Pseudomyxoma peritonei (PMP) is a rare clinical condition characterized by the presence of extracellular mucin in the abdominal cavity. Histologically, it is classified as adenomucinosis, hybrid adenomucinosis or mucinous adenocarcinoma.1,2
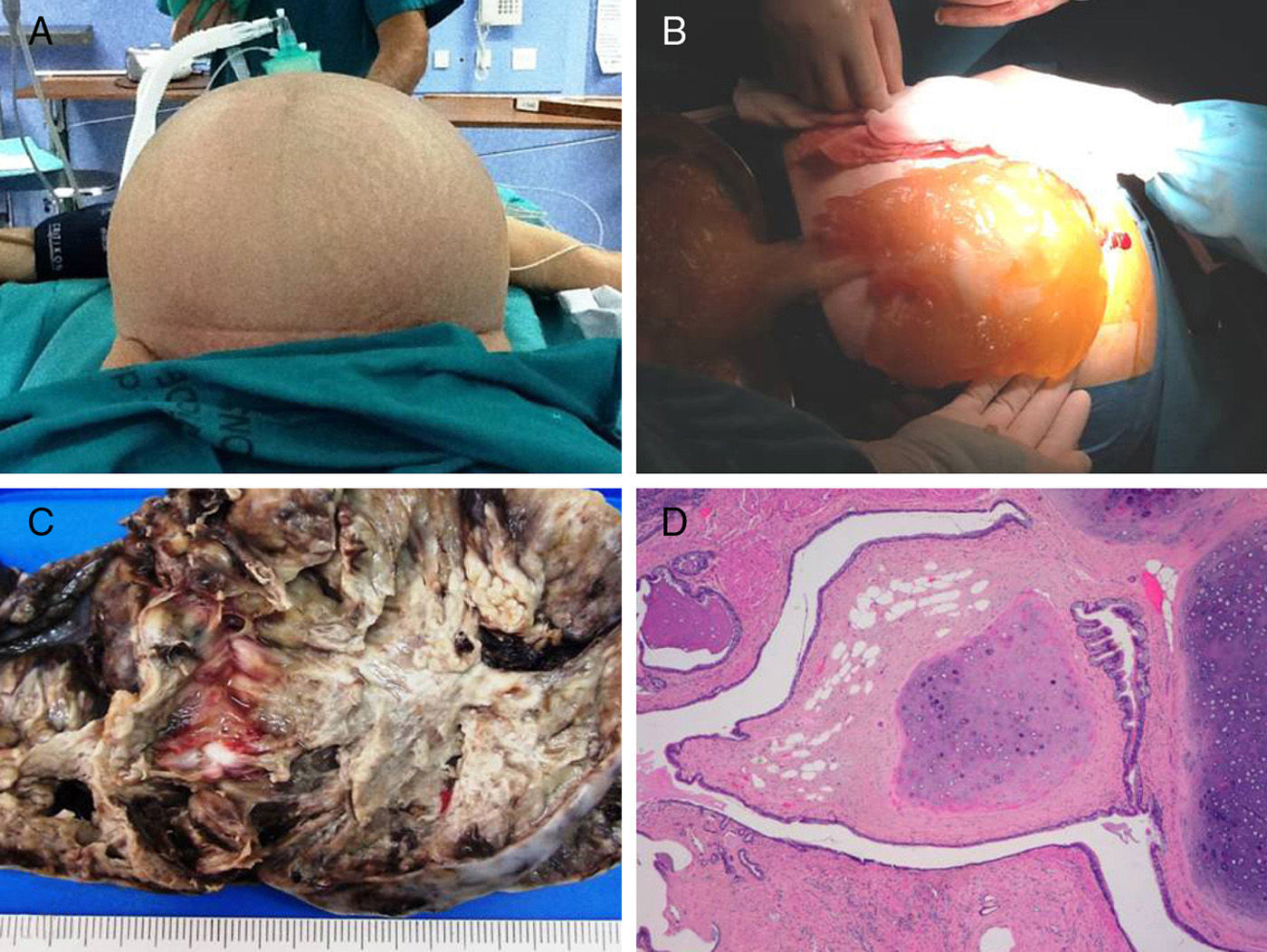
We present the case of a 76-year-old woman with a 3-month history of pain and abdominal distension (Fig. 1A) accompanied by anorexia. Upon examination, we observed an extremely distended abdomen that was painful and tender, which made it impossible for the patient to remain in any position. Abdominal ultrasound reported a large mass (probably mucinous) occupying the entire abdominal cavity, whose origin was not be to identify. Abdominal-pelvic CT scan showed evidence of a large mass that was compressing the abdominal organs. The presumed diagnosis was PMP originating in the appendix or mucinous tumour of the left ovary. We removed 15kg of thick mucinous material (Fig. 1B). The procedure also involved: bilateral adnexectomy; total omentectomy; right parietal, left parietal and pelvic peritonectomies; appendectomy; and resection of several implants. After complete cytoreductive surgery, HIPEC was done following the coliseum technique (Sugarbaker).
Postoperative progress was favourable, and the patient was discharged on the 12th day. At the last follow-up, which was 20 months after surgery, the patient was disease-free and had normal tumour marker levels.
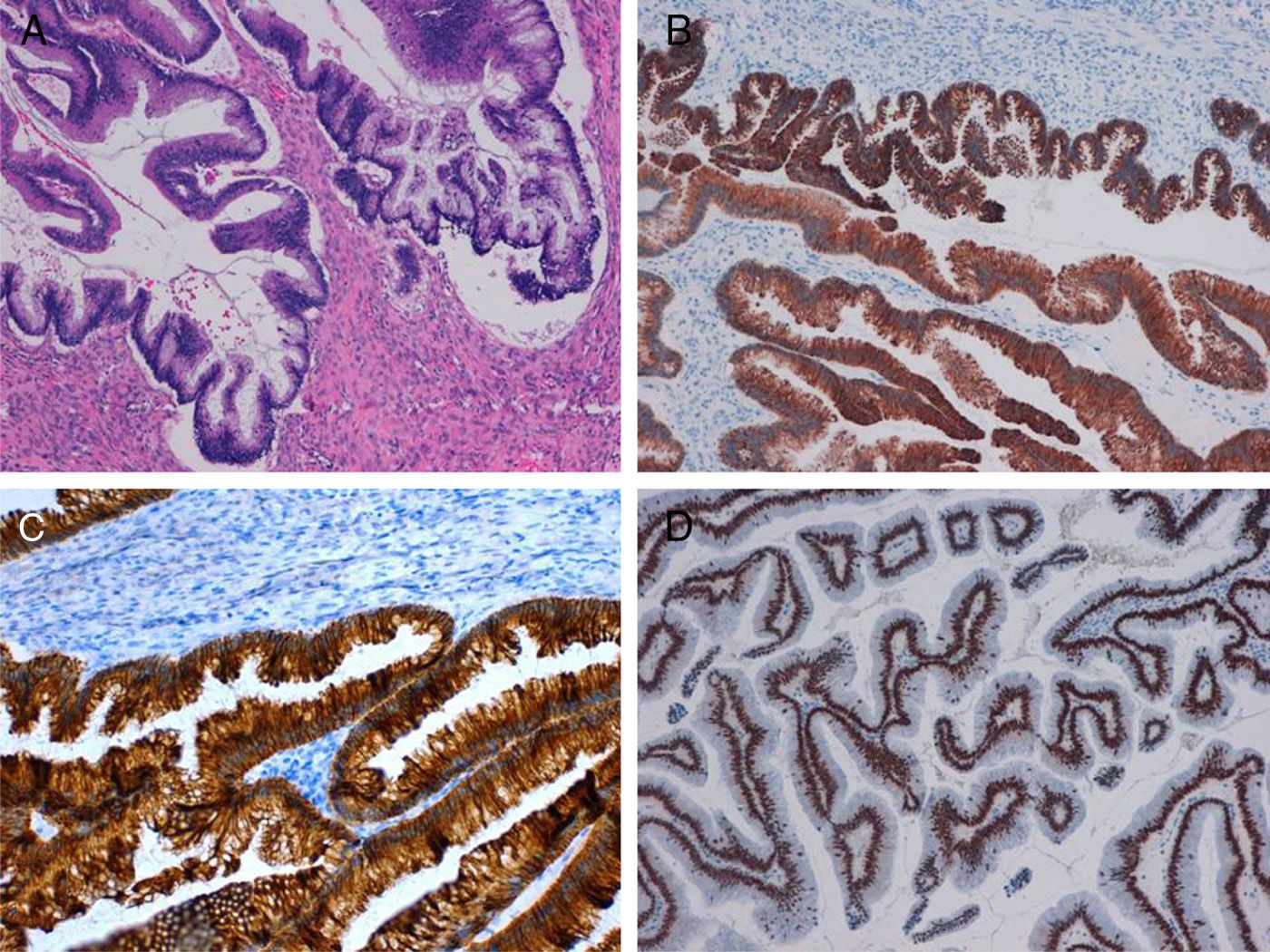
The macroscopic study demonstrated a multilocular, mucinous tumour of the left ovary that measured 18×10cm, with capsular rupture (Fig. 1C), while the cecal appendix was normal. The microscopic study of that ovary confirmed the presence of a low-grade malignant mucinous neoplasm (Fig. 2A) developed in digestive-type tissue derived from the endodermal germ layer of a mature cystic teratoma of the ovary, with positive immunoexpression for CK20, CDX2 and CK7 (Fig. 2B–D) and negative for CA125.
The mucinous neoplasm adopted an expansive neoplastic growth, without destruction of the stroma, associated with paucicellular parietal and extramural mucus. The rest of the teratoma presented mature tissue elements also derived from the endodermal germ layer (respiratory epithelium and tracheobronchial mucus glands) and from the mesodermal layer (cartilage, bone, fatty tissue and vascular structures) (Fig. 1D). The contralateral ovary contained an incidental mature cystic teratoma measuring 2cm, with mature tissue from 2 germ layers (endodermal and mesodermal) but no detected tissue derived from the ectodermal germ layer. The histology study of the peritoneum, omentum and implants confirmed the existence of large quantities of paucicellular mucin with few mucinous epithelial cells and no atypia, which correlated with disseminated peritoneal adenomucinosis (DPAM).
From a histological and immunohistochemical standpoint, it is not easy to determine whether an ovarian mucinous tumour derives from the epithelium of the ovarian surface, is secondary to a mucinous tumour of the lower gastrointestinal tract, or whether it is a tumour with teratomatous histogenesis.3,4 The existence of epithelial elements of the gastrointestinal tract in the teratomatous component and other tissues from different germ layers adjoining with the mucinous tumour, the lack of a previous or synchronous lower gastrointestinal tumour with a normal appendix, as well as immunoexpression positive for CDX2, CK20 and co-expression for CK7 support the diagnosis of a primary ovarian mucinous tumour associated with a mature cystic teratoma.4,5 The immunophenotype of the ovarian mucinous tumour showed similarities with mucinous tumours of the lower gastrointestinal tract, fundamentally with low-grade appendicular tumours that are usually associated with PMS, as in this case.3,4,6–8
Although the association of low-grade appendicular mucinous tumours/mucoceles with PMP is accepted,6–8 the true origin of PMP in women who have an appendicular tumour and ovarian tumour simultaneously has been a subject of controversy.3–5 Thus, advances made in molecular genetics and immunohistochemistry techniques have provided convincing evidence that the origin of PMP is the appendicular mucinous tumour, and that the ovarian tumour would be a metastasis of the latter.3–5,9 In our case, the appendix was normal, which favoured the theory of a primary ovarian mucinous tumour as the origin of the PMP.
Mucinous tumours are present in 2%–11% of mature ovarian cystic teratomas, and 3%–8% of mucinous ovarian tumours are associated with teratomas.4 Primary ovarian mucinous tumours are usually CK7+ and CK20−, mucinous tumours derived from the lower gastrointestinal tract are CK20+ and CK7−, although some tumours of the right colon and appendix may also express CK7. Mucinous tumours that develop in teratomas present variable immunostaining, with frequent positivity for both cytokeratins, CK20 and CK7, in a diffuse or focal staining pattern.4,5,8
We conclude that the term PMP should be reserved exclusively to define the clinical situation characterized by the massive presence of mucinous material in the peritoneal cavity, regardless of its tumour origin. According to the Ronnet classification, there are 3 histologic types: diffuse peritoneal adenomucinosis, disseminated peritoneal mucinous carcinoma, and intermediate or hybrid forms. Several studies and consensus advocate the use of complete cytoreductive surgery combined with perioperative intraperitoneal chemotherapy as the treatment of choice for this type of patients.10 We therefore recommend that these patients should be referred to one of the peritoneal oncology reference centres in our country.
Please cite this article as: Motos Micó J, Velasco Albendea FJ, Ferrer Márquez M, Medina Estévez E, Torres Melero J. Tumour mucinoso desarrollado en un teratoma ovárico maduro: presentación inusual de pseudomixoma peritoneal. Cir Esp. 2015;93:e69–e71.