Groove pancreatitis (PS) is an uncommon clinical situation and radiologically it can mimic carcinoma of the periampullary area. The aim of this paper is to study a series of 8 patients who underwent surgery with preoperative diagnosis of pancreatic head mass and subsequent pathological diagnosis of PS.
MethodsIn our series, 6 were men and 2 were women, with an average age of 51.9 years. Before surgery, all patients had epigastric abdominal pain requiring analgesia at high doses. The preoperative analytical CEA and Ca 19.9 were normal in all patients. Imaging studies showed intrapancreatic solid lesions in 6 of the 8 patients, and in the remaining 2 one papillary mass of 5 and 6cm, respectively, that caused stenosis in the duodenal luz. EUS neoplastic cells were negative in all patients.
ResultsThe immediate postoperative evolution was satisfactory, there are no complications. In our series, no patients have died. The long-term follow-up, in 7 of the 8 patients, has been excellent with disappearance of abdominal pain and improvement of nutritional status. The remaining patient had frequent recurrent episodes of acute pancreatitis, and at 60 months, presented a pseudocyst that has required a Roux-en-Y cystojejunostomy.
ConclusionsPS must be included in the differential diagnosis of pancreatic lesions, which may include carcinoma of the periampullary area and other causes of chronic pancreatitis.
La pancreatitis del surco (PS) es una entidad poco frecuente que clínica y radiológicamente puede simular un carcinoma del área periampular. El objetivo de este trabajo es presentar una serie de 8 pacientes que fueron intervenidos quirúrgicamente con el diagnóstico preoperatorio de masa en cabeza pancreática, con un diagnóstico anatomopatológico definitivo de PS.
MetodosEn nuestra serie, 6 eran hombres y 2 mujeres y tenían una edad media de 51,9 años. Previamente a la cirugía, todos los pacientes presentaban dolor abdominal en epigastrio que requería analgesia a altas dosis. En la analítica preoperatoria, el CEA y el Ca 19.9 fueron normales en todos los pacientes. Los estudios de imagen mostraron lesiones sólidas intrapancreáticas en 6 de los 8 pacientes, y en los 2 restantes una masa mamelonada de 5 y 6cm, respectivamente, que estenosaba la luz duodenal. La ecoendoscopia fue negativa para células neoplásicas en todos los pacientes.
ResultadosLa evolución durante el postoperatorio inmediato fue satisfactoria, sin complicaciones. En nuestra serie, no ha fallecido ningún paciente. La evolución a largo plazo, en 7 de los 8 pacientes, ha sido excelente, con desaparición del dolor abdominal y mejoría del estado nutricional. El caso restante ha tenido frecuentes episodios de pancreatitis aguda recidivante y, a los 60 meses, ha presentado un seudoquiste que ha precisado una quistoyeyunostomía en Y de Roux.
ConclusionesLa PS debe incluirse en el diagnóstico diferencial de lesiones pancreáticas, que pueden incluir carcinoma del área periampular y otras causas de pancreatitis crónica.
Groove pancreatitis (GP) is a type of chronic pancreatitis that is considered a pseudotumor lesion that is located in the area of the groove between the head of the pancreas, duodenum, and common bile duct. Although uncommon, clinically, and radiologically it can simulate periampullary carcinoma; therefore, the importance of a correct diagnosis.
In the literature, several terms have been used to describe GP. The first description is from 1970 in an article by Potet and Duclert,1 who published a study of 4 patients with what they called “cystic dystrophy of the pancreas”. In 1973, Becker2 used the term GP for the first time in a series of 117 patients. In 1982, Solte et al.3 incorporated the term “groove pancreatitis” to refer to a special form of chronic pancreatitis characterized by a fibrous layer located in the anatomic space situated between the head of the pancreas, duodenum, and main bile duct, described in a series of 30 patients. Afterwards, in 1991, Becker and Mischke4 defined a “pure” form, which only affected the area of the groove, and a “segmental” form in which, in addition to the scar tissue in the groove, there is involvement of the head of the pancreas in the dorsal–cranial portion and occasional stenosis of the pancreatic duct. In 2004, Adsay and Zambani5 called this pathology “paraduodenal pancreatitis”. Other terms used have been: duodenal wall cyst and hamartoma of the Brunner glands.
GP appears in the 4th–5th decades of life, and it is more frequent in men.6 The etiology is unknown, although it is usually associated with alcohol, smoking, peptic ulcer, previous gastric resections, etc.6–9 Clinically, it presents with recurring postprandial abdominal pain accompanied by nausea and vomiting when there is predominant duodenal involvement and, on rare occasions, with intermittent jaundice when there is also involvement of the intrapancreatic common bile duct.8–10 The radiological findings depend on the stage of the disease when found.11–15 In most cases, treatment involves surgery, as it is difficult to make a precise preoperative diagnosis of the disease because it may seem a neoplastic process.5,7,9,16
The objective of this study is to present a series of 8 patients who were treated surgically after preoperative diagnosis of a mass in the head of the pancreas, which was confirmed to be GP in the definitive pathology study. In addition, we review the literature about this anatomo-clinical entity.
MethodsFrom January 2003 to December 2014, 8 patients were treated at our hospital after a pathology diagnosis of GP. In our series, mean age at the time of the intervention was 51.9±8 years (range: 39–70). Out of the 8 patients, 6 were males (75%) and 2 were females (25%). Table 1 shows the patients’ symptoms and risk factors for developing the disease. As for alcohol use, 5 male patients had had moderate–severe alcoholism for several years. The 2 women in our series were not drinkers, but they were heavy smokers (more than 20 cigarettes/day). In addition, one presented a Roux-en-Y cystojejunostomy due to a pancreatic pseudocyst more than 10 years earlier.
Age, Sex, Clinical Data, and Risk Factors of our Series.
| Case | Age | Sex | Symptoms | Diabetes | Alcohol | Smoker |
|---|---|---|---|---|---|---|
| 1 | 39 | M | Diffuse pain, weight loss, and steatorrhea | No | Yes | Yes |
| 2 | 41 | F | Epigastric pain and steatorrhea | No | No | Yes |
| 3 | 50 | M | Epigastric pain, vomiting, and weight loss | No | No | Yes |
| 4 | 70 | M | Epigastric pain, asthenia | No | Yes | Yes |
| 5 | 40 | M | Epigastric pain, jaundice | No | Yes | Yes |
| 6 | 62 | M | Pain in right hypochondrium, weight loss | Yes | Yes | No |
| 7 | 55 | M | Epigastric pain vomiting | No | Yes | No |
| 8 | 58 | F | Epigastric pain, vomiting | No | No | Yes |
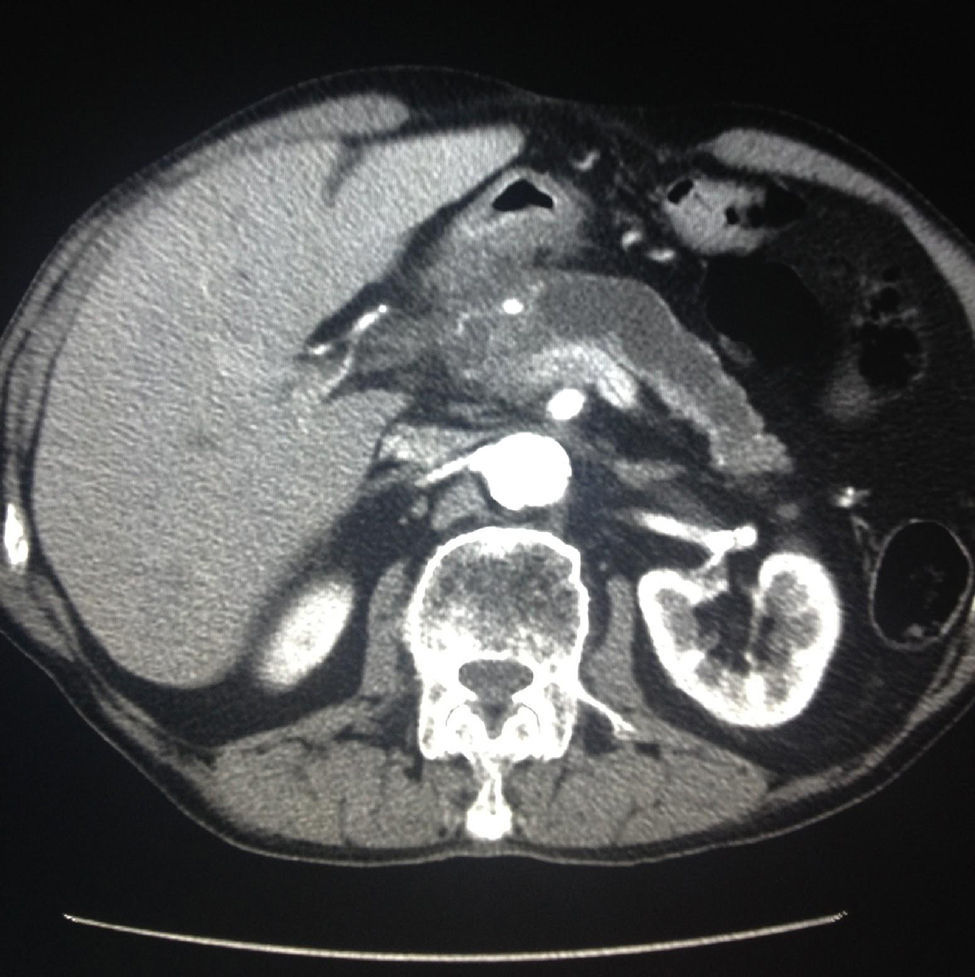
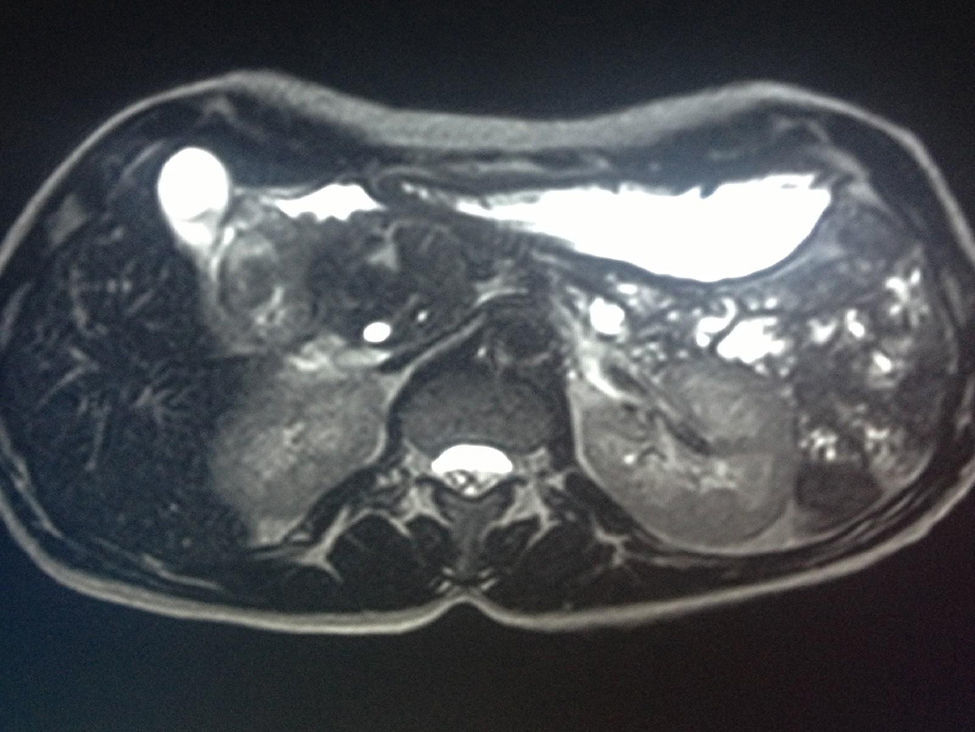
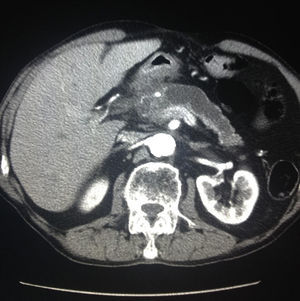
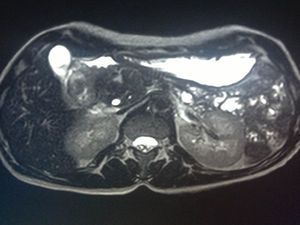
All patients had abdominal pain located in the epigastrium and required high doses of analgesia. Two of the 8 patients presented associated weight loss of more than 10kg in the previous month, one due to uncontrollable vomiting and the other due to anorexia. In only one patient the pain was accompanied by progressive jaundice, with direct bilirubin levels of 15mg/dL. Tumor markers, CEA and Ca 19.9, were normal in all patients. The radiological findings in our series (thoracoabdominal CT, MRI, endoscopic ultrasound, and PET/CT) are shown in Table 2 (Figs. 1–3). In our series, 4 patients underwent endoscopic ultrasound-guided fine needle aspiration: only one case presented a histology study that confirmed the diagnosis of GP. None of the patients presented neoplastic cells. PET/CT scans were done in 4 out of the 8 patients: in one case there was an observed metabolic increase in the head of the pancreas (SUV 4.5) and, in the remaining 3 cases, the uptake was in the 2nd portion of the duodenum (SUV 4, 5, and 5.5, respectively).
Data From Imaging Studies of Our Series.
| Case | CT | MRI | Endoscopic ultrasound | PET/CT |
|---|---|---|---|---|
| 1 | Cystic mass in head; dilated bile duct | Dilated Wirsung | No | No |
| 2 | Papilliform duodenal mass | Distal common bile duct narrowing; duodenal thickening | Yes | Increase in duodenal wall; SUV: 4 |
| 3 | Focal pancreatitis; calcifications | No | Yes | No |
| 4 | Multicystic mass in head of pancreas | Mass in the head; dilated Wirsung | No | No |
| 5 | Mass in head with duodenal infiltration; dilated bile duct | Dilated common bile duct | Yes | Increase in head; SUV: 4.5 |
| 6 | Multicystic mass infiltrating superior mesenteric artery | Dilated Wirsung; normal common bile duct | Yes | No |
| 7 | Duodenal mass; mild dilatation of Wirsung | No | No | Increase in duodenal wall; SUV: 5 |
| 8 | Duodenal mass; gastric dilatation | No | No | Increase in duodenum; SUV: 5.5 |
The surgical indication in 7 of the 8 patients was due to a pancreatic mass with suspected neoplasm. Only one patient (case 3) was diagnosed preoperatively with GP. In this patient, surgery was indicated because of abdominal pain that was not treatable at home, a weight loss of 20kg in one month and several hospitalizations. As for the surgical techniques, in 7 patients pancreaticoduodenectomy (PD) was conducted according to the Whipple technique. The patient who underwent total pancreaticoduodenectomy (case 8) presented a history of previous pancreatic surgery, specifically a cystojejunostomy 10 years earlier due to a pseudocyst in the tail of the pancreas and, in addition to the mass in the head, fibrotic nodules were observed in the body and tail of the pancreas during the second intervention, associated with atrophied pancreatic parenchyma.
ResultsPathologic CharacteristicsMacroscopic DescriptionAll cases presented pathological similarities. During dissection, an important amount of thickening of the duodenal wall was observed in multiple transversal cuts. The pancreatic tissue of the head showed cystic formations between 0.3 and 0.5cm, immersed in a stroma that was whitish and fibrous in appearance, with uneven edges and elastic consistency.
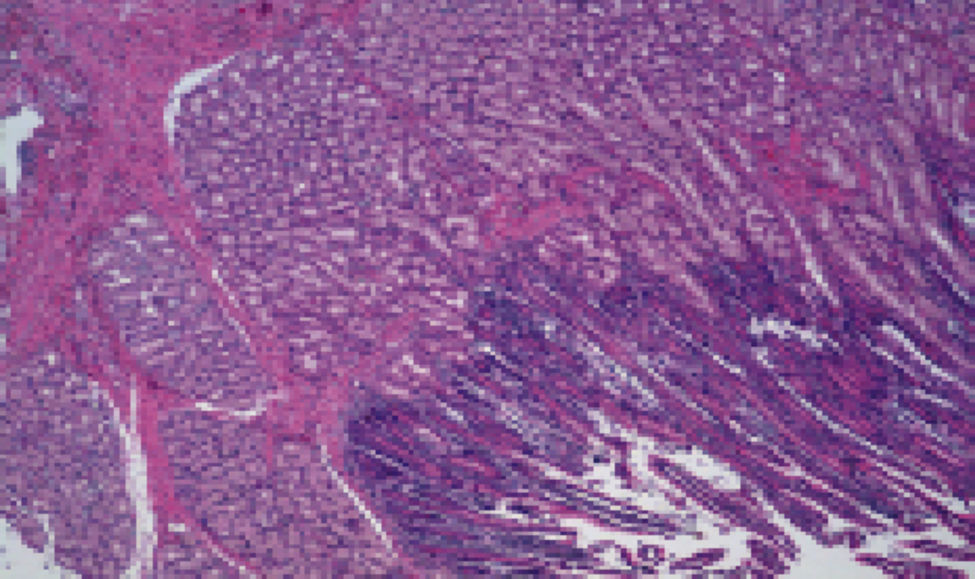
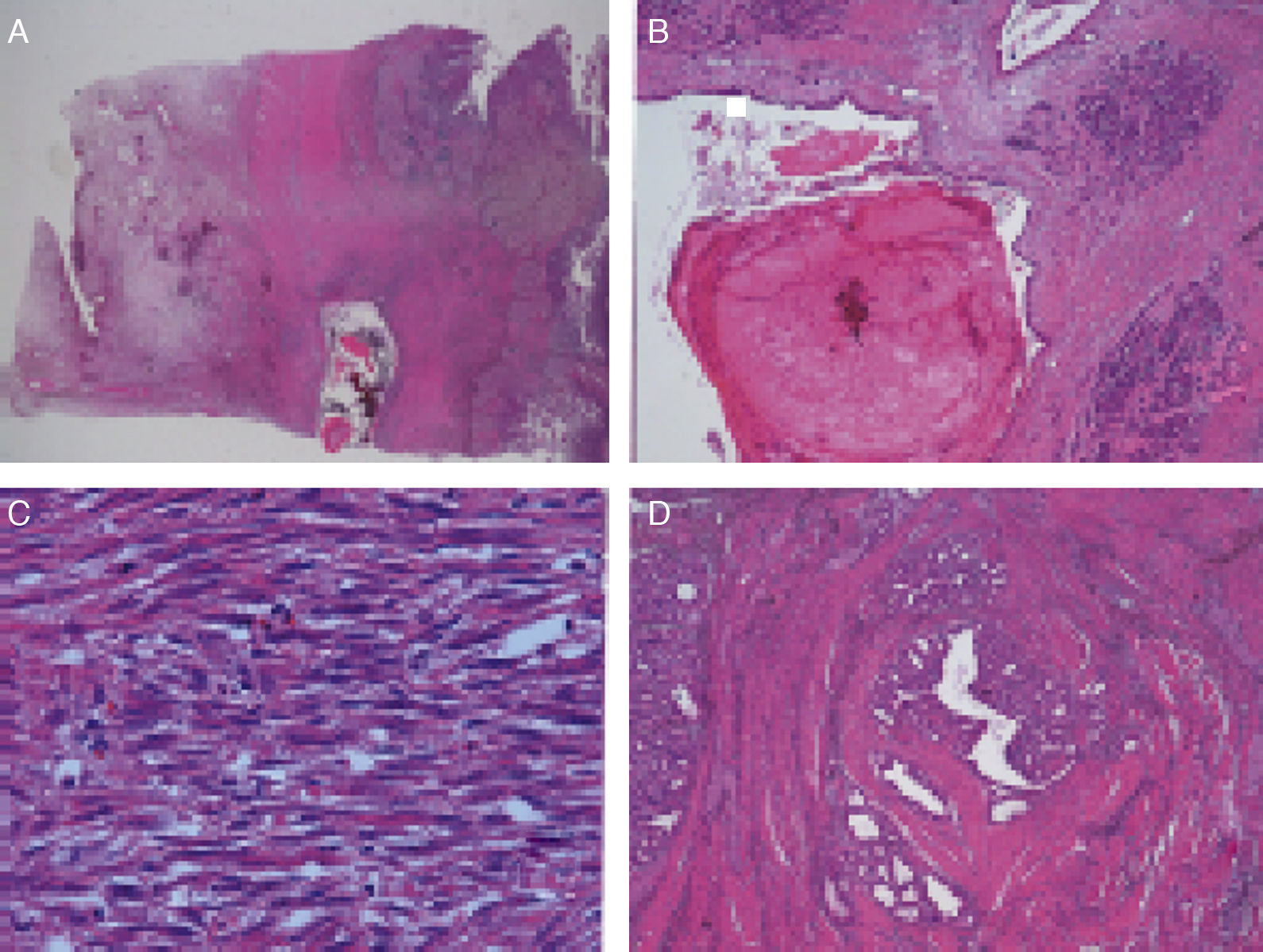
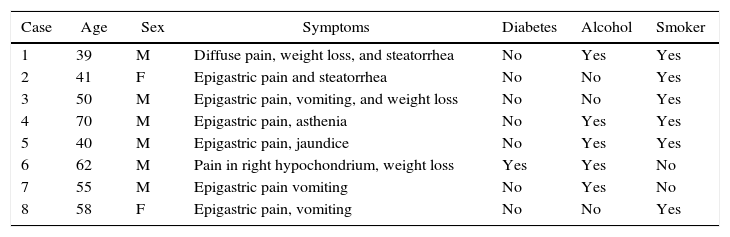
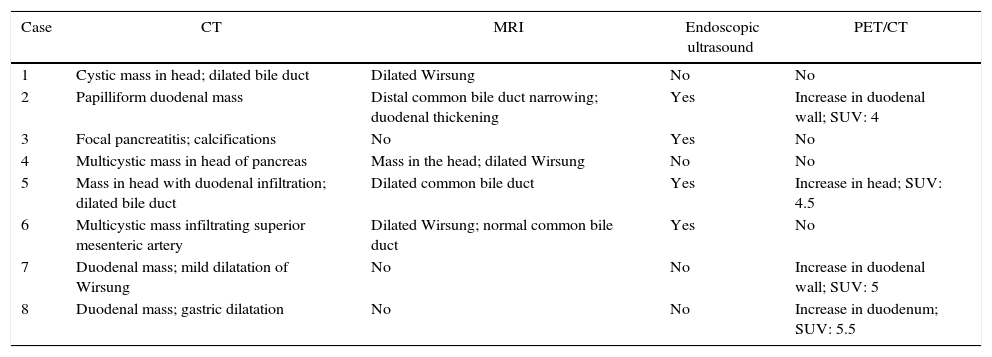
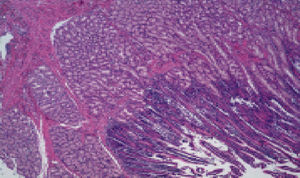
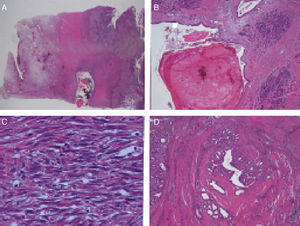
Microscopic DescriptionThe histology study showed thickening of the duodenal submucosa, mainly at the expense of severe hyperplasia of the Brunner glands. In all patients, extensive areas of fibrosis were identified, characterized by a proliferation of spindle cells, with no atypia and disorganized in irregular sheaves, with positivity for smooth muscle actin. In the middle of this myofibroblastic proliferation, there were multiple cystic formations as well as dilated pancreatic ducts, with no epithelial layer and very dense eosinophilic secretion in the lumen (with the appearance of pancreatic cell islets with chronic inflammation) (Figs. 4 and 5A–D).
(A) Cyst with thick secretions situated in the muscle plane beneath a very thick mucosa with prominent Brunner glands; (B) dilated duct with secretions in the atrophic pancreatic tissue; (C) active myofibroblastic proliferation with eosinophils and some mitoses; (D) heterotopic pancreatic tissue in the duodenal wall.
Patient progress during the immediate postoperative period was satisfactory, with no complications of interest. In our series, no there were no patient deaths.
Long-term Follow-upIn 7 of the 8 patients, the long-term evolution was excellent, with disappearance of the abdominal pain and a spectacular improvement of the patients’ nutritional state. Nonetheless, case 2 has continued to have frequent crises of abdominal pain in the area of the mesogastrium, associated with abdominal distension, which has caused moderate malnutrition. Furthermore, 60 months after PD, in the radiological studies of the abdomen, a 10-cm pseudocyst was detected in the tail of the pancreas that, after 6 months, required another surgical intervention to perform a Roux-en-Y cystojejunostomy.
DiscussionThe importance of GP lies in its diagnostic difficulty and, therefore, determining which treatment is best. This is due to the anatomic crossroads where it lies, which is a space created by the head of the pancreas medially, the second duodenal portion laterally, the third portion of the duodenum and the inferior vena cava, and the upper portion outlined by the duodenal bulb. This virtual space is occupied by the intrapancreatic common bile duct, the pancreatic duct, the duct of Santorini and the papilla. Vascularization must also be considered (gastroduodenal and superior pancreaticoduodenal arteries), as well as the retroperitoneal and peripancreatic lymph nodes.10 Therefore, the main clinical implication of GP lies in the large number of pathologic processes, both malignant as well as benign, that can settle in this region. The most important of the malignant processes is adenocarcinoma of the head of the pancreas,7,11 whose preoperative differentiation can be difficult, especially when the tumor presents an important fibrotic component. Other malignant tumors are duodenal adenocarcinoma, ampulloma, distal cholangiocarcinoma, functional or non-functional pancreatic neuroendocrine tumors, duodenal stroma tumors, carcinoid tumors, and intraductal papillary mucinous tumors. All these tumors are hypervascular, and preoperative diagnosis is complicated. As for the differential diagnosis of benign processes, this should include other causes of chronic pancreatitis (alcohol, autoimmune, hereditary, etc.), choledochal cysts, duodenal diverticulum, etc.
The incidence of GP is unknown,8,9 although it is very rare in women.6 More than 5% of the pancreatectomies performed with a preoperative diagnosis of carcinoma are non-tumor lesions in the end.13 The pathogenesis of GP is not well defined,6,14 although there are triggering factors, such as alcohol abuse,15,16 which causes altered secretions in the duct of Santorini and increased intraductal pressure that facilitates the formation of pseudocysts and the leakage of pancreatic juices to the area of the groove.17 Other triggering factors include the presence of a heterotopic pancreas, peptic ulcers (gastric and duodenal), gastric resections, duodenal wall or head of pancreas cysts, etc.
With regards to diagnosis, clinical symptoms are non-specific although there is occasionally epigastric abdominal pain with nausea, vomiting, and weight loss if there is a predominance of duodenal obstruction. One datum that is helpful is the chronicity of the symptoms, with a mean duration of 3–6 months, and jaundice, which usually fluctuates in the case of GP.18 Another important data is lipase, which is not usually elevated in GP and is an analytical datum that differentiates GP from other types of pancreatitis.6,13
The radiological diagnosis depends on the stage in which the disease is found. On abdominal ultrasound, prominent duodenal folds are observed, which are hyperechoic and have a normal muscle layer. In the segmental form, there may be heterogenous echogenicity in the head of the pancreas.9,11,19 According to Wronski et al.,19 a pathognomonic ultrasound finding is hyperechogenicity of the dorsal–cranial portion of the head of the pancreas with anechoic ductal structures due to fibrosis of the head and myomatous proliferation. On CT, pure GP is usually observed as a hypodense mass in the groove due to the fibrotic tissue. Cystic formations of different sizes can also be observed in the medial duodenal wall. The pancreatic duct is normal. In the segmental form, the pancreatic duct is seen to be dilated, as well as the intra- and extrahepatic bile duct, with a stenotic distal common bile duct. There is no vascular infiltration, not even in advanced cases of the disease. The segmental form poses the most problems for diagnosis as it is very complicated to distinguish from pancreatic cancer.11 Magnetic resonance cholangiopancreatography is essential and, for some authors, it is the best diagnostic method.11,12,20 The most characteristic finding is a laminar hypointense mass in T1 between the head of the pancreas and the 2nd portion of the duodenum, with the remaining pancreas hyperintense. In T2, it can be hypo-, iso- or hyperintense, depending on the chronicity of the process. In initial phases, because of edema, it is hyperintense; when fibrosis has become established, the appearance is hypointense. These data are similar to pancreatic cancer if there is a fibrotic component. In advanced stages with parenchyma atrophy, hypointensity can be observed in the head or in the entire gland in T1, with hyperintense cysts in T2 in the groove. This thickening of the duodenal wall and the cystic changes are a common finding of GP, which are rare in malignant processes.
With regards to the use of endoscopic ultrasound and biopsy sampling, there is little information about its use in GP, despite the growing use for the diagnosis of other pancreatic processes, which will depend on the area biopsied.21 Fibrotic changes can be observed, with hyperplasia of Brunner gland cells, a circumstance that does not enable us to rule out a neoplasm.5 In our series, this was done in 4 patients associated with FNA and, in only one case, the histology study confirmed the diagnosis of GP but was not conclusive in the 3 remaining cases.
ERCP can only be done in patients who do not present duodenal stenosis, and the common bile duct can be visualized. It is difficult to distinguish slow, progressive stenosis from the typical irregular stenosis of neoplasias.22
There are no data in the literature about the use of PET/CT in GP. In our series, it was used in 4 patients for extension studies given a high suspicion of neoplastic disease, in 3 of the 4 patients, there was increased metabolic activity observed in the duodenal wall, with a maximum SUV of 4.5 and 5.5, respectively. In the remaining patient, the increased metabolism appeared in the head of the pancreas, with a maximum SUV of 4.5. The preoperative interpretation of these maximum SUV values is controversial as it may correspond with a benign or malignant lesion.
As no extensive studies have been published in the literature, we find that treatment can be quite varied. If the diagnosis of GP is confirmed, the management can initially be conservative, with analgesia and with enteral nutrition if there is no duodenal obstruction and abstinence of alcohol and tobacco consumption.6,9,23–25 Endoscopic treatment involves drainage of the pancreatic duct, dilatation of the stenosis and stent placement; it can be carried out either in initial stages of the disease, before the establishment of fibrosis,23 or as a “bridge” treatment before surgery. The disadvantage of endoscopic treatment is the high rate of recurrences and complications derived from the procedure. Therefore, most authors6,9,23,24 prefer surgery as it better controls abdominal pain and leads to weight gain. Surgery is considered the treatment of choice in GP, especially if symptoms are not controlled or there are diagnostic uncertainties that indicate malignancy.23–25 It has been demonstrated that PD is the definitive treatment for GP because it improves symptoms.25 Non-resective interventions, such as biliary or digestive bypass, are not effective and a high rate of recurrences have been observed.24,26,27 In 2009, Casetti et al.23 performed PD in 58 patients. In the histology studies of the surgical specimens, they only found one patient with duodenal adenocarcinoma and one other patient with a neuroendocrine tumor in the head of the pancreas. In their experience, 76% of the patients experienced improved symptoms. In 2014, Egorov et al.27 published a series of 62 patients, 10 of whom received medical treatment and 52 were operated on. In 29 of the 52 surgical patients, PD was performed, and in the remaining 23 other derivative surgical techniques were used. Control of the symptoms was 85% in patients treated with PD. During follow-up, one patient presented malignization of the duodenal dystrophy 5 years later.
In our series, 8 patients were treated with surgery: 7 underwent PD and one patient total pancreaticoduodenectomy because of previous surgery (cystojejunostomy) and the fibrotic appearance associated with the presence of nodules in the body and tail of the pancreas. There were no serious complications and no cases of death related with the surgical procedure. Symptoms improved notably in 7 of the 8 patients (87.5%), and we have had no cases with malignization.
In conclusion, the determination of GP or ADC is usually difficult because, in most cases, surgery is indicated due to the impossibility to make a reliable diagnosis of benign disease. Only in cases where clinical and radiological data provide absolute certainty of GP can treatment be initially medical, with rest, enteral nutrition, and abstinence from alcohol and tobacco. If there is no evident clinical improvement with these measures, then surgery is indicated. In this case, the most useful surgical technique is PD.
Authorship/CollaboratorsFrancisco Sánchez Bueno and Gloria Torres Salmerón helped in study design. Francisco Sánchez Bueno, Gloria Torres Salmeron and Wilfredo Víctor Gutiérrez Zárate contributed in data collection. Francisco Sánchez Bueno, Gloria Torres Salmerón, Jesús de la Peña, Eduardo Ortiz, Matilde Fuster and María Antonia Claver helped in analysis and interpretation of the results. Francisco Sánchez Bueno and Gloria Torres Salmerón contributed in composition of the article. Francisco Sánchez Bueno and Pascual Parrilla Paricio helped in critical review and approval of the final version.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez-Bueno F, Torres Salmerón G, de la Peña Moral J, Ortiz Ruiz E, Fuster Quiñonero M, Gutiérrez Zárate WV, et al. Pancreatitis del surco versus adenocarcinoma de páncreas: a propósito de 8 casos. Cir Esp. 2016;94:346–352.