Tumor seeding after a surgical procedure is an uncommon finding that occurs during the extraction of a surgical specimen or the introduction/extraction of surgical instruments through the incision during surgery. This is unlike skin metastasis, which is caused by infiltration of the skin or surrounding soft tissues through the lymphovascular pathway.1
The first tumor seeding in a surgical wound associated with laparoscopy was published in 1978, after ovarian cancer surgery.2 The incidence varies between 0.6% and 1.6% and in lung cancer between 1% and 12%, with presentation between 14 days or several months after surgery.3 The accepted mechanism is direct seeding of neoplastic cells during surgery.
The management of seeded tumors includes surgery, chemotherapy and radiotherapy. The use of intraoperative skin protection devices and extraction bags can reduce the risk of this complication.
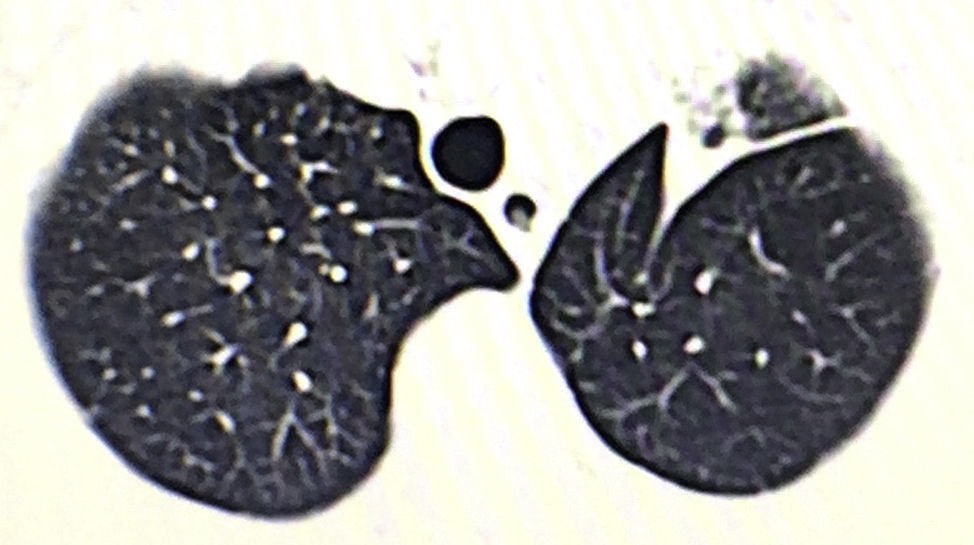
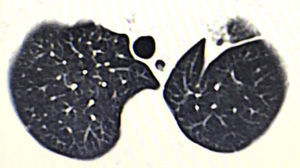
We present the case of a 76-year-old male patient with a lung tumor in the left upper lobe (Fig. 1). Video-assisted thoracoscopy was carried out with 2 ports measuring 15mm and 45mm in the eighth and fifth intercostal spaces along the anterior axillary line, respectively. Culmenectomy was performed with lobe-specific lymph node dissection of lymph node stations 5, 7, 10 and 11. The pathology study reported an umbilicated lesion measuring 2×2cm that was identified as a mucin-producing pulmonary adenocarcinoma with a predominantly acinar pattern (90%), and lymph nodes with mixed hyperplasia (T1a, N0, M0; stage IA). After 3 days of hospitalization, the patient was discharged with no postoperative complications and lung re-expansion of 100%.
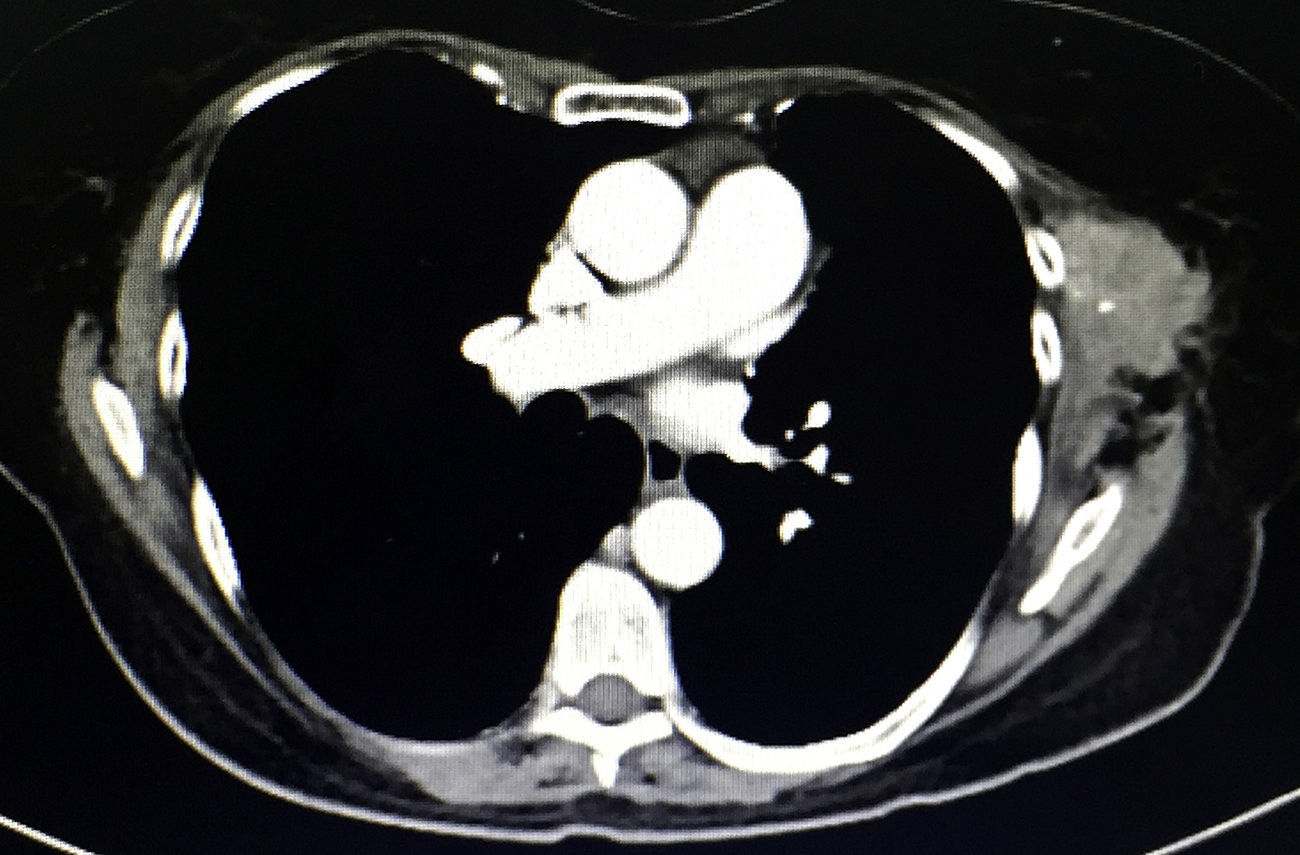
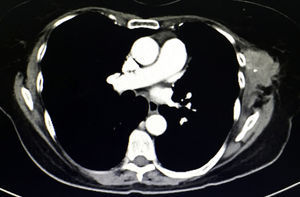
During physical examination 18 months after surgery, a palpable mass measuring 6×4cm was detected under the previous surgical port-site scar, adhered to adjacent tissue, fixed and painful, which the patient stated had increased in size in recent weeks. The CT scan (Fig. 2) showed a heterogeneous multilobulated soft tissue tumor in the previous surgical wound.
A 6×4cm lesion was completely resected with disease-free margins. The mass infiltrated the subcutaneous cellular tissue and the muscular plane, extending to the intercostal muscles. The pathology study identified the tumor as a mucin-producing adenocarcinoma in striated muscle; immunohistochemistry TTF1+, CK7+ and CK20+. After the withdrawal of a subcutaneous drain on the second day, the patient was discharged without complications on the third day of surgery.
Downey et al.4 surveyed 55 surgeons to determine tumor seeding after video-assisted thoracoscopy. The survey was completed by 48 participants, reporting 21 cases. The interval from surgery to cutaneous recurrence ranged from 14 days to 29 months (mean: 7.6 months). The incision was the most frequent recurrence site (14), including 6 adenocarcinomas, 3 squamous cell and 5 mesotheliomas. The study does not report the total number of patients, so the incidence cannot be determined. With regard to the techniques for preventing the contact of the removed tumor tissue with the skin, ports or collection bags were used in 7 patients, not used in 9, and there was no report of their use in 5.
The Parekh et al. study,5 which included 410 patients who underwent video-assisted thoracoscopy for malignancy with follow-up in 91% of the cases (mean 25 months), only reported one case of tumor seeding (0.26%). They did not specify whether the surgical specimens were removed with a collection bag or not. Jancovici et al.6 reported 2 port recurrences out of 148 procedures (1.4%). One year later, Swanson et al.7 reported 475 thoracoscopies without surgical port-site recurrences. In our experience, this is the first case of tumor seeding by direct implantation in lung resections using video-assisted thoracoscopy. The use of collector bags to withdraw the surgical specimen is standardized in our practice; however, the use of intraoperative skin protectors is not a habit. This factor should be taken into account, since the moment of implantation in a surgical wound directly from a neoplasm not only occurs when removing the specimen, but also during its manipulation and when interchanging instruments through the ports, as in our case.
Collard and Reymond8 propose preventive measures: extraction using a protective device, extension of the extraction incision, irrigation of the cavity and aspiration of the lavage liquid at the end of the procedure, use of smooth instruments instead of forceps (non-contact dissection), etc. Many issues are still being debated, such as the negativity of the surgical margins of the specimen, tumor staging at the time of resection and the frequency of implants compared to open surgery.
The collected literature needs comparisons with more recent studies, but such studies are very scarce.
In conclusion, video-assisted thoracoscopy is the procedure of choice for early-stage lung cancer and metastatic tumors. However, its advantages, disadvantages and possible complications must be taken into account, including the development of tumor seeding in the surgical site. The use of incision protectors and extractor bags is recommended, although there is no universal consensus. More numerous series with longer follow-ups are necessary to standardize the approach.
Please cite this article as: Mier JM, Víctor-Valdivia Z, Ríos S. Siembra tumoral en herida quirúrgica tras culmenectomía pulmonar izquierda videotoracoscópica. Cir Esp. 2017;95:620–621.