To determine the usefulness of mortality risk scores for the endovascular treatment of ruptured abdominal aortic aneurysms.
MethodsRetrospective study of 61 patients undergoing endovascular repair between 2009 and 2014. Preoperative variables and in-hospital mortality were collected. The Hardman, GAS, Vancouver and ERAS scales were calculated.
ResultsIn-hospital mortality was 45.9%. The univariate predictors obtained were age, male sex, hypertension, smoking, chronic obstructive pulmonary disease, systolic blood pressure <90mmHg, heart rate, and loss of consciousness. After completing the multivariate analysis, significant variables were age (P=.021), systolic blood pressure <90mmHg (P=.004) and heart rate (P=.050). The GAS (76.79±9.88 vs 90.43±14.76, P=.001), Vancouver (4.41±0.62 vs 4.83±0.55, P=.007), and ERAS scales (0.06±0.24 vs 0.86±0.76, P=.001) were statistically different between the groups. The scale resulting from the following formula: 0.083+0.158 (if age>80 years)+0.701 (if systolic blood pressure<80mmHg)+0.598 (if heart rate<70 beats/min); obtained an area under the curve of 0.95.
ConclusionsAge, systolic pressure and heart rate, are predictors of hospital mortality of patients treated with endovascular repair of ruptured abdominal aortic aneurysms. Applying the scale proposed in this study, in combination with GAS, Vancouver, and ERAS scales, allows the detection of patients who would not benefit from endovascular treatment.
Conocer la utilidad de las escalas de riesgo de mortalidad para el tratamiento endovascular de los pacientes con aneurisma de aorta abdominal roto. Diseñar una escala de riesgo específica.
MétodosEstudio retrospectivo de 61 pacientes intervenidos mediante reparación endovascular de aneurisma de aorta abdominal roto entre 2009 y 2014. Se recogieron variables preoperatorias y de mortalidad intrahospitalaria, así como las escalas Hardman, GAS, Vancouver y ERAS.
ResultadosLa mortalidad intrahospitalaria fue del 45,9%. El estudio univariante obtuvo como factores pronósticos la edad, el sexo varón, la hipertensión arterial, el hábito tabáquico, la enfermedad pulmonar obstructiva crónica, la tensión arterial sistólica<90mmHg, la frecuencia cardiaca y la pérdida de conciencia. Tras la realización del análisis multivariante, la variables significativas fueron la edad (p=0,021), la presión arterial sistólica (p=0,004) y la frecuencia cardiaca (p=0,050). Las escalas GAS (76,79±9,88 vs. 90,43±14,76; p=0,001), Vancouver (4,41±0,62 vs. 4,83±0,55; p=0,007) y ERAS (0,06±0,24 vs. 0,86±0,76; p=0,001) resultaron estadísticamente diferentes en los pacientes fallecidos. La escala resultante de la siguiente fórmula: 0,083+0,158 (si edad >80 años)+0,701 (si tensión arterial<80mmHg)+0,598 (si frecuencia cardiaca<70 lat/min) obtuvo un área bajo la curva de 0,95.
ConclusionesEdad, presión sistólica y frecuencia cardiaca constituyen factores predictores de mortalidad intrahospitalaria de los pacientes con aneurisma de aorta abdominal roto tratados mediante exclusión endovascular. La aplicación de la escala propuesta en el presente estudio, en combinación con las escalas GAS, Vancouver y ERAS, permite conocer los pacientes que no se beneficiarían de tratamiento endovascular.
A ruptured aorta is a fatal event. The percentage of patients who make it to the hospital alive ranges between 40% and 70%.1,2 In spite of advances made in critical care, the treatment of ruptured abdominal aortic aneurysms (AAA) continues to present elevated mortality rates. Meanwhile, the mortality rate of open surgical repair for ruptured AAA ranges from 25% to 50%, depending on the series.3,4
In recent decades, interest in minimally invasive surgery has led to the development of endovascular aneurysm repair (EVAR). This is an attractive therapeutic option in patients with elevated comorbidity, including patients with ruptured AAA.5,6 Endovascular procedures offer several potential advantages over open surgery, as they are less invasive, eliminate the risk of injury to periaortic or abdominal structures, diminish the bleeding of surgical dissection and minimize hypothermia. Thirty-day mortality rates range between 10% and 45%, although most series still possess a relatively low number of patients compared to reports in the literature about open surgery applications.7
Several studies have tried to establish tools to identify patients that could potentially benefit from surgical treatment and those with no possibilities for survival (Hardman, Vancouver, Glasgow Aneurysm Score [GAS], Edinburgh Ruptured Aneurysm Score [ERAS], Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity [POSSUM], Vascular Study Group of New England [VSGNE], etc.). These preoperative risk scales were initially designed and validated to try to identify patients at high risk for open surgery, and some authors have tried to apply them to EVAR.
The objective of our study was to analyze preoperative risk factors for mortality in patients undergoing endovascular treatment for ruptured AAA and to apply the risk scales currently available for open surgery, while trying to develop a preoperative predictive scale for mortality. In this manner, we intend to obtain information that will enable us to identify patients with elevated risk for mortality who would not benefit from endovascular treatment.
MethodsOurs is a retrospective observational study of patients treated in the Angiology and Vascular Surgery Unit at the Hospital Clínico Universitario in Valladolid, Spain, from January 2009 until December 2014. Included in the study were those patients with ruptured AAA diagnosed by CT angiography using i.v. contrast and treated with EVAR. Excluded from the study were those patients who presented refractory shock to vasoactive drugs or cardiorespiratory arrest with no response to advanced cardiopulmonary resuscitation maneuvers. The study included demographic variables, comorbidity, lab analyses and vital signs upon arrival to the Emergency Room (Table 1).
Demographics, Comorbidities, Baseline Vitals and Preoperative Analytical Values of the Sample Studied.
| Mean | Range | |
|---|---|---|
| Age | 73.5 | 49–88 |
| n | % | |
|---|---|---|
| Sex | ||
| Males | 53 | 86.9 |
| Females | 8 | 13.1 |
| Arterial hypertension | 39 | 63.9 |
| Tobacco habit | 34 | 55.7 |
| Cardiac comorbidity | 20 | 32.8 |
| Hypercholesterolemia | 16 | 26.2 |
| Chronic obstructive pulmonary disease | 12 | 20.3 |
| Arrhythmia | 10 | 16.4 |
| Diabetes mellitus | 10 | 16.4 |
| Chronic renal failure | 8 | 13.1 |
| Peripheral arterial disease | 4 | 6.6 |
| Cerebrovascular comorbidity | 2 | 3.3 |
| Consciousness | 57 | 93.4 |
| Heart failure | 23 | 37.1 |
| ST depression | 2 | 3.3 |
| Mean | SD | |
|---|---|---|
| Systolic arterial pressure (mmHg) | 107.1 | 45.3 |
| Heart rate (bpm) | 79.9 | 21.3 |
| Hemoglobin (g/dL) | 11.6 | 2.5 |
| Creatinine (mg/dL) | 1.4 | 0.6 |
Cardiac comorbidity: previous history of myocardial infarction, cardiac surgery, angina or arrhythmia; cerebrovascular comorbidity: previous history of cerebrovascular accident or transitory ischemia attack.
Values are shown as percentages and mean (standard deviation, SD).
Patients were treated by open surgery if they did not meet the following anatomic requirements for EVAR obtained during CT angiography: proximal aortic neck diameter >17mm and <32mm, length >10mm, with an angle between the suprarenal and juxtarenal aorta <60°, angle between the juxtarenal aorta and aneurysm sac <60–90°, circumferential thrombus <50% and circumferential calcification <50%; aortic bifurcation diameter >18mm if bifurcated stent (if <18mm, aortouniiliac stent and femorofemoral bypass); iliac arteries diameter >7mm, with distal neck diameter <22mm, distal neck length >15mm, angle between the AAA and iliac artery <60° and with non-circumferential calcification.8
During the study period, 93 patients with a diagnosis of ruptured AAA were admitted to hospital. Two died in the Emergency Room. CT-angiography studies were performed in 91 patients, 61 of whom met the anatomic requirements for endovascular treatment (67%). The main reason for exclusion was problems in the proximal neck length or diameter. Out of the 30 patients who would not have benefitted from endovascular repair, 21 were treated with open surgery, 4 died before the intervention and 5 were treated conservatively. The stents used were Talent® and Endurant® (Medtronic, Santa Rosa, CA, USA).
The risk scales included in the study are explained in detail below. Other scales, such as POSSUM, were not included as they contain surgery-related variables.
GASThe GAS scale score was calculated with the following formula: age (yrs)+7 for cardiac comorbidity (defined as a previous history of myocardial infarction, cardiac surgery, angina or arrhythmia)+10 for cerebrovascular comorbidity (defined as a previous history of cerebrovascular accident or transitory ischemic accident)+17 for shock (defined as systolic pressure <80mmHg)+14 for renal failure (defined as preoperative serum creatinine >160μmol/L)+7 for open surgery.
Vancouver ScaleThe Vancouver Scale was calculated with the formula: age (yrs)×0.062+loss of consciousness (yes=1/no=−1)×1.14+heart failure (yes=1/no=−1)×0.6.
ERASThe formula applied for ERAS was: +1 for the Glasgow scale assessment in hospital <15, +1 for in-hospital systolic pressure <90mmHg, +1 for a preoperative hemoglobin level <5.6mmol/L. A score of 0–1 has a predicted risk of death for open surgery of 30%, a score of 2 has a 50% risk, and a score of 3 has a risk of 80%.
Hardman IndexThe Hardman index was calculated with the following formula: +1 for age >76, +1 for loss of consciousness during hospitalization, +1 for preoperative serum creatinine >190μmol/L, +1 for hemoglobin level <5.6mmol/L, +1 for electrocardiographic signs of ischemia (defined as a ST segment depression of more than one millimeter or an associated change in the T wave determined by a cardiologist). An index of 3 or more has a predicted risk of death of 100% for open surgery.
VSGNEThe formula of the Vascular Study Group of New England (VSGNE) scale is: age >76 years (2points)+heart failure (2 points)+loss of consciousness (1 point)+suprarenal impingement (1 point) (Robinson).
Statistical AnalysisThe statistical analysis was conducted with SPSS 20.0 software. Means and intervals were calculated for the continuous variables. For the qualitative variables, a univariate analysis was done using the chi-squared and Fisher's exact test; for the quantitative variables, Student's t test was used. The multivariate analyses were performed by means of a logistic regression study, in addition to a resampling study with bootstrapping as a validation technique. In order to define cut-points for the scores from the scales that were significant after the results of the multivariate study, receiver operating characteristic (ROC) curves were used. A P<.05 was considered statistically significant.
ResultsThe mean age of the patients analyzed was 73.5 (range 49–88), and the majority were men (86.9%). The main comorbidities presented at admittance were arterial hypertension (63.9%) and tobacco habit (55.7%); other comorbidities are shown in Table 1. Most patients were conscious upon admittance (93.4%), with hemodynamic and analytic values within normal ranges (Table 1). A total of 33 patients survived and 28 died during hospitalization (mortality rate 45.9%).
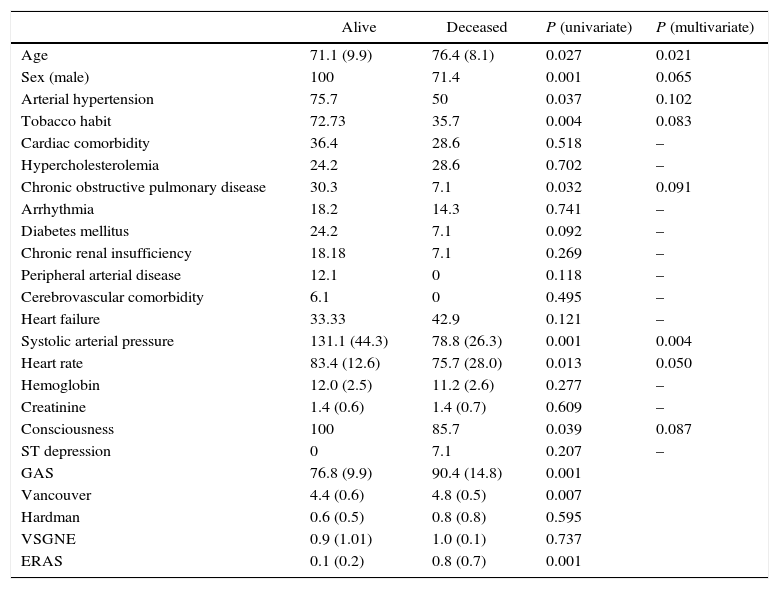
We studied the differences between patients who survived and those who had deceased. Age, male sex, arterial hypertension, tobacco habit, chronic obstructive pulmonary disease, systolic arterial tension, heart rate and consciousness presented statistically significant differences in the univariate study (Table 1). After the multivariate analysis, significant results were observed for age (P=.021), systolic blood pressure (P=.004) and heart rate (P=.050) (Table 2).
Univariate and Multivariate Studies of Preoperative Variables.
| Alive | Deceased | P (univariate) | P (multivariate) | |
|---|---|---|---|---|
| Age | 71.1 (9.9) | 76.4 (8.1) | 0.027 | 0.021 |
| Sex (male) | 100 | 71.4 | 0.001 | 0.065 |
| Arterial hypertension | 75.7 | 50 | 0.037 | 0.102 |
| Tobacco habit | 72.73 | 35.7 | 0.004 | 0.083 |
| Cardiac comorbidity | 36.4 | 28.6 | 0.518 | – |
| Hypercholesterolemia | 24.2 | 28.6 | 0.702 | – |
| Chronic obstructive pulmonary disease | 30.3 | 7.1 | 0.032 | 0.091 |
| Arrhythmia | 18.2 | 14.3 | 0.741 | – |
| Diabetes mellitus | 24.2 | 7.1 | 0.092 | – |
| Chronic renal insufficiency | 18.18 | 7.1 | 0.269 | – |
| Peripheral arterial disease | 12.1 | 0 | 0.118 | – |
| Cerebrovascular comorbidity | 6.1 | 0 | 0.495 | – |
| Heart failure | 33.33 | 42.9 | 0.121 | – |
| Systolic arterial pressure | 131.1 (44.3) | 78.8 (26.3) | 0.001 | 0.004 |
| Heart rate | 83.4 (12.6) | 75.7 (28.0) | 0.013 | 0.050 |
| Hemoglobin | 12.0 (2.5) | 11.2 (2.6) | 0.277 | – |
| Creatinine | 1.4 (0.6) | 1.4 (0.7) | 0.609 | – |
| Consciousness | 100 | 85.7 | 0.039 | 0.087 |
| ST depression | 0 | 7.1 | 0.207 | – |
| GAS | 76.8 (9.9) | 90.4 (14.8) | 0.001 | |
| Vancouver | 4.4 (0.6) | 4.8 (0.5) | 0.007 | |
| Hardman | 0.6 (0.5) | 0.8 (0.8) | 0.595 | |
| VSGNE | 0.9 (1.01) | 1.0 (0.1) | 0.737 | |
| ERAS | 0.1 (0.2) | 0.8 (0.7) | 0.001 |
Values are shown as mean (standard deviation, SD) and percentages.
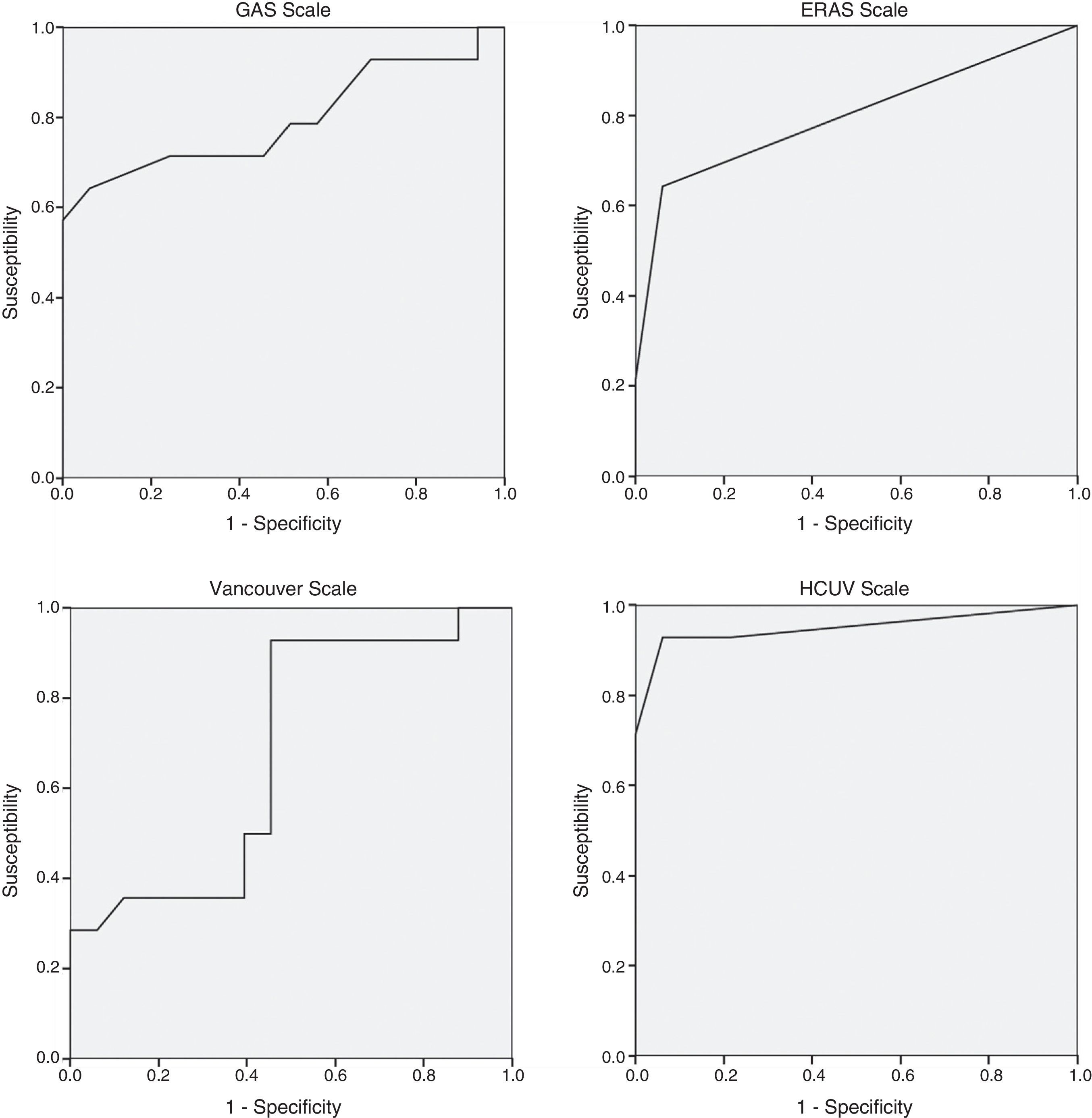
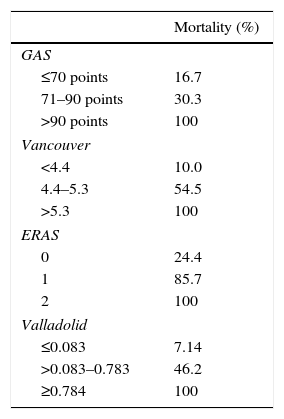
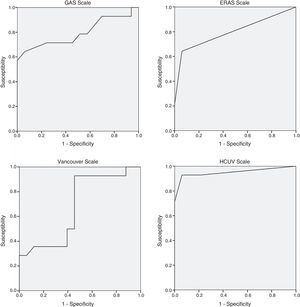
Table 3 shows the mortality rates according to the scales available in the literature for open surgery. The GAS, Vancouver and ERAS scales were statistically different in the deceased patients. A GAS scale score >90 points, Vancouver scale score >5.3 and ERAS score of 2 were associated with predicted mortality rates of 100% (Table 3).
The regression study offered the following formula for mortality: 0.083+0.158 (if age is >80)+0.701 (if systolic arterial tension is <80mmHg)+0.598 (if heart rate is <70bpm) with an R2 value=0.69. The significance of the coefficients after the resampling with bootstrapping was: age >80 (P=.039; 95%CI: 0.020–0.309), systolic arterial tension <80mmHg (P=.001; 95%CI: 0.428–0.875) and heart rate <70bpm (P=.003; 95%CI: 0.389–0.795). The area under the curve of the present model reached 0.950, which is superior to other scales studied: GAS 0.794, Vancouver 0.680, ERAS 0.798 (Fig. 1). A score of ±0.784 presented a mortality rate of 100%.
DiscussionIn spite of the advances made in medical care in recent decades, ruptured AAA are still an important threat to patients’ lives, with an overall mortality rate of 90% and hospital mortality rates of 30%–50%. The introduction of EVAR in the treatment of AAA has resulted in a reduction in perioperative mortality in elective surgery, and the implementation of this technique has therefore grown enormously.9
In different hospitals, between 28% and 79% (mean 49.1%) of ruptured AAA cases are treated with endovascular procedures.3,10 Furthermore, the proportion of patients treated with EVAR has increased in recent years, specifically in high-risk, unstable and hypotensive patients, which is a patient group with a higher incidence of perioperative mortality.4
Current treatment choices for ruptured AAA are not clearly in favor of EVAR. In 2013, Antoniou et al.11 published a meta-analysis with almost 60000 patients, in which endovascular treatment was associated with lower hospital mortality rates, less respiratory complications and a lower incidence of acute renal failure. Nonetheless, these affirmations have not been confirmed by the Cochrane Review published in 2014 based on 3 randomized studies, which did not find differences in hospital mortality rates even after 30 days.12 As for one-year mortality, there was a slight, non-significant difference in favor of endovascular repair versus open surgery (38.6% vs 42.8%, respectively).13 Moreover, although endovascular treatment did not provide a clear benefit for one-year survival, it does provide faster hospital discharge and better quality of life, which are the hallmarks of a cost-effective treatment option.14
Karkos et al.15 and Conroy et al.16 published studies about the usefulness of the Hardman index for predicting 30-day mortality in patients with ruptured AAA treated with endovascular techniques. Conroy et al. found that only the loss of consciousness was an independent risk factor for mortality, while Karkos et al. found this only to be true of the local anesthesia variable. In both studies, the Hardman index predicted an elevated risk for mortality, but with a probability that was much lower than in patients treated with open surgery. In our study, the Hardman index could not be considered a predictor for postoperative mortality (P=.595), and the loss of consciousness reached differences in the univariate study that disappeared in the multivariate study. One conclusion of this study, which concurs with the Karkos et al. study from 2008, is the inability to use the Hardman index to exclude patients who would not benefit from endovascular treatment for ruptured AAA. In our setting, with the application of the Valladolid scale and the remaining scales available that reached significant differences (EVAR, GAS, Vancouver), we were able to identify this population.
The GAS scale was the subject of research in a Greek multicenter study published in 2014,17 which included a total of 113 patients treated with EVAR for ruptured AAA. The scale was not able to predict postoperative mortality as it presented an area under the curve of 0.64 (0.79 in our study). Although the scores of the GAS scale were similar in both populations (86.0±1.30 vs 83.1+14.04, respectively), the percentage of patients over the age of 80 was higher in the Antonopoulos et al. study than in ours (38.1% vs 24.6%). In the last year, another study published with a cohort of 27 patients also showed the lack of utility of the GAS scale.18
By applying the Valladolid scale, we would not have treated 20 patients, which represents 32.8% (those with a score ≥0.784, predicted mortality 100%). This number of patients is higher than those with a 100% mortality risk on other scales: GAS >90 points: 16 patients; Vancouver>5.3:8 patients; ERAS=2:6 patients. A total of 4 patients met the criteria for a predicted 100% mortality on all 4 scales.
The mortality rate of 45.9% observed in our study may seem elevated compared to some published series. Nonetheless, it must be mentioned that the only patients treated conservatively were those who decided on this therapeutic option (either themselves or their families) after having received medical information about the surgical risk.
Possible limitations of our study include the fact that it is a non-multicenter study with a limited sample size. The research has been focused on the study of preoperative parameters, so other scales (such as POSSUM19) have not been contemplated because they contain intraoperative variables,.
Ethical considerations involved in treating patients with ruptured AAA are difficult and delicate.20,21 In senior patients or those with severe comorbidities (dementia or advanced heart disease, severe chronic obstructive pulmonary disease, etc.), the open surgical option has traditionally been ruled out. As ruptured AAA is a fatal disease in 100% of cases treated non-surgically, risk scales have been developed to try to resolve which patients would not benefit from treatment. Endovascular treatment, however, is a less aggressive therapeutic option that can be considered in practically all patients, although it is not free of risks of complications or patient mortality. The development of prognostic scales and studies about risk factors that influence the evolution of these patients can help us confront this ethical and clinical dilemma.
In conclusion, the predictive factors found in this study about hospital mortality in patients with endovascular treatment of ruptured AAA are age, systolic blood pressure and heart rate. There are 3 prognostic scales described for the open surgical treatment of ruptured AAA that are also valid for endovascular treatment: the GAS, Vancouver and ERAS scales. The scale proposed in the present study is able to stratify preoperative risk for these patients in a more precise manner. The application of the mentioned scales is able to identify which patients would not benefit from endovascular surgical treatment and who should perhaps be treated with conservative management.
Conflict of InterestThe authors have no conflict of interests to declare.
Please cite this article as: San Norberto EM, Fuente R, García-Saiz I, Revilla A, Martín-Pedrosa M, Vaquero C. Nueva escala de predicción de mortalidad en los aneurismas de aorta abdominal rotos. Cir Esp. 2016;94:339–345.