Cystic dystrophy of the duodenal wall is a rare complication of the aberrant pancreas. It is usually asymptomatic, although there may be symptoms such as gastrointestinal hemorrhage, acute and chronic pancreatitis, biliary obstruction or cystic dystrophy. This is a rare condition that almost exclusively affects the duodenal wall and is characterized by the presence of intramural cystic lesions associated with fibrosis and a notable inflammatory component. In most cases, it poses important diagnostic and therapeutic difficulties as it frequently simulates tumors of the head of the pancreas.1–4
We present a case of cystic dystrophy of the duodenal wall in which the definitive diagnosis was determined by pathology studies.
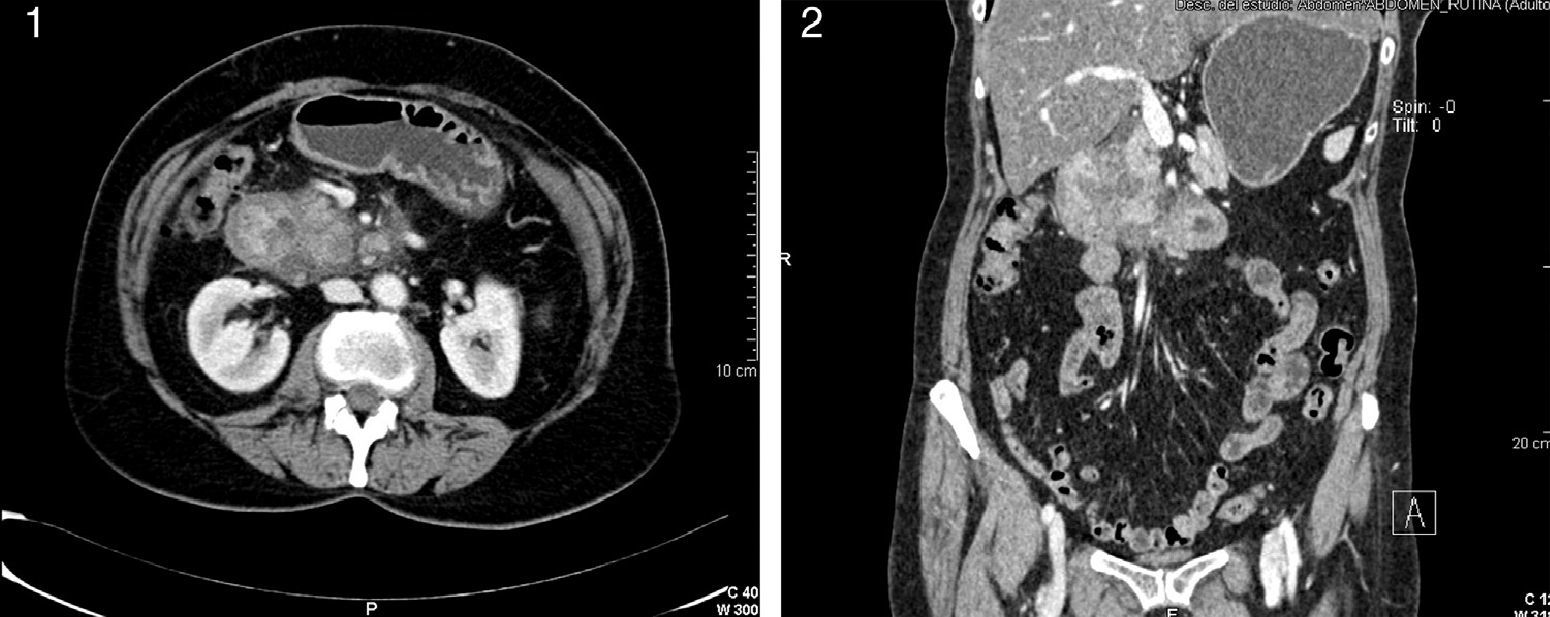
The patient was a 60-year-old woman who complained of abdominal pain with vomiting and weight loss over the course of the last 3 months, with greater intensity in recent weeks. Abdominal ultrasound showed evidence of a hypoechoic solid mass measuring 4–6cm adjacent to the head of the pancreas that seemed to be dependent upon the 2nd portion of the duodenum. Abdominal CT scan demonstrated concentric wall thickening of the second portion of the duodenum, important narrowing of the lumen and occupation of the pancreaticoduodenal groove; cystic structures were identified in the wall of the duodenum (9mm) and in the uncinate process of the pancreas (12mm) (Figs. 1 and 2). Gastrointestinal endoscopy of the second portion of the proximal duodenum showed a 2cm segment that was slightly distensible, stenotic and with erythematous and edematous mucosa. The appearance was one of extrinsic compression. Magnetic resonance cholangiopancreatography revealed an altered signal in the region of the second portion of the duodenum, without being able to distinguish whether the disease was inflammatory or tumor-related. Finally, during endoscopic ultrasound-guided fine needle aspiration biopsy, the pancreas was seen to be discretely heterogenous in the body and tail with dilatation of the pancreatic duct, while the head presented signs of chronic pancreatitis and thickening of the pancreaticoduodenal groove. The duodenal wall did not seem to show signs of significant alterations. A hypoechoic area was identified in the head of the pancreas, with irregular edges measuring 27cm×32cm. The fine-needle aspiration was not effective for diagnosis as not enough material was obtained. Tumor markers Ca 19.9 and CEA were within normal limits, and the octreotide scan was negative.
Pancreaticoduodenectomy was performed and the pathological study revealed chronic pancreatitis. Furthermore, in the duodenal wall there was inflammation and cystic dilatations both in the Brunner glands of the duodenal wall as well as in groups of ducts located in the submucosa of the duodenum, compatible with ectopic pancreatic tissue. These lesions were compatible with cystic dystrophy of the duodenal wall. As an incidental finding, a carcinoid tumor measuring 0.6cm in diameter was found in the duodenal submucosa, which had not been detected in any of the preoperative tests, with positivity for the neuroendocrine markers chromogranin and synaptophysin. The postoperative period was uneventful, and the patient was discharged on the 12th day post-op.
In 80% of cases, cystic dystrophy of an aberrant pancreas is associated with chronic pancreatitis. It is characterized by the irregular thickening of the duodenal wall and the presence of cysts of varying sizes in the interior of the thickened wall. The cystic lesions, located in the submucosa or muscle itself, are outlined by cuboidal epithelial cells. Meanwhile, in the underlying ectopic pancreatic tissue, fibrosis is observed along with chronic inflammatory changes similar to those described in chronic pancreatitis.5 It may be asymptomatic, but on most occasions the symptoms are abdominal pain with associated vomiting. In this case, there was also a neuroendocrine tumor, an association that has been described by other authors.6
Treatment is controversial7,8 and options include medical treatment with transitory results using somatostatin analogs and parenteral nutrition, endoscopic fenestration of the cysts when they are few and superficial (which is not usual) and surgery. Surgical treatment is usually the best option for the definitive treatment of the symptoms, including pancreaticoduodenectomy, gastric bypass or duodenal resections with pancreatic preservation.9 Pancreaticoduodenectomy is the classically used procedure and should be considered when, despite different diagnostic techniques, the nature of the tumor continues to be uncertain.
Please cite this article as: Jiménez-Fuertes M, Costa-Navarro D. Distrofia quística de pared duodenal. De la incertidumbre diagnóstica a la confirmación anatomopatológica. Cir Esp. 2014;92:498–499.