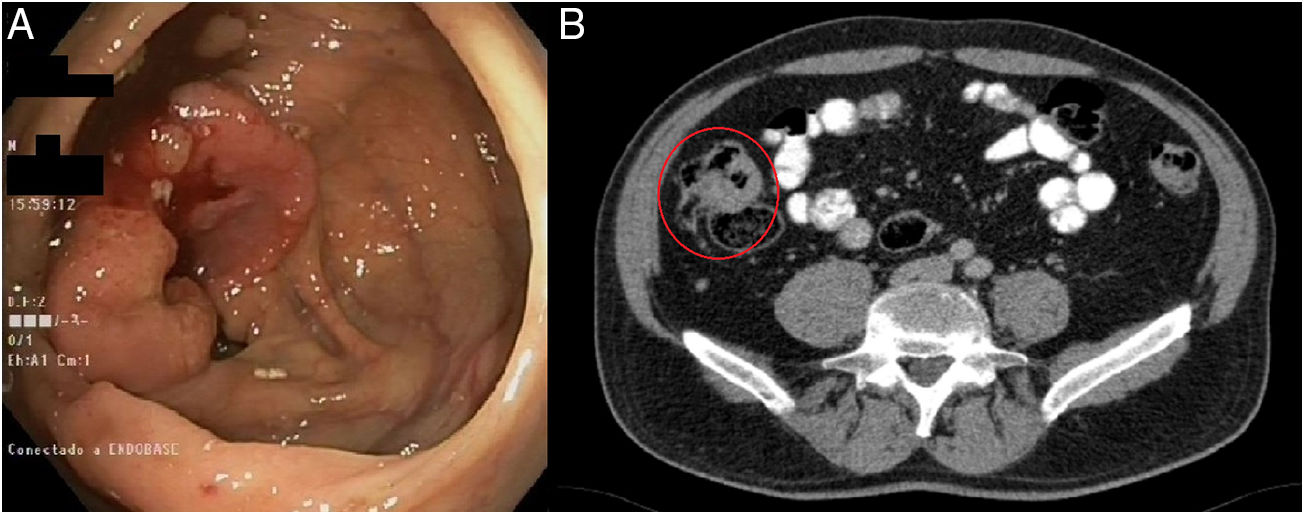
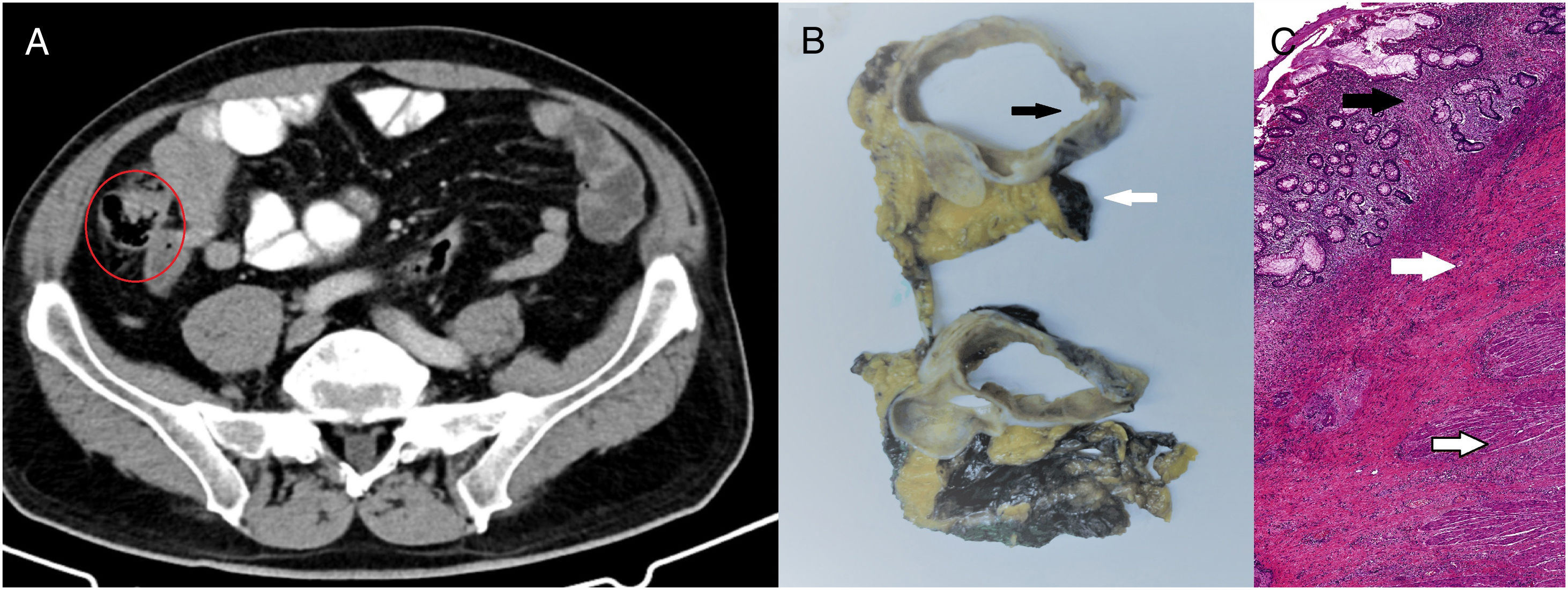
We present a case from the ELECLA clinical trial (Spanish Registry of Clinical Studies: 2016-002970-10), in which the role of neoadjuvant chemotherapy is analyzed in locally advanced colon cancer, considered T4 or T3 with more than 5mm of extramural invasion, with or without lymph node involvement and no evidence of distant metastasis on CT. The patient is a 62-year-old male who underwent colonoscopy as part of an anemia study, in which an infiltrating adenocarcinoma was observed in the cecum (Fig. 1A). In the staging CT scan, the cecal tumor was observed to have invaded the ileocecal valve: T3N1M0 (Fig. 1B). After being informed of the possibility of participating in the trial and signing the corresponding informed consent, the patient was included in the study and randomly assigned to receive 3 preoperative cycles of chemotherapy based on the XELOX regimen. The tumor volume was reduced by 71% to become a T2N0M0 tumor, as evaluated by the preoperative CT scan (Fig. 2A). Four weeks after the end of chemotherapy, a right hemicolectomy was performed laparoscopically. The patient was discharged on the third postoperative day with no surgical complications. The pathology results reported the absence of residual tumor, with complete pathological response (degree of tumor regression 0 on the modified Ryan scale) (Fig. 2B and C). After a 12-month follow-up, the patient is asymptomatic and remains disease free.
Diagnostic tests: (A) colonoscopy: neoplasm in right colon adjacent to the cecum and ileocecal valve, with histological confirmation of invasive adenocarcinoma; (B) CT scan: mass in the cecum with involvement of the ileocecal valve measuring 7×3.6×3cm, with invasion of the adjacent fat and satellite lymphadenopathies – T3N1M0.
Response assessment tests: (A) CT scan after neoadjuvant chemotherapy showing reduction in tumor mass, now measuring 4×2.2×2.5cm, with the disappearance of the speculation of the edges and reduced lymphadenopathies – T2N0M0; (B) macroscopic image showing colon wall without the tumor (black arrow), mesocolon marked with stain (white arrow), 0/39 affected lymph nodes; (C) microscopic image with hematoxylin–eosin stain (40×) showing complete pathological response and mucosa with regenerative changes, without evidence of dysplasia or malignancy (black arrow). Submucosa shows notable signs of fibrosis (white arrow). Muscularis propia is normal (emptied arrow).
This case report demonstrates an example of the efficacy of neoadjuvant chemotherapy in locally advanced colon cancer. In the various clinical trials that are currently being carried out, complete pathological response rates have been reported between 2% and 4.6%, with different regimens based on capecitabine – or folinic acid and fluorouracil – and oxaliplatin, with or without panitumumab.8–10 It is necessary to determine whether the time between the end of neoadjuvant chemotherapy and surgery (reportedly between 24 and 61 days8,10) and the addition of monoclonal antibodies can influence pathological response rates. It is necessary to complete the clinical trials that are currently underway and have a long-term follow-up to determine the real impact that this type of findings may have on survival.
The ELECLA clinical trial received the 2018 Grant of the Spanish Foundation of Coloproctology for research projects as well as a grant from the Regional Health Administration of Castilla y León (GRS 1890/A/18).
Conflict of InterestsNone.
Please cite this article as: Arredondo J, Simó V, Castañón C, Suárezc MJ, Álvarez MC. Respuesta patológica completa tras quimioterapia neoadyuvante en cáncer de colon localmente avanzado. Cir Esp. 2020;98:168–170.