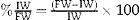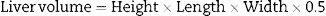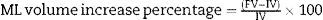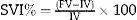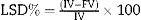Liver failure might be a cause of death after major hepatectomies. The ALPPS technique appears to be a promising strategy to avoid it, however no experimental studies supporting this procedure have been previously described. The aim was to develop an experimental model of ALPPS in rats.
MethodExperimental. A total of 30 Sprague Dawley rats were used. To develop the ALPPS procedure, ligation of the left portal branch of the middle lobe (LM) was performed. This demarcates the left side (SILM) from the right side (SDLM); parenchyma transection was performed following the demarcated line. The animal's weight, volume and weight of both LM were analyzed. Sacrifice at 3, 7 and 14 days after the procedure (10 per group) was performed.
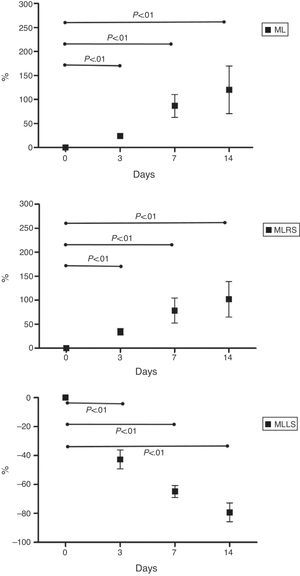
ResultsNo bleeding or ascites was observed during the postoperative period. The LM increased by 24.1%, 86.9% and 120.4% at 3, 7 and 14 days. The SDLM increased by 34.4%, 78.8% and 102.0% at 3, 7 and 14 days. The SILM decreased 42.6%, 64.8%, and 79.3% at day 3, 7 and 14 days respectively.
ConclusionThe ALPPS procedure can be performed in rats, achieving the expected results. Comparison studies to 2-staged hepatectomy will be necessary.
La insuficiencia hepática postresección es una de las principales causas de muerte en el postoperatorio de una hepatectomía mayor. La técnica ALPPS aparece como una estrategia prometedora para evitarla, pero no existen estudios experimentales al respecto. El objetivo del trabajo es desarrollar un modelo experimental de ALPPS en ratas.
MétodoSe desarrolló un modelo experimental de ALPPS en 30 ratas Sprague Dawley. Se realizó la ligadura de la rama portal izquierda del lóbulo medio (LM), con lo cual se demarca el sector izquierdo (SILM) y derecho (SDLM); posteriormente se realizó la transección parenquimatosa por la línea isquémica. Se evaluaron el peso del animal, el volumen y peso del LM y de ambos. Sacrificio a los 3, 7 y 14 días (10 por grupo).
ResultadosNo se presentaron complicaciones hemorrágicas ni ascitis en el postoperatorio. El incremento del volumen del LM fue del 24,1%; 86,9% y 120,4% a los 3, 7 y 14 días. El SDLM (no ligado) se incrementó un 34,4%; 78,8% y 102,0% a 3, 7 y 14 días. El SILM disminuyó un 42,6%; 64,8%, y 79,3% en los días 3, 7 y 14.
ConclusiónLa realización del ALPPS fue posible en ratas, logrando los resultados esperados. Futuros estudios son necesarios para compararlo con la técnica de hepatectomía en 2 tiempos.
The need to perform large hepatic resections has led surgical teams to devise strategies to reduce the development of postoperative liver failure. Two-stage hepatectomy with portal vein ligation or embolisation is a universally accepted process applied in the event that the future liver remnant is small. In the first stage (first surgery), the tumours on the side to be hypertrophied must be resected, performing contralateral portal vein ligation or embolisation. After a 4–6 week period, and confirmation of hypertrophy on the non-embolised side, the second stage is completed (second surgery) and liver resection with portal vein occlusion is performed.1–5 This allows us to reduce the possibility of hepatic failure due to small liver.6,7
However, this technique is not sufficient at times to achieve the required hypertrophy. Recently a new technique has been developed to perform 2-stage hepatectomies. For this, a parenchymal transection is added to portal vein ligation between the liver to be resected and the future remnant liver as part of the “first stage”. Then, the “second stage” must be completed after 7 days.8 This technique is known as ALPPS (associating liver partition and portal vein ligation for staged hepatectomy).9–11 The advantage ALPPS provides is the seemingly bigger and faster liver hypertrophy, compared to that obtained with portal vein occlusion only.12 Enthusiasm from these findings has led different surgical groups, including ours, to perform this procedure lacking basic supporting evidence. Several clinical communications have demonstrated an increase in ALPPS morbidity and mortality in comparison to conventional 2-stage hepatectomy.9–11
In 2000, Great Britain's Medical Research Council (MRC) established the provisions for evaluating complex surgical procedures and the development of new procedures. MRC recommendations include: developing and accessing, through iterative phases, the use of experimental models instead of observational designs whenever possible, measuring results, reporting surgeries in detail for better reproducibility, summarising evidence and achieving wider application.13–15 However, the need to resolve clinical problems has led to innovation before developing study models, as is the case with ALPPS.
This study aims to develop an ALPPS technique for an experimental model on rats as a first step toward a physiological evaluation of liver regeneration mechanisms involved in this new technique.
Materials and MethodsMethodological DesignThe study was configured as a controlled experiment. A total of 30 Sprague Dawley male rats underwent the ALPPS technique. Animals were sacrificed on days 3, 7 and 14, in sets of 10 per sacrifice group. At that time, liver samples were taken for future histological assessment.
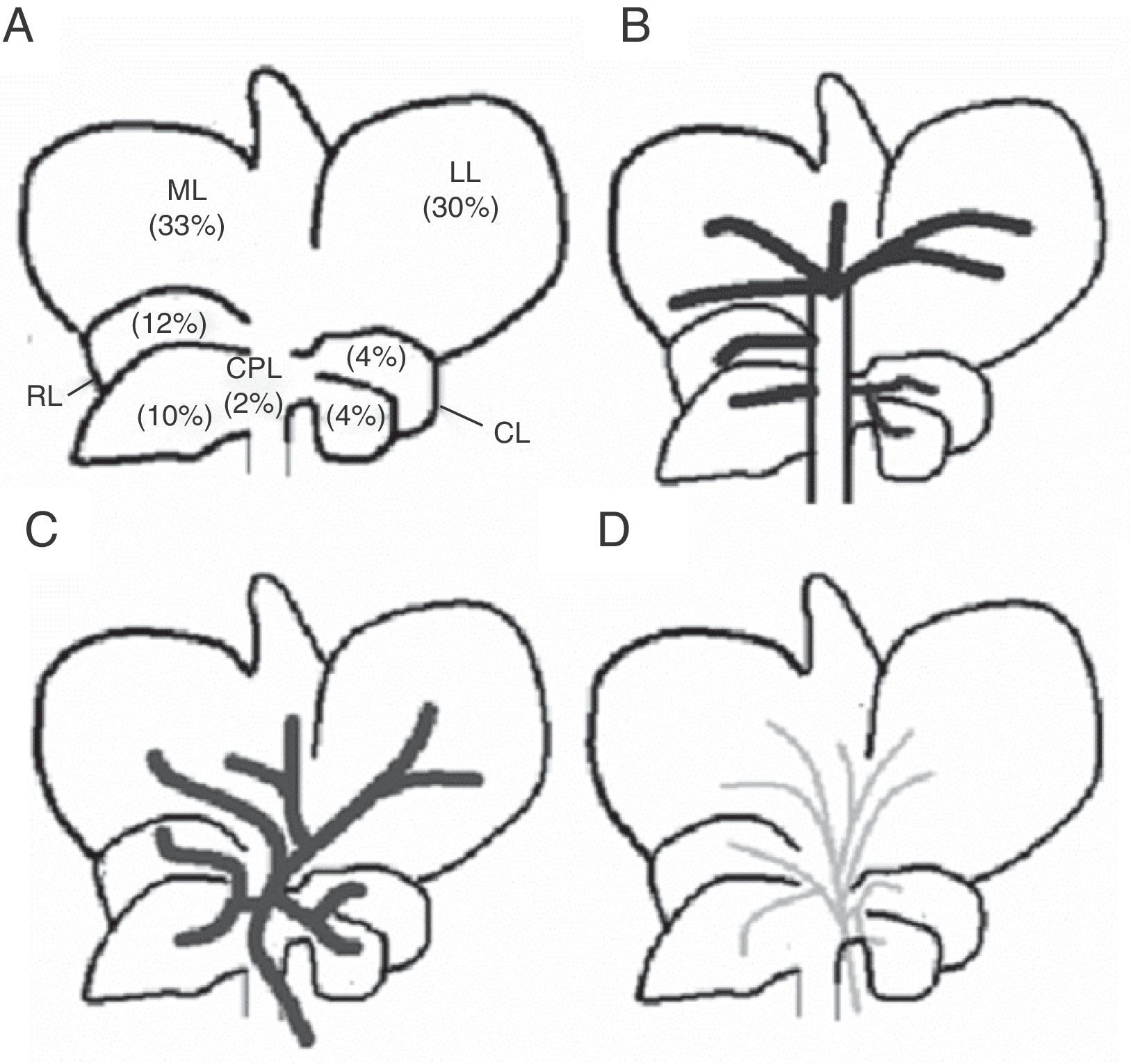
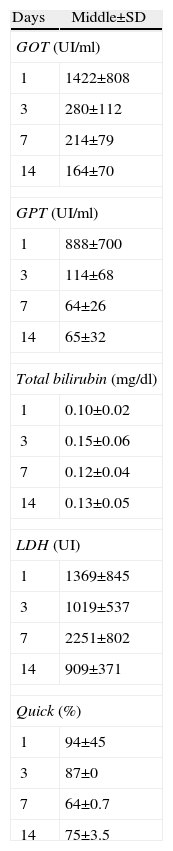
Rat livers have 5 lobes: right, middle left, caudate, and paracaval (Fig. 1). Each is irrigated by its own portal pedicle and drained by its own hepatic veins. In turn, the middle lobe has 2 separate portal vein branches: middle lobe right branch and middle lobe left branch. Based on the above, we developed the ALPPS experimental model, seeking a way to turn the rat liver anatomy into a variant similar to that of the human liver in order to be able to conduct the ALPPS.16
TechniqueThe developed experimental model is in line with the Comité Institucional para el Cuidado y Uso de Animales de Laboratorio [Institutional Committee for Care and Use of Laboratory Animals] (CICUAL) accepted standards for handling lab animals. A Carl Zeiss® 10× surgical microscope was used to develop the technique.
All animals were anaesthetised with isoflurane and oxygen (concentrate of isoflurane 1.5% and oxygen at 0.5l/min) using a Herlam® Isovap 2000 vaporizer (Herlam Laboratories, Argentina). For postoperative analgesia, morphine was administered subcutaneously at a rate of 2.5mg/kg.
Middle line laparotomy and section of the falciform ligament were performed in a sterile setting. After the liver was exposed, the caudate lobe pedicle was dissected and its portal vein was ligated with 7-0 silk string. Then, the same procedure was performed in the portal branches of the right and left lobe, respectively. Subsequently, the portal vein left branch that feeds the middle lobe was dissected and ligated. The correct ligation of the portal branch was verified by a change in parenchyma coloration, which was most remarkable in the middle lobe where preservation of right side portal vein flow yielded the ischemic demarcation line. Then, a hepatic parenchyma transection was performed along the ischemic demarcation line. It was performed by placing parallel 7-0U-stich polypropylene sutures, then sectioning the parenchyma with scissors; these steps were performed successively until the paracaval lobe was reached (Fig. 2).17 This last lobe was left unscathed. Arterial circulation and biliary duct branches were maintained in all cases.
Schematic representation of the technical steps to adapt the rat's middle lobe to portal vein occlusion techniques (black: occluded portal vein flow; grey: current portal flow). (A) Rat liver showing its respective lobes and normal portal vein irrigation (see description in text). (B) Liver showing portal vein pedicle ligation of the right, left, and caudate lobes. (C) Liver with portal middle lobe left portal vein branch ligation, delimiting 2 sectors: middle lobe right sector and middle lobe left sector; the ischemic demarcation line will be the transection site for the ALPPS technique.
Blood samples were obtained sequentially by retro-ocular plexus puncture on days 1, 3, 7 and 14, according to the animal sacrifice sequence. Sacrifice was performed via exsanguination under anaesthesia, by direct puncture of the inferior vena cava; during this procedure we took a blood sample for biochemical analysis.
MeasurementsWeight of the AnimalEach rat was weighed at the time of first surgery and at the time of sacrifice.
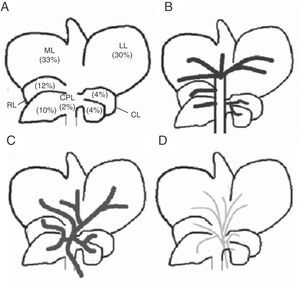
Final Weight (FW)/Initial Weight (IW) RatioThis variable was calculated as a clinical assessment measurement according to the following formula:
Liver VolumeWe recorded the middle lobe total volume (MLTV), the middle lobe right sector volume (RSV), and left sector volume (LSV). Calculation was performed according to the following formula18:
Measurements were conducted directly at the time of surgery using a calliper (Vernier® 0–150mm Calliper, China), always in the same direction and by the same operator. They were taken at the time of surgery and at sacrifice to compare them.
ML Volume Increase Percentage (MLI%)We calculated the percent ratio between final volume (FV) and initial volume (IV) according to the following formula:
MLRS volume increase percentage (SVI%): the same above formula is applied.MLLS Volume Decrease Percentage (LSD%)In this case a reduction of volume after portal vein pedicle ligation is assumed. FV reduction in relation to IV is calculated as a percentage according to the following formula:
Biochemical AnalysesWe conducted biochemical analyses for glutamic-oxaloacetic transaminase (GOT) doses, glutamic-pyruvic transaminase (GPT), total bilirubin (TB), Lactate Dehydrogenase (LDH), and prothrombin concentration (Quick).
Statistical AnalysisResults are shown as mean±its standard deviation. A P≤.05 was considered significant. SPSS statistical software v17.0 was used (Release 17.0.0; 2008).
ResultsThe technique was carried out successfully in the suggested experimental model. No bleeding complications occurred from the cut surface or ascites during the postoperative period.
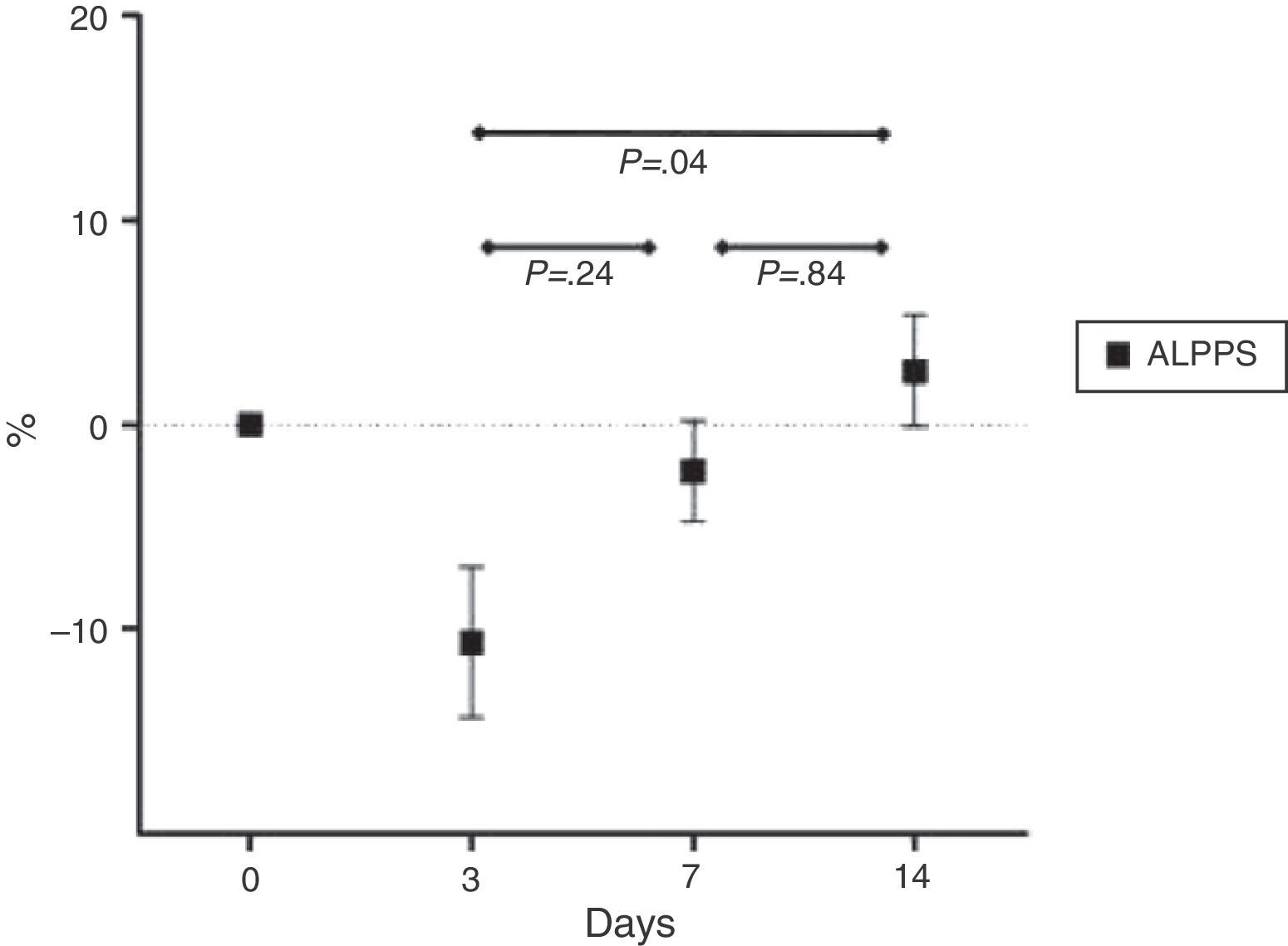
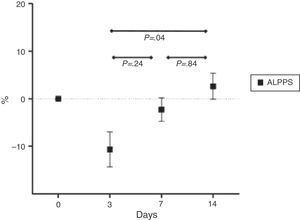
Mean percentage ratios between the animal's initial and final weight are shown in Fig. 3.
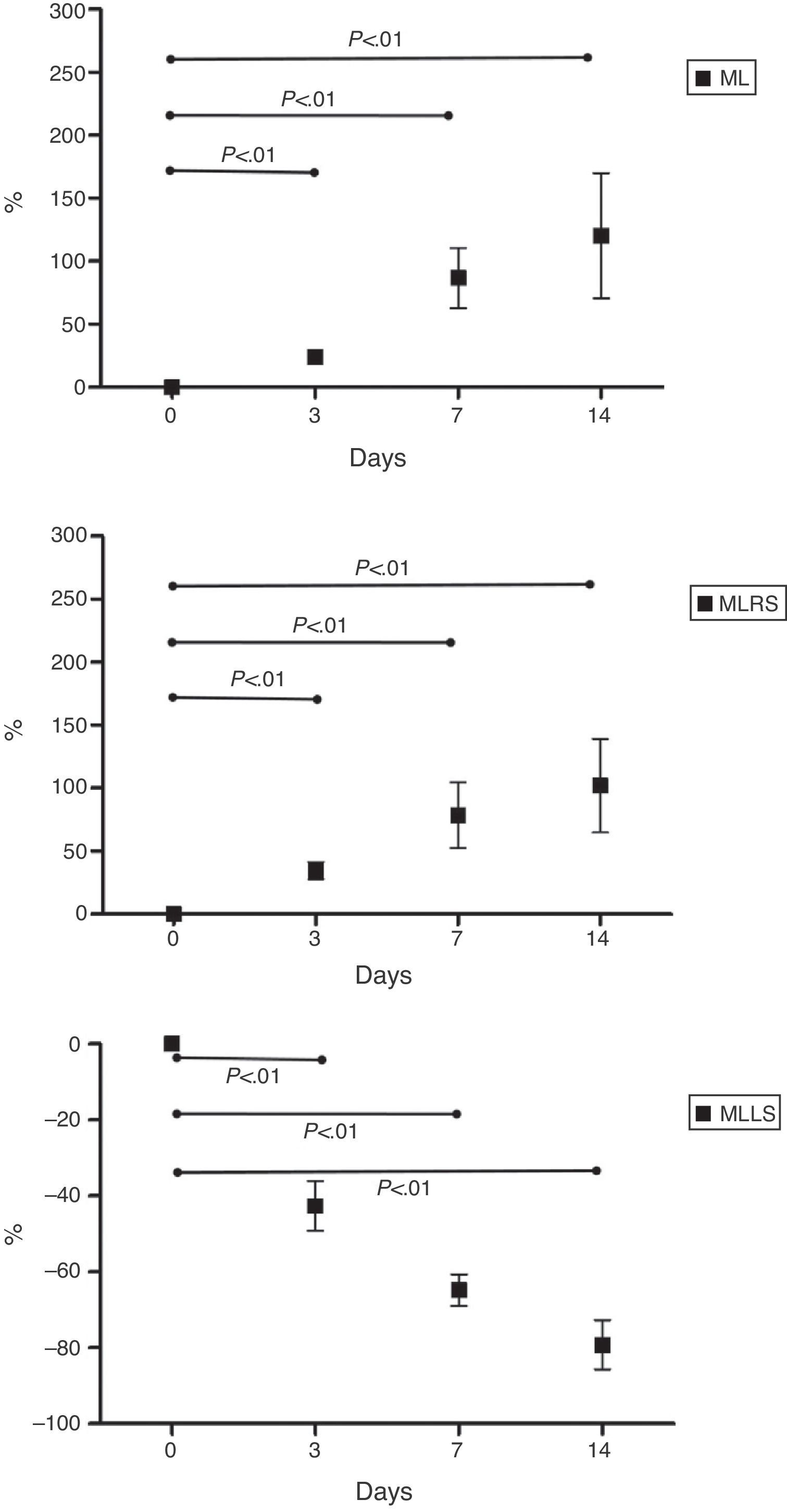
ML mean percentage increase was 24.1%±8.8% at 3 days, 86.9%±47.8% at 7 days, and 120.4%±99.5% at 14 days. SDML mean percentage increase was 34.4%±21.6% at 3 days, 78.8%±81.7% at 7 days and 102.0%±117.7% at 14 days. MLLS mean percentage reduction was 42.6%±13.1% at 3 days, 64.8%±8.4% at 7 days and 79.3%±12.9% at 14 days. Results are represented in Fig. 4. We have also noticed a volumetric reduction on the right, left and caudate lobes; these were ligated as part of the procedure.
Table 1 lists the values of biochemical determinations.
Analysed Biochemical Determination Values.
| Days | Middle±SD |
| GOT (UI/ml) | |
| 1 | 1422±808 |
| 3 | 280±112 |
| 7 | 214±79 |
| 14 | 164±70 |
| GPT (UI/ml) | |
| 1 | 888±700 |
| 3 | 114±68 |
| 7 | 64±26 |
| 14 | 65±32 |
| Total bilirubin (mg/dl) | |
| 1 | 0.10±0.02 |
| 3 | 0.15±0.06 |
| 7 | 0.12±0.04 |
| 14 | 0.13±0.05 |
| LDH (UI) | |
| 1 | 1369±845 |
| 3 | 1019±537 |
| 7 | 2251±802 |
| 14 | 909±371 |
| Quick (%) | |
| 1 | 94±45 |
| 3 | 87±0 |
| 7 | 64±0.7 |
| 14 | 75±3.5 |
Shown as mean±standard deviations.
P significant ≤.05.
The main objective of this study was to develop the first ALPPS experimental model, since the literature consulted failed to show basic research studies on this subject for the study of physiological regeneration mechanisms associated with this surgical innovation. This is why we only described the development of the technique, and left out the comparison to portal vein ligation, and without the second-surgical stage, which would be the second hepatectomy. The above, added to the functional studies, plus the histological study, were part of the second part of this project.
The first step to be able to develop this model was to “humanise” the rat liver. As stated above, rat livers have 5 lobes. We were able to ligate one of the 2 portal branches of the middle lobe, and conduct parenchymal transection, simulating a whole liver, to which a portal vein was ligated and the ALPPS procedure was conducted. Ligating the middle lobe left branch was decided based on its technical feasibility. It is noteworthy that this procedure requires training in experimental surgery on rats, since it is essential to preserve arterial and biliary duct irrigation to the lobes where the portal vein ligation is performed.19 This procedure should be conducted with some type of image enlarging means.
We were able to find that at 3 days after surgery, there was a significant hepatic volume increase of the future liver remnant (middle lobe right sector), which continued to grow at 7 and 14 days. This increase was directly related to a reduction in volume of the liver sector that underwent portal ligation (left sector of the left lobe and all other liver lobes). The foregoing was directly related to lack of portal vein flow. This demonstrated that the model reproduced the changes that occurred in humans; therefore, we considered that we reached our objective.
An important detail we observed is the impact of the procedure on the animal's weight. We have noticed that on postoperative day 3, weight reduces significantly, caused by the catabolic state of the animal. Recovery from this state happens about 14 days from the procedure. We believe that this detail bears great importance and that we can relate it to what occurs in human beings. One of the most criticised aspects of ALPPS is that patients have to undergo 2 complex surgeries in a short time period. The second hepatectomy is usually conducted one week after the first procedure. At this stage, the patient remains in a catabolic state; therefore, it is possible that he/she may not be in the best clinical conditions, due to the immediate postoperative period from the first complex surgery. This fact can be associated with the increase in morbidity and mortality shown by patients treated with ALPPS. It is quite clear that ALPPS is an excellent technique to achieve future liver remnant hypertrophy. However, we did not know what would happen if instead of operating on patients in the first postoperative week, we waited 2–3 weeks for patients to be in better clinical condition.
We understand that increases obtained in AST and ALT biochemical determinations are due to the parenchyma transection. Enzymatic elevation during the postoperative period is found in clinical practice after a hepatectomy with subsequent postoperative normalisation.
To progress in the study of the regeneration phenomenon related to ALPPS, further analyses are required that are already undergoing and exceeding the primary objective of this publication, which is the technical determination of the model; without doubt they will help build the foundation for the knowledge to develop this new and promising technique. We can thus conclude that the ALPPS experimental model is a reality today; it is reproducible and would help as a physiopathological comparison base for other liver resection techniques.
Conflict of InterestAuthors declare having no conflict of interest.
Please cite this article as: Almau Trenard HM, Moulin LE, Padín JM, Stringa P, Gondolesi GE, Barros Schelotto P. Desarrollo de un modelo experimental de ligadura portal asociada a transección parenquimatosa (ALPPS) en ratas. Cir Esp. 2014;92:676–681.
Work presented at the 10th E-AHPBA Biannual Congress, Belgrade, Serbia.