The recipient of an adult living donor liver transplant (ALDLT) is subjected to great hemodynamic changes that could lead to the appearance of a “small-for-size” syndrome in the post-operative period due to portal hyperflow. The aim of this article is to evaluate these changes, and try to correlate them with portal vein flow during reperfusion.
Material and methodsA protocol for monitoring various liver hemodynamic data of the ALDLT recipient before, during and after surgery has been used since the year 2003. The hemodynamic outcome of the recipient after the transplant, as well as the correlation between the portal vein flow during reperfusion and the collected hemodynamic data is analyzed.
ResultsThere was no small for size syndrome. A significant relationship was found between the portal flow during reperfusion and the portal vein pressure at the beginning of the operation (r=0.46, P<.006) and with the portocaval shunt flow during the anhepatic phase (r=0.55, P<.001). The recipients showed a normal splanchnic hemodynamic state at 3 months after the transplant.
ConclusionsHemodynamic monitoring of the ALDLT recipient is essential to prevent portal hyperflow. The relationship between flow during reperfusion and flow through the portocaval shunt means that patients with a higher risk of hyperflow can be identified and can be modified before reperfusion.
El receptor de un trasplante hepático de donante vivo en adulto (THDVA) está sometido a grandes cambios hemodinámicos que pueden determinar la aparición del síndrome de «small-for-size» en el postoperatorio como consecuencia de un hiperaflujo portal. El objetivo de este trabajo es evaluar dichos cambios e intentar correlacionarlos con el flujo de la vena porta durante la reperfusión.
Material y métodosDesde el 2003 se lleva a cabo un protocolo de monitorización de distintos datos hemodinámicos hepáticos del receptor de THDVA antes, durante y después de la intervención quirúrgica. Se analiza la evolución hemodinámica del receptor tras el trasplante, y se correlaciona el flujo de la vena porta durante la reperfusión con dichos datos hemodinámicos.
ResultadosNo hubo ningún síndrome de small-for-size. Se halló una correlación significativa del flujo portal durante la reperfusión con la presión de la vena porta al inicio de la intervención (r=0,46; p=0,006) y con el flujo del shunt portocava durante la fase anhepática (r=0,55; p<0,001). Los receptores mostraron un estado hemodinámico esplácnico normal a los 3 meses del trasplante.
ConclusionesLa monitorización hemodinámica del receptor de THDV es fundamental para evitar el hiperaflujo portal. La correlación entre flujo durante la reperfusión y el flujo a través del portocava nos va a permitir identificar a los pacientes con mayor riesgo de hiperaflujo y poder modularlo antes de la reperfusión.
The use of partial grafts in adult living donor liver transplantation (ALDLT) implies two important aspects: donor safety and the quantity of liver tissue to be transplanted.
On the one hand, donor safety is one of the most important factors in the process of ALDLT, and the safety of these donors should be guaranteed.1 Donor morbidity varies according to the series published, but it is estimated to be between 20% and 50%.2 Surgical complications are closely connected to the magnitude of the hepatectomy performed in the donor. This has been reflected in a recent publication showing the results in more than 1200 living donors, with a greater incidence of biliary complications in those donors who underwent right hepatectomy than in those in whom the left liver was used as a graft (44.2% vs 18.8%; P<.05).3 Biliary complications were also more frequent in the right liver donors (12.2% vs 4.9%; P<.05). Finally, it should be emphasized that the living liver donation process is not free of mortality, with a declared mortality rate ranging between 0.15% and 0.2%.4
On the other hand, the size of liver mass, or rather the proportion between the weight of the graft implanted and that of the recipient's (graft to body weight ratio [GBWR]) is directly associated with postoperative mortality. Several studies have demonstrated poorer survival in recipients with grafts whose GBWR were less than 0.8%.5–9 The reason for this reduced survival is the appearance of the “small-for-size syndrome” (SFSS) in those grafts with a GBWR less than 0.8%. In spite of the lack of diagnostic criteria with total consensus, SFSS is defined as the presence of signs and symptoms of liver failure (prolonged hyperbilirubinemia, altered coagulation tests, production of ascites and encephalopathy) in absence of other justifying factors in a patient with a graft whose GBWR is less than 0.8%.6 The importance of SFSS is its high mortality of up to 50%5 in the postoperative period, mostly as a consequence of infectious complications.
Usually, the recipient of a liver transplant is a patient with terminal-phase chronic hepatopathy who presents portal hypertension and hyperdynamic circulation. In these patients, fundamentally due to elevated cardiac output, there is increased splanchnic circulation and, as a consequence, portal vein flow, with the presence of important hemodynamic changes during transplantation.10 Therefore, in the case of ALDLT, two factors converge that could influence the development of SFSS: high portal flow (PF) and a diminished vascular bed due to the partial graft. Both factors contribute to a high relative PF (PF divided by liver mass), and SFSS is produced.
During ALDLT, if excessive PF is determined, there is the possibility to lower it using different surgical or pharmacological maneuvers, known as modulation of portal graft inflow.11,12 Until now, inflow modulation has been done after reperfusion of the graft and after finding excess PF. Given that injury due to hyperflow occurs immediately,13 even temporary high PF may condition the graft results.
In 2003, we initiated a hemodynamic monitoring program for ALDLT recipients in order to characterize the evolution of the recipient from a hemodynamic standpoint before, during and after transplantation. Our aim was to determine hemodynamic factors related with graft PF in order to be able to make modifications before reperfusion.
Material and MethodsIn 2003, the hemodynamic monitoring protocol for ALDLT recipients was begun. Since then, a total of 45 procedures have been performed. Hemodynamic data were collected prospectively and analyzed retrospectively. Table 1 summarizes the demographic characteristics of the recipients.
Demographic Characteristics of Living Donor Liver Transplant Recipients.
| Age (years) (mean±SD) | 54.7±9.2 |
| Sex (M/F) | 32/13 |
| Patient weight (kg) (mean±SD) | 70.6±14.9 |
| Native liver weight (g) (mean±SD) | 1119.6±224.6 |
| Graft weight (g) (mean±SD) | 710.7±126.6 |
| GBWR (mean±SD) | 1.04±0.2 |
| Origen of the hepatopathy (n) | |
| HCV infection | 30 |
| Alcoholic cirrhosis | 9 |
| HBV infection | 1 |
| HBV+HCV infection | 1 |
| Cryptogenic cirrhosis | 1 |
| NASH | 1 |
| Primary biliary cirrhosis | 1 |
| Autoimmune hepatitis | 1 |
| Child-Turcotte-Pugh score | |
| A | 11 |
| B | 21 |
| C | 13 |
| MELD score (mean±SD) | 13.5±4.1 |
| Hospital stay (mean±SD) | 32.4±18.9 |
| Median follow-up (months) | 44.0 |
The ALDLT surgical procedure involved two interventions. First, the liver graft was obtained for the recipient, including Couinaud segments V–VIII; to do so, a right hepatectomy was carried out in the donor, while constantly maintaining circulation in the future graft and preserving the middle hepatic vein in the donor. After having completed the hepatectomy, the graft was perfused with preservation fluid and kept refrigerated at 4°C until the time of implantation.
In the recipient, firstly a total hepatectomy of the diseased liver was performed and splanchnic circulation was preserved with a partial portocaval shunt. Subsequently, the graft was irrigated with warm saline solution and the implantation was begun. Initially, the right hepatic vein of the graft was anastomosed to the right hepatic vein of the recipient; then, the temporary portocaval shunt was sectioned, the portal vein anastomosis was performed and the graft was reperfused. Afterwards, the arterial anastomosis was completed, and lastly the biliary anastomosis was performed.
Protocol for Hemodynamic MonitoringHemodynamic monitoring in recipients included 3 well-defined aspects: preoperative, intraoperative and postoperative monitoring.
Preoperative MonitoringThis was done in the hepatic hemodynamics laboratory at the hospital 24h before transplantation. Both hepatic and systemic hemodynamic variables were monitored under conscious sedation, although these latter variables were only collected in 28 patients (Table 2).
Variables Analyzed in the Hemodynamic Monitoring Protocol.
| Preoperative monitoring |
| Hepatic hemodynamics |
| Wedged hepatic venous pressure (WHPV) |
| Free hepatic venous pressure (FHVP) |
| Hepatic Venous Pressure Gradient (HVPG) |
| Cardiopulmonary hemodynamics |
| Pulmonary artery pressure (PAP) |
| Pulmonary capillary pressure (PCP) |
| Right atrial pressure (RAP) |
| Cardiac output (CO) |
| Mean arterial pressure (MAP) |
| Heart rate (HR) |
| Cardiac index (CI) |
| Systolic ejection volume (SEV) |
| Systemic vascular resistance (SVR) |
| Systemic Vascular Resistance Index (SVRI) |
| Pulmonary vascular resistances (PVR) |
| Pulmonary vascular resistance index (PVRI) |
| Perioperative Monitoring | |
| Time of measurement | Variables |
| Start of intervention | Portal vein pressure |
| After portocaval shunt | Portal vein flow |
| After portal reperfusion | Hepatic artery flow |
| After arterial reperfusion | Cardiac output |
| After ligation of the splenic artery | Mean arterial pressure |
| End of intervention | Central venous pressure |
| Vascular resistances | |
| Post-operative monitoring |
| Wedged hepatic venous pressure (WHVP) |
| Free hepatic venous pressure (FHVP) |
| Hepatic venous pressure gradient (HVPG) |
After laparotomy, the first step involved the placement of a catheter (Certofix Mono S 330, 16G, L 30cm, Braun, Barcelona, Spain) in a mesenteric vein up to the portal vein in order to be able to monitor portal pressure (PP) during transplantation. The portal and arterial flows were determined with the transit time flow measurement method, using 8–12mm catheters for the portal vein and 3–5mm for the hepatic artery, which were monitored using the Medi-Stim VeriQ System® device (Medi-Stim ASA, Oslo, Norway). All the measurements were taken 5min after completing the anastomosis in order to achieve the best possible stability. The hemodynamic measurements were done in different phases of the intervention which, together with the hemodynamic data collected during the transplantation, are summarized in Table 2.
Post-Operative MonitoringHepatic hemodynamics were analyzed both 3 days and 3 months after the transplantation in order to guarantee normalization after transplantation.
Ligation of the Splenic ArteryAfter the portal and arterial reperfusion phase, the portal and arterial flows were measured. In agreement with other publications,14,15 a PF of more than approximately 2,000mL/min or 260mL/min/100g of hepatic tissue was considered excessive. An arterial flow of less than 100mL/min was considered insufficient. In this latter case, the portal vein was temporarily clamped in order to rule out a technical problem in the anastomosis with a significant increase in blood flow. In case of insufficient arterial or excessive portal flow, splenic artery ligation (SAL) was done at its origin in the celiac artery.
Clinical and Analytical Evolution of the Recipient After TransplantationAfter the intervention, the ALDLT recipients were transferred to the intensive care unit, where they remained until the fifth day post-transplantation and were latter moved to a conventional hospitalization ward. During the hospital stay, daily liver function analyses were ordered (GOT, GPT, alkaline phosphatase, GGT, bilirubin, prothrombin time) along with clinical monitoring. The presence of SFSS was evaluated in accordance with the criteria defined by Dahm et al.6 (Table 3).
Criteria for Small-for-Size Syndrome.
| Dysfunction due to small sizeDysfunctiona of a small-sized graft (GBWR <0.8%) during the first week post-op, after the exclusion of other causesb |
| Malfunction due to small sizeFailurec of a small-sized graft (GBWR <0.8%) during the first week post-op after the exclusion of other causesb |
Source: adapted from Dahm et al.6
Graft dysfunction: presence of 2 of the following criteria for 3 consecutive days: bilirubin >100μmol/l; INR >2; hepatic encephalopathy grade 3 or 4.
The statistical differences between groups were analyzed with the Student's t test for numerical variables. The statistical correlations between continuous variables were analyzed with Pearson's correlation. All the results are expressed as means±standard deviation, except when specified to the contrary. Measurement were considered statistically significant when P<.05. The statistical analysis of the data was performed with SPSS Statistics 17.0 (SPSS, Chicago, IL, USA).
ResultsGeneral ResultsThe analytical evolution of the ALDLT recipients was similar to that of recipients of cadaveric donor liver transplants, with a maximum peak in transaminases between the first and second day post-op, coagulation levels that progressively raised to normal levels at about one week post-op and bilirubin levels that tended to normalize.
Mean GBWR was 1.03%±0.22%, with a median of 1.03% and a range from 0.66% to 1.83%. Despite the fact that 4 patients presented a GBWR lower than 0.8%, none of the patients presented clinical or analytical criteria for SFSS according to the Dahm et al. classification.6 No immediate retransplantations were necessary. With a median follow-up of 39 months, only one patient out of 45 recipients needed to be retransplanted as a consequence of secondary biliary cirrhosis. Two patients died 3 months after the intervention, one due to acute hepatitis of unknown etiology and another due to complications derived from CMV disease.
As for vascular complications, there was only one hepatic artery thrombosis that required surgical reintervention 12h post-op. A total of 22 patients presented a biliary complication, specifically leaks. The origin of the leak was mainly the biliary anastomosis in 17 cases, followed by transection surface in 4 cases and finally one patient whose leak was produced by the Kehr drain. The initial treatment was conservative in 18 patients and surgical in 4. All the patients evolved favorably with resolution of their symptoms.
Patient 1-year, 3-year and 5-year survival rates were 93,2%, 84.2% and 74.2%, respectively.
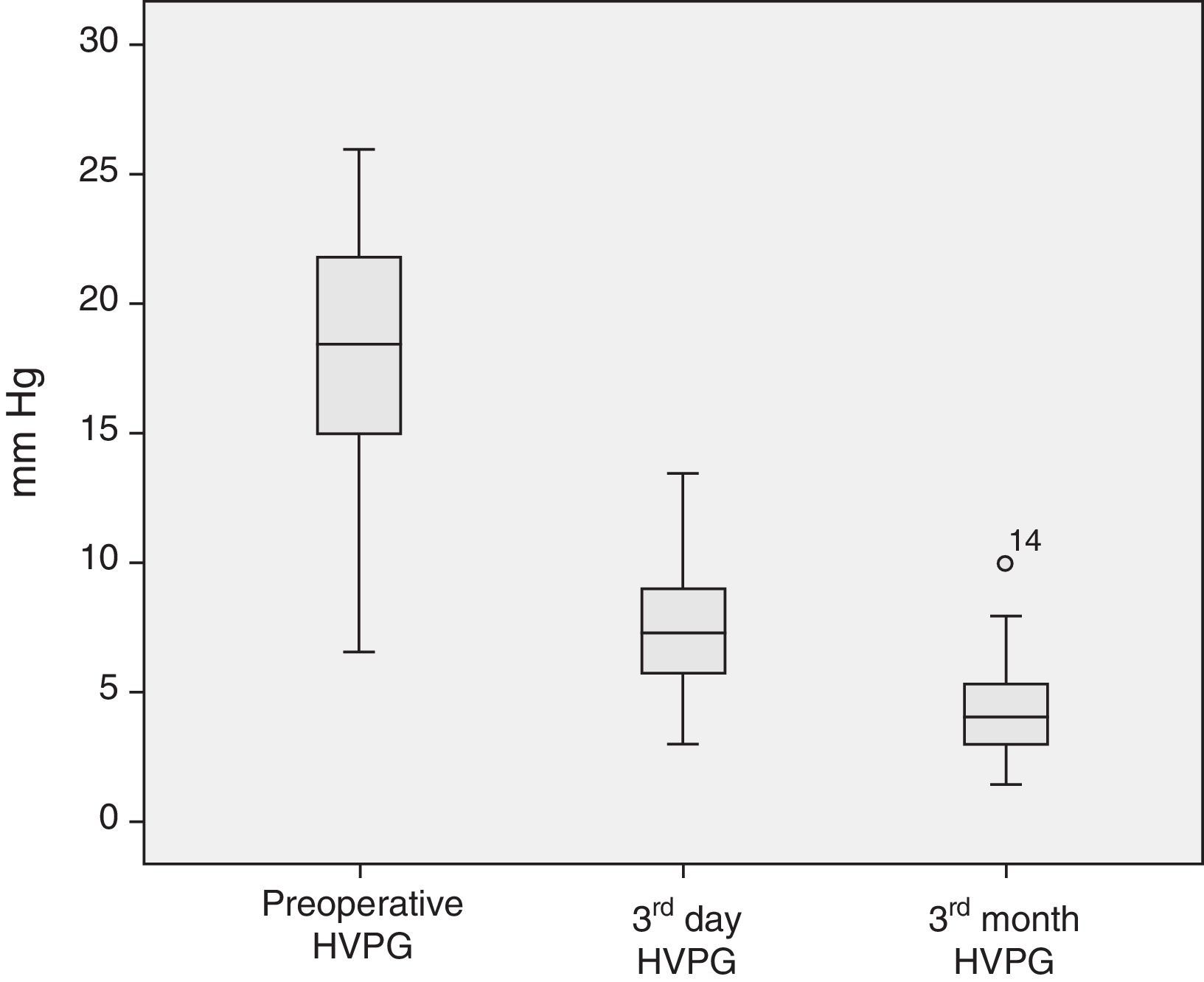
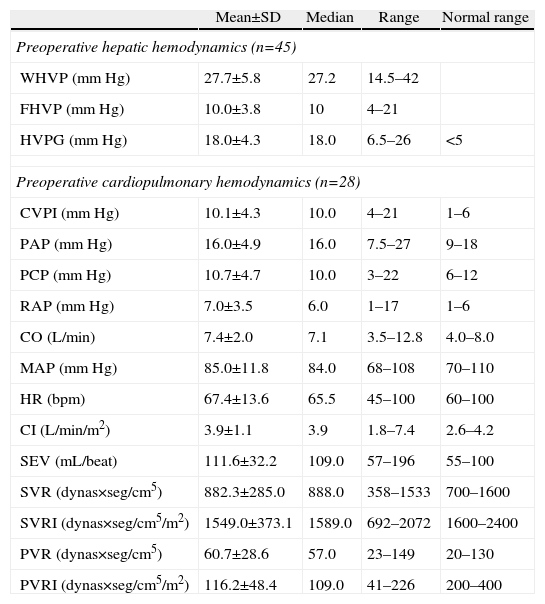
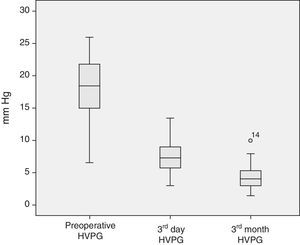
Descriptive Analysis of the Hemodynamic MonitoringPreoperative MonitoringAll of the patients presented portal hypertension during the pre-operative hemodynamic monitoring. Moreover, 40 of the patients presented severe portal hypertension (HVPG >12mmHg). The analysis of the cardiopulmonary hemodynamic parameters showed that the ALDLT recipients overall presented a tendency toward hyperdynamic circulation, with a cardiac output at the upper limits of normal values and pulmonary and systemic vascular resistances at the lower limit of normal range (Table 4).
Results of the Preoperative Hemodynamic Parameters.
| Mean±SD | Median | Range | Normal range | |
| Preoperative hepatic hemodynamics (n=45) | ||||
| WHVP (mmHg) | 27.7±5.8 | 27.2 | 14.5–42 | |
| FHVP (mmHg) | 10.0±3.8 | 10 | 4–21 | |
| HVPG (mmHg) | 18.0±4.3 | 18.0 | 6.5–26 | <5 |
| Preoperative cardiopulmonary hemodynamics (n=28) | ||||
| CVPI (mmHg) | 10.1±4.3 | 10.0 | 4–21 | 1–6 |
| PAP (mmHg) | 16.0±4.9 | 16.0 | 7.5–27 | 9–18 |
| PCP (mmHg) | 10.7±4.7 | 10.0 | 3–22 | 6–12 |
| RAP (mmHg) | 7.0±3.5 | 6.0 | 1–17 | 1–6 |
| CO (L/min) | 7.4±2.0 | 7.1 | 3.5–12.8 | 4.0–8.0 |
| MAP (mmHg) | 85.0±11.8 | 84.0 | 68–108 | 70–110 |
| HR (bpm) | 67.4±13.6 | 65.5 | 45–100 | 60–100 |
| CI (L/min/m2) | 3.9±1.1 | 3.9 | 1.8–7.4 | 2.6–4.2 |
| SEV (mL/beat) | 111.6±32.2 | 109.0 | 57–196 | 55–100 |
| SVR (dynas×seg/cm5) | 882.3±285.0 | 888.0 | 358–1533 | 700–1600 |
| SVRI (dynas×seg/cm5/m2) | 1549.0±373.1 | 1589.0 | 692–2072 | 1600–2400 |
| PVR (dynas×seg/cm5) | 60.7±28.6 | 57.0 | 23–149 | 20–130 |
| PVRI (dynas×seg/cm5/m2) | 116.2±48.4 | 109.0 | 41–226 | 200–400 |
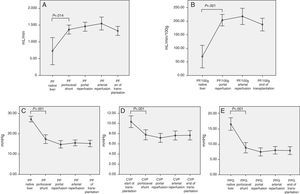
The intraoperative evolution of the hemodynamic parameters during transplantation had an inflection point during the anhepatic phase. During this surgical time, total hepatectomy of the native liver was carried out and splanchnic circulation was preserved through the temporary portocaval shunt.
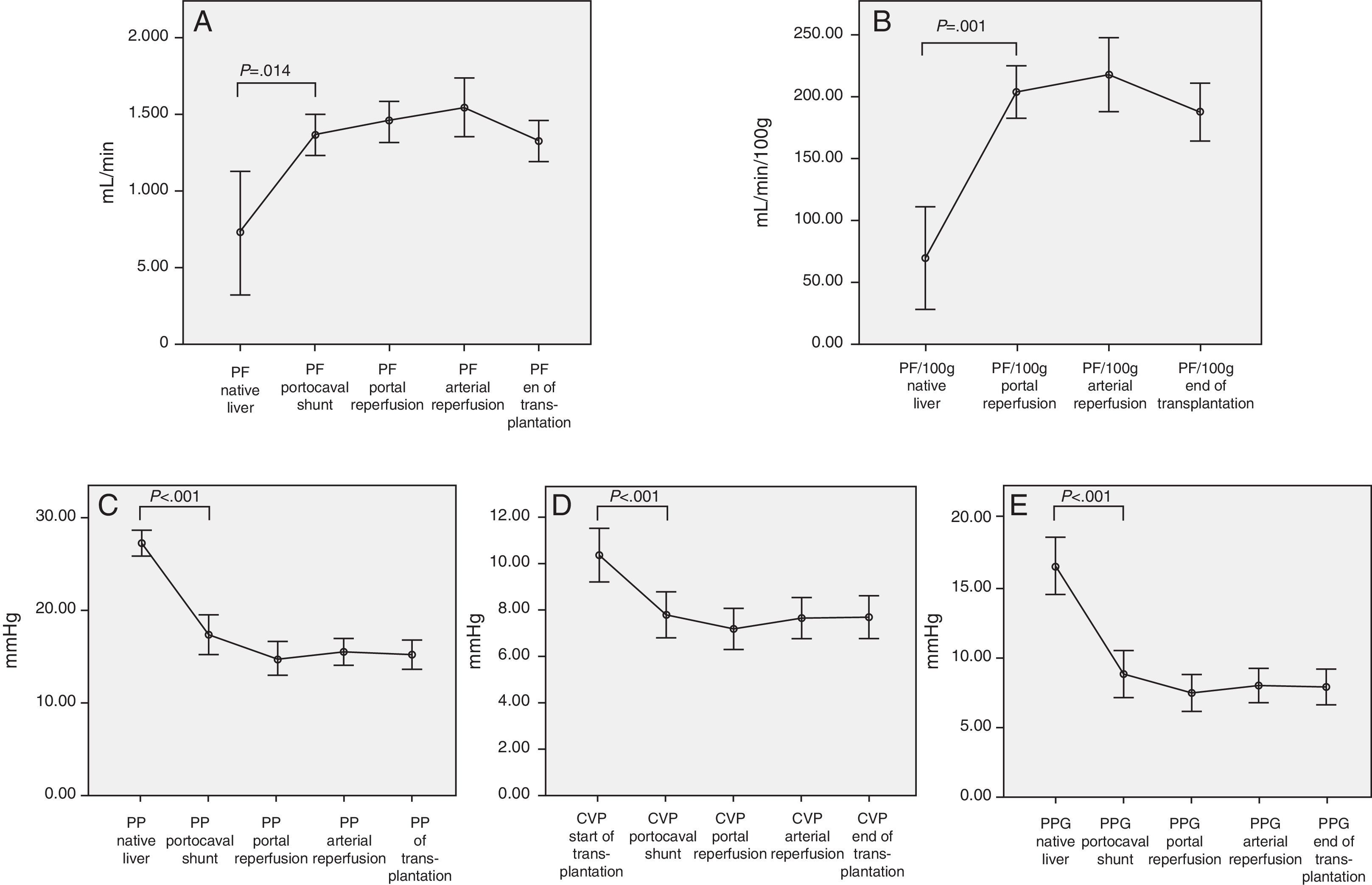
Therefore, we can observe significant hemodynamic changes in the following parameters compared with the baseline situation and using the temporary portocaval shunt (Fig. 1):
- -
Increased total portal vein flow (727±596 vs 1362.8±410mL/min; P=.014) or related with graft weight (69±62 vs 204.2±72mL/min/100g; P<.001).
- -
Reduced portal vein pressure (27.2±4.2 vs 17.4±6.1mmHg, P<.001).
- -
Reduced central venous pressure (10.4±3.7 vs 7.8±3.2; P>.001).
- -
Reduced PP slope (16.4±5.9 vs 8.8±5.3mmHg, P<.001).
The analysis of the post-operative hepatic hemodynamic evolution shows a disappearance of the state of portal hypertension prior to surgery, and normal levels are reached in the third month after transplantation (Fig. 2).
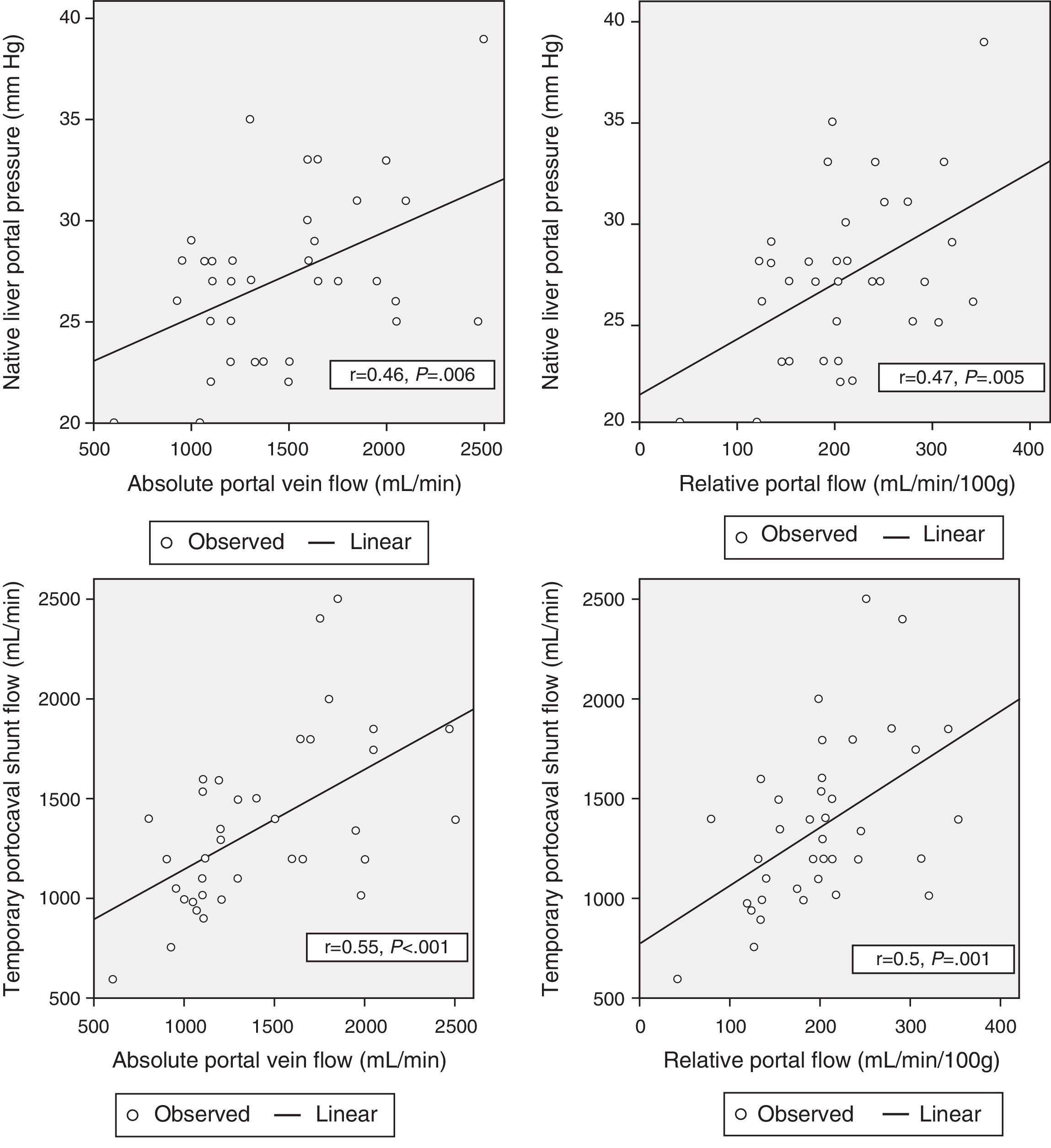
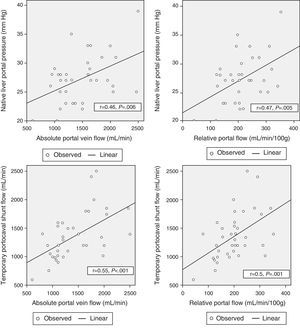
Correlation Between Hemodynamic Parameters Prior to Reperfusion and Portal FlowWith the aim to correlate the portal vein flow during reperfusion and the different hemodynamic parameters prior to reperfusion, significant correlations were found between the pressure of the portal vein of the native liver at the start of the intervention and absolute PF related to graft weight (r=0.46; P=.006 and r=.47; P=.005, respectively). The portal flow during reperfusion correlated with the flow of the portal vein through the temporary portocaval shunt during the anhepatic phase, both in terms of absolute PF (r=.55, P<.001) as well as related to graft weight (r=.5, P=.001) (Fig. 3).
There was no correlation found between preoperative hemodynamic variables and PF after graft reperfusion.
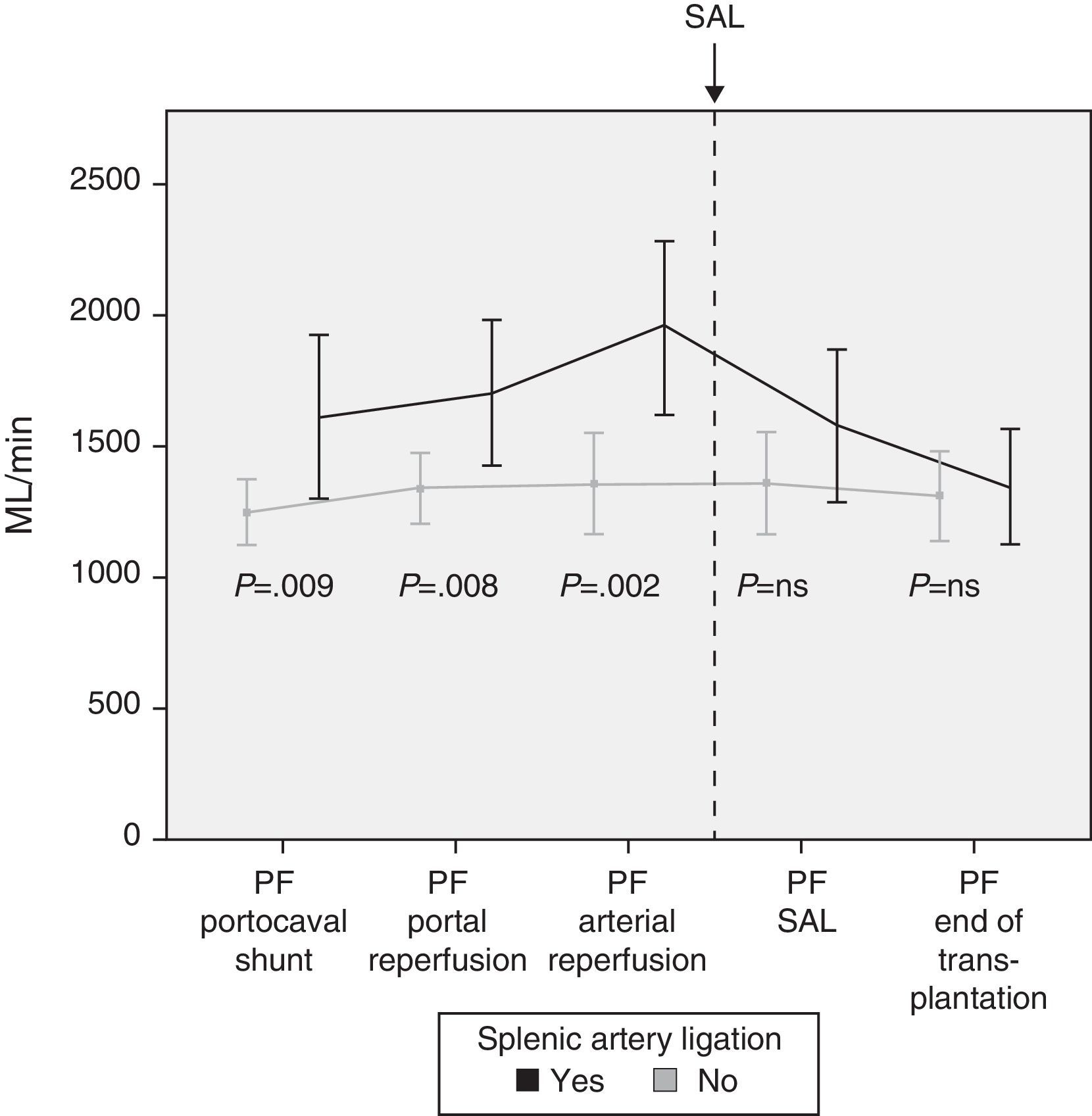
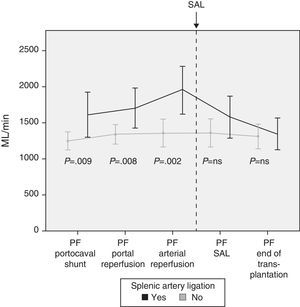
Modulating Portal Flow: Splenic Artery LigationFollowing the criteria established in the Methods section, SAL was performed in a total of 13 patients (29%) during surgery. The cause for ligature was due to excessive portal vein flow in 4 patients, insufficient hepatic artery flow in 5 patients and for both reasons in 4 patients. After SAL, the portal vein flows, which had been significantly greater in those patients who required it, showed no differences at the end of the intervention with those patients who did not require SAL (Fig. 4).
DiscussionSFSS has been studied both in experimental as well as clinical settings, and it seems clear that the key factor for its appearance is an excess of PF during graft reperfusion. This excess of flow produces an acute histologic lesion characterized by periportal edema and hemorrhage and loss of the normal alignment of the portal endothelial cells that could even extend to the centrilobular veins.13,16 It is postulated that this lesion, which impedes the normal irrigation of the hepatocytes, is the cause of later failure of liver function.
Graft overperfusion in SFSS has been measured objectively with PP or with PF during reperfusion. Asian groups are partial to monitoring PP. In this way, they have been able to find improved survival in those recipients who present PP levels below 20mm Hg at the end of the transplantation.17 Recently, the same group published their experience with ALDLT using small-sized grafts (even grafts with GBWR lower than 0.6%) with intentional control of PP in order to obtain values <15mmHg at the end of the trasplantation.18 In western countries, however, the preferred method for assessing excess perfusion is the PF measurement. Experimental data have been able to demonstrate, on one hand, that the flow better reflects splanchnic hemodynamic state and that PP and PF do not necessarily have to be related14; also, an increase in flow of twice the baseline is necessary to obtain good results.16 From a clinical perspective, both elements have been confirmed and discrepancies have been reported between the two parameters, with normal PP and excessive PF, and with up to 25% of patients with excessive PP (>20mm Hg) and low PF (lower than 90mL/min/100g), which could compromise the viability of the graft.14 As for the moderate increase in PF in the ALDLT recipient, this has been well documented in the literature with levels 2 to 3 times the baseline values.19
Given that the main problem for the development of SFSS is hemodynamic, the hemodynamic monitoring protocol was initiated with the aim to analyze the hemodynamic changes that occur in recipients over the course of ALDLT and to be able to determine a hemodynamic factor that would be capable of predicting PF during reperfusion. Both in one instance as well as in another, we have been able to identify the importance of temporary portocaval shunts in living-donor liver transplantations.
In our case, we routinely use a temporary portocaval shunt during the anhepatic phase. Initially described by Belghiti et al. in complete-graft liver transplantation, either elective20 or emergency,21 its usefulness has been observed in later studies that have demonstrated its superiority over the veno-venous bypass technique in terms of hemodynamic stability, fewer transfusions required and preserved renal function.22
In our experience, in addition to the previously mentioned benefits, we have found another reason to systematically use temporary portocaval shunts. After analyzing hemodynamic evolution during transplantation, it is clear that there is a before and after effect with the use of temporary portocaval shunts. During the anhepatic phase, the hemodynamic state of the transplant recipient, with portal hypertension evolving over a long period of time, is suddenly altered by the disappearance of the native liver that created resistance against the portal blood flow. During this phase, the pressure drops significantly and the blood flow through the portal vein increases, which will later correlate with PF. This change compared with the baseline hemodynamic situation supports the fact that there is no preoperative hemodynamic parameter that presents a correlation with PF after reperfusion.
In case of demonstrated high PF in the operating room, different maneuvers can be carried out to decrease the flow. Our first option is performing an SAL as it is an effective and relatively straight-forward method. Thirteen patients required SAL after completing portal and arterial anastomoses and analyzing the flows. The results show the effectiveness of SAL for reducing PF, as a significant decrease in PF was observed afterwards.
Due to the vast amount of literature on the association of SFSS with small-sized grafts, an essential requirement for accepting a potential liver donor is that the future GBWR for the recipient should be greater than 0.8%. Nonetheless, as there may be small errors in the volumetric calculation of the grafts, out of the total of 45 recipients, 4 presented a lower GBWR, and fortunately there were no major consequences. Nevertheless, the demonstrated correlation of the portocaval shunt flow and final PF will enable us to identify those patients at risk for presenting excess flow during reperfusion and thus be able to modulate the flow before endothelial damage occurs, leading to SFSS. As a consequence of all this, the hemodynamic monitoring of ALDLT recipients will allow us to deliberately use smaller-sized grafts with the expected decrease in donor morbidity, while the results in the recipient are maintained.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Sánchez Cabús S, et al. Protocolo de monitorización hemodinámica hepática en el trasplante hepático de donante vivo en adultos. Cir Esp. 2013;91:169-76.