Fournier's gangrene (FG) is the necrotizing fasciitis of the perineum and genital area and presents a high mortality rate. The aim was to assess prognostic factors for mortality, create a new mortality predictive scale and compare it with previously published scales in patients diagnosed with FG in our Emergency Department.
MethodsRetrospective analysis study between 1998 and 2012.
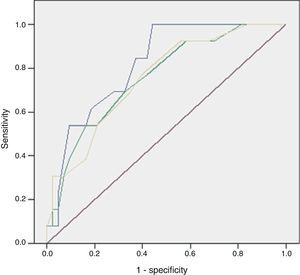
ResultsOf the 59 patients, 44 survived (74%) (S) and 15 died (26%) (D). Significant differences were found in peripheral vasculopathy (S 5 [11%]; D 6 [40%]; P=.023), haemoglobin (S 13; D 11; P=.014), haematocrit (S 37; D 31.4; P=.009), white blood cells (S 17,400; D 23,800; P=.023), serum urea (S 58; D 102; P=.001), creatinine (S 1.1; D 1.9; P=.032), potassium (S 3.7; D 4.4; P=.012) and alkaline phosphatase (S 92; D 133; P=.014). Predictive scores were: Charlson index (S 1; D 4; P=.013), severe sepsis criteria (S 16 [36%]; D 13 [86%]; P=.001), Fournier's Gangrene Severity Index Score (FGSIS) (S 4; D 7; P=.002) and Uludag Fournier's Gangrene Severity Index (UFGSI) (S 9; D 13; P=.004). Independent predictive factors were peripheral vasculopathy, serum potassium and severe sepsis criteria, and a model was created with an area under the ROC curve of 0.850 (0.760–0.973), higher than FGSIS (0.746 [0.601–0.981]) and UFGSI (0.760 [0.617–0.904]).
ConclusionsFG showed a high mortality rate. Independent predictive factors were peripheral vasculopathy, potassium and severe sepsis criteria creating a predictive model that performed better than those previously described.
La gangrena de Fournier (GF) es la fascitis necrosante del periné y área genital que presenta una elevada mortalidad. El objetivo es analizar los factores pronósticos de mortalidad, creación de una nueva escala predictiva de mortalidad y compararla con las ya validadas en los pacientes diagnosticados de GF en nuestro Servicio de Urgencias.
MétodosEstudio analítico, retrospectivo entre 1998 y 2012.
ResultadosDe los 59 casos, 44 sobrevivieron (74%) (S) y 15 fallecieron (26%) (E). Se encontraron diferencias significativas en la vasculopatía periférica (S 5 [11%]; E 6 [40%]; p=0,023), haemoglobin (S 13; E 11; p=0,014), haematocrito (S 37; E 31,4; p=0,009), leucocitos (S 17.400; E 23.800; p=0,023), urea (S 58; E 102; p<0,001), creatinina (S 1,1; E 1,9; p=0,032), potasio (S 3,7; E 4,4; p=0,012) y fosfatasa alcalina (S 92; E 133; p=0,014). Escalas predictivas: índice de Charlson (S 1; E 4; p=0,013), criterios de sepsis grave (S 16 [36%]; E 13 [86%]; p=0,001), Fournier's gangrene severity index score (FGSIS) (S 4; E 7; p=0,002) y Uludag Fournier's Gangrene Severity Index (UFGSI) (S 9; E 13; p=0,004). Los factores predictores independientes fueron la vasculopatía periférica, el potasio sérico y criterios de sepsis grave, creando un modelo con área bajo la curva de 0,850 (0,760-0,973) superior al FGSIS (0,746 [0,601-0,981]) y al UFGSI (0,760 [0,617-0,904]).
ConclusionesLa GF presentó una tasa de mortalidad elevada cuyos factores predictores independientes fueron la vasculopatía periférica, el potasio sérico y criterios de sepsis grave, creando un modelo con una capacidad discriminativa superior al resto.
Fournier's gangrene (FG) is the polymicrobial necrotizing fasciitis of the perineum and genital area, which may be of colorectal, genitourinary, traumatic or idiopathic aetiology. It primarily affects males in their 50s to 70s who, in most cases, have predisposing factors. It requires a multidisciplinary treatment, including haemodynamic support in the intensive care unit (ICU), broad-spectrum antibiotic therapy and surgical debridement; occasionally, a colostomy, suprapubic cystostomy or orchiectomy is required. It has an elevated mortality rate (7%–75%); the validated predictive scales are complex and they can potentially be improved, since they are partially composed of variables that have not shown significant differences, thus reducing their predictive capacity.1–4
The objective of this study is to analyse the prognostic factors of mortality, to create a new mortality predictive scale and to compare it to the already validated scales in a series of patients diagnosed with FG at our Emergency Department.
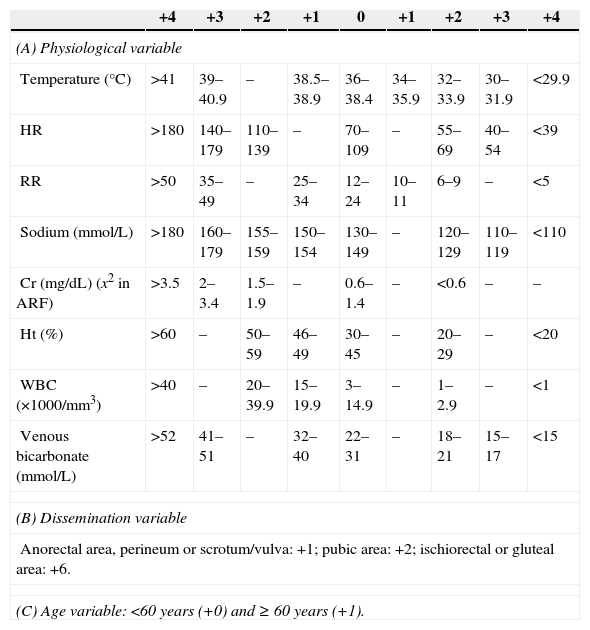
MethodsAn observational, 15-year retrospective (1998–2012) cohort study was conducted at the Emergency Department of Hospital General Universitario Gregorio Marañón [Gregorio Marañón University General Hospital]. All patients clinically diagnosed with FG at the Emergency Department were included and the diagnosis was confirmed by a radiological test when applicable. Patients with an incomplete medical record were excluded. The independent variables collected were age, sex, associated comorbidities, Charlson comorbidity index (CCI) and age-adjusted Charlson comorbidity index (AACCI),5 aetiology, vital signs (temperature, blood pressure, heart rate and respiratory rate), symptomatology, lab tests (the serum potassium values provided by our laboratory were considered normal in the range of 3.5–4.5mmol/L), systemic inflammatory response syndrome (SIRS) (defined as compliance with two or more of the following criteria: heart rate >90bpm, respiratory rate >20bpm or PaCO2 <32mmHg, temperature >38°C or <36°C, WBCs >12,000/mm3, <4000/mm3 or >10% of immature forms), severe sepsis (defined as sepsis [SIRS in addition to a clinically or microbiologically documented infection] associated with organ dysfunction, hypotension or tissue hypoperfusion). Organic dysfunction variables were: renal [creatinine >2mg/dL, >0.5mg/dL increase, or diuresis <0.5mL/kg/h for at least 2h], pulmonary [arterial hypoxaemia with PaO2/FIO2 <300], liver [platelets <100000/mm3, total bilirubin >2mg/dL, INR >1.5 or activated partial thromboplastin time >60s]. Arterial hypotension variables were: systolic blood pressure <90mmHg, mean blood pressure <70mmHg or decreased systolic blood pressure >40mmHg with respect to its baseline value. Tissue hypoperfusion variables were: lactate >3mmol/L),6 stay at the ICU and use of vasoactive drugs, antibiotic used, affected body surface (ABS) measured in the normogram used in burned patients according to which penis (1%), each scrotum (1%), perineum (1%) and each ischiorectal fossa (2.5%),3,4 surgical treatment associated with the debridement (colostomy, cystostomy or orchiectomy), microbiological culture and predictive scales (Fournier's Gangrene Severity Index Score [FGSIS] and Uludag Fournier's Gangrene Severity Index [UFGSI]) (Table 1).2–4 The dependent variable collected was mortality. Data collection was performed by reviewing medical records. The study protocol was approved by the Clinical Research Ethics Committee, which considered it a risk-free investigation given its retrospective nature in which no surgery was performed.
Fournier's Gangrene Severity Index.
| +4 | +3 | +2 | +1 | 0 | +1 | +2 | +3 | +4 | |
|---|---|---|---|---|---|---|---|---|---|
| (A) Physiological variable | |||||||||
| Temperature (°C) | >41 | 39–40.9 | – | 38.5–38.9 | 36–38.4 | 34–35.9 | 32–33.9 | 30–31.9 | <29.9 |
| HR | >180 | 140–179 | 110–139 | – | 70–109 | – | 55–69 | 40–54 | <39 |
| RR | >50 | 35–49 | – | 25–34 | 12–24 | 10–11 | 6–9 | – | <5 |
| Sodium (mmol/L) | >180 | 160–179 | 155–159 | 150–154 | 130–149 | – | 120–129 | 110–119 | <110 |
| Cr (mg/dL) (x2 in ARF) | >3.5 | 2–3.4 | 1.5–1.9 | – | 0.6–1.4 | – | <0.6 | – | – |
| Ht (%) | >60 | – | 50–59 | 46–49 | 30–45 | – | 20–29 | – | <20 |
| WBC (×1000/mm3) | >40 | – | 20–39.9 | 15–19.9 | 3–14.9 | – | 1–2.9 | – | <1 |
| Venous bicarbonate (mmol/L) | >52 | 41–51 | – | 32–40 | 22–31 | – | 18–21 | 15–17 | <15 |
| (B) Dissemination variable | |||||||||
| Anorectal area, perineum or scrotum/vulva: +1; pubic area: +2; ischiorectal or gluteal area: +6. | |||||||||
| (C) Age variable: <60 years (+0) and ≥ 60 years (+1). | |||||||||
A, Fournier's Gangrene Severity Index Score; A+B+C: Uludag Fournier's Gangrene Severity; ARF, acute renal failure; Cr, creatinine; HR, heart rate; Ht, haematocrit; RR, respiratory rate.
The statistical analysis was conducted with the SPSS® programme version 21.0 for Windows; the qualitative variables were defined by frequency and percentage and the quantitative variables were defined by the median and the 25–75 percentiles. The statistical tests employed in the univariate analysis were the chi-square test and Fisher's exact test for the qualitative variables and the Mann–Whitney and Kruskal–Wallis tests for the quantitative variables. The multivariate study was performed with a binary logistic regression model, including the significant variables in the univariate study (P=.05). The ROC area under the curve (AUC) was used to assess the discriminatory capacity of both the created model and the existing models, and the Sidak test from the statistical programme STATA for Windows was used to compare those capacities.
ResultsOf the 59 enrolled patients, 53 were males (90%) and 6 were females (10%) with a median age of 68 years (51–73), and prior medical history in 48 of them (81%) included high blood pressure in 29 (49%), diabetes mellitus (DM) in 25 (36%), heart disease in 17 (29%), immunodepression in 14 (24%) (10 neoplasms, 2 chemotherapeutic treatments, 1 corticosteroid treatment and 1 acquired immunodeficiency syndrome), alcoholism in 12 (20%), smoking habit in 12 (20%), kidney disease in 9 (15%) and lung disease in 8 (14%), with a CCI of 1 (0; 3) and an AACCI of 4 (2; 6).
The aetiology was colorectal in 31 cases (52%) (26 abscesses and 5 neoplasms), genitourinary in 12 (20%) (10 orchiepididymitis and 2 bladder neoplasms), traumatic in 9 (15%) (5 pressure ulcers, 2 bladder catheters, 1 traffic accident and 1 perforation by foreign body) and idiopathic in 7 (12%) cases.
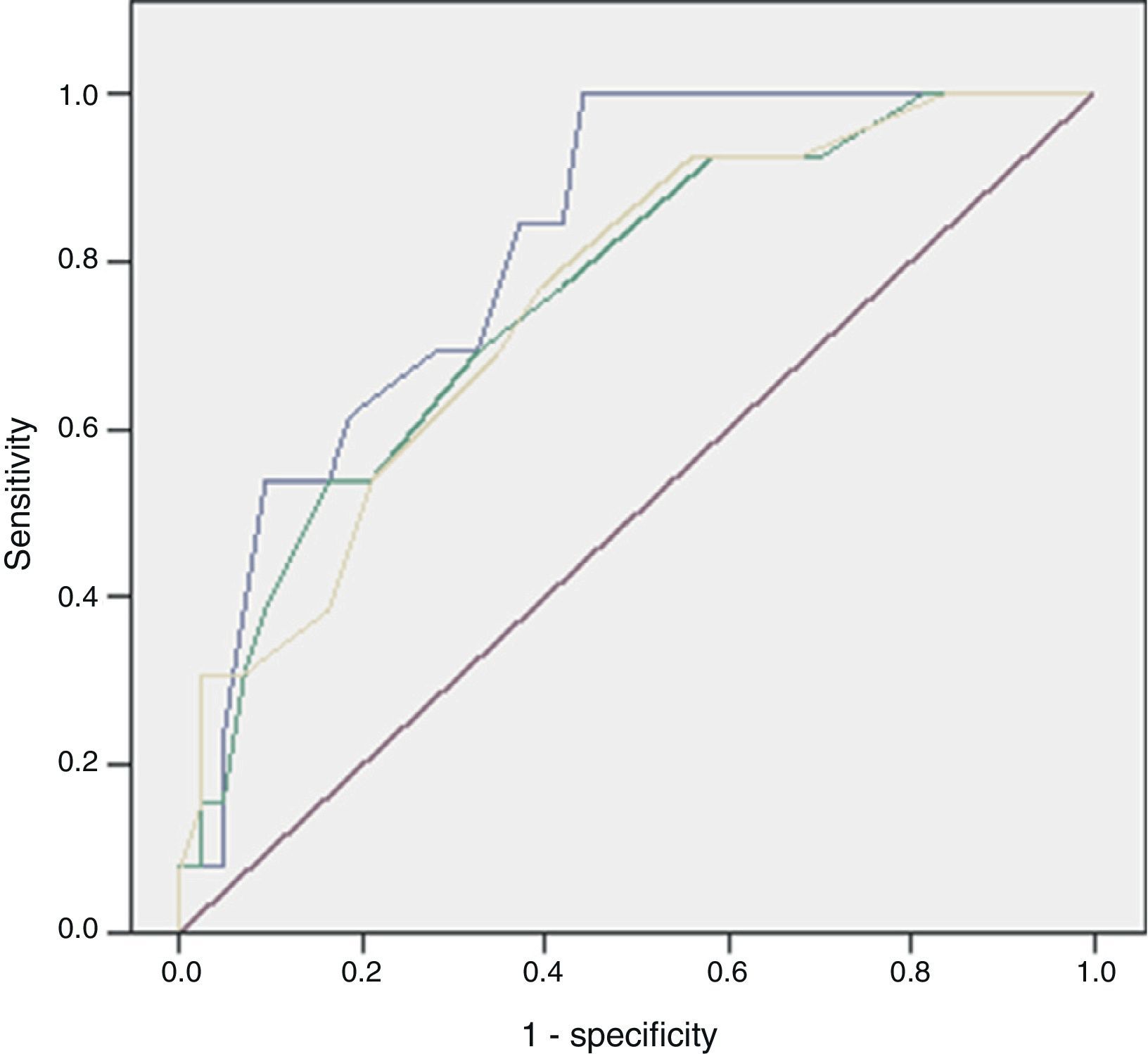
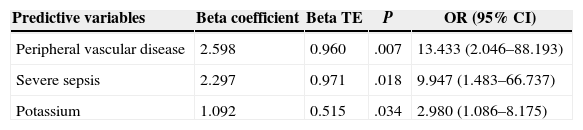
Of the 59 patients, 44 (74%) survived and 15 (25%) died with a hospital stay of 30 (18–56) and 8 (4–16) days, respectively. The aetiology in mortality cases was: colorectal in 5 cases (16%), genitourinary in 6 (50%), traumatic in 4 (44%) and idiopathic in 0 (0%), P=.095. The causes of death were: multiple organ failure in 12 (80%), pneumonia in 1, pulmonary thromboembolism in 1 and acute myocardial infarction in 1. The analysis of the mortality predictive factors is shown in Tables 2 and 3. The multivariate analysis showed that the independent predictive variables were the presence of peripheral vascular disease, high serum potassium levels, and severe sepsis criteria (Table 4). The model created was characterised by: constant of −7.566, adequate internal calibration (Hosmer–Lemeshow: P=.405) and an AUC of 0.850 (0.760–0.973).
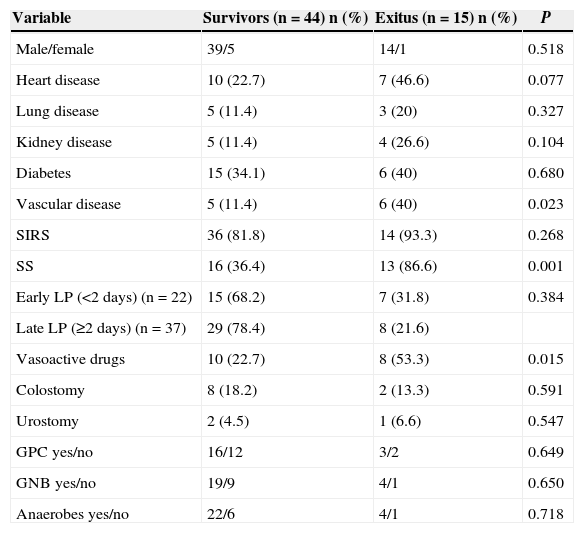
Univariate Analysis of Mortality-predictive Qualitative Variables.
| Variable | Survivors (n=44) n (%) | Exitus (n=15) n (%) | P |
|---|---|---|---|
| Male/female | 39/5 | 14/1 | 0.518 |
| Heart disease | 10 (22.7) | 7 (46.6) | 0.077 |
| Lung disease | 5 (11.4) | 3 (20) | 0.327 |
| Kidney disease | 5 (11.4) | 4 (26.6) | 0.104 |
| Diabetes | 15 (34.1) | 6 (40) | 0.680 |
| Vascular disease | 5 (11.4) | 6 (40) | 0.023 |
| SIRS | 36 (81.8) | 14 (93.3) | 0.268 |
| SS | 16 (36.4) | 13 (86.6) | 0.001 |
| Early LP (<2 days) (n=22) | 15 (68.2) | 7 (31.8) | 0.384 |
| Late LP (≥2 days) (n=37) | 29 (78.4) | 8 (21.6) | |
| Vasoactive drugs | 10 (22.7) | 8 (53.3) | 0.015 |
| Colostomy | 8 (18.2) | 2 (13.3) | 0.591 |
| Urostomy | 2 (4.5) | 1 (6.6) | 0.547 |
| GPC yes/no | 16/12 | 3/2 | 0.649 |
| GNB yes/no | 19/9 | 4/1 | 0.650 |
| Anaerobes yes/no | 22/6 | 4/1 | 0.718 |
GNB, gram-negative bacilli; GPC, gram-positive cocci; LP, latency period (onset of symptoms until diagnosis); SS, severe sepsis; SIRS, systemic inflammatory response syndrome.
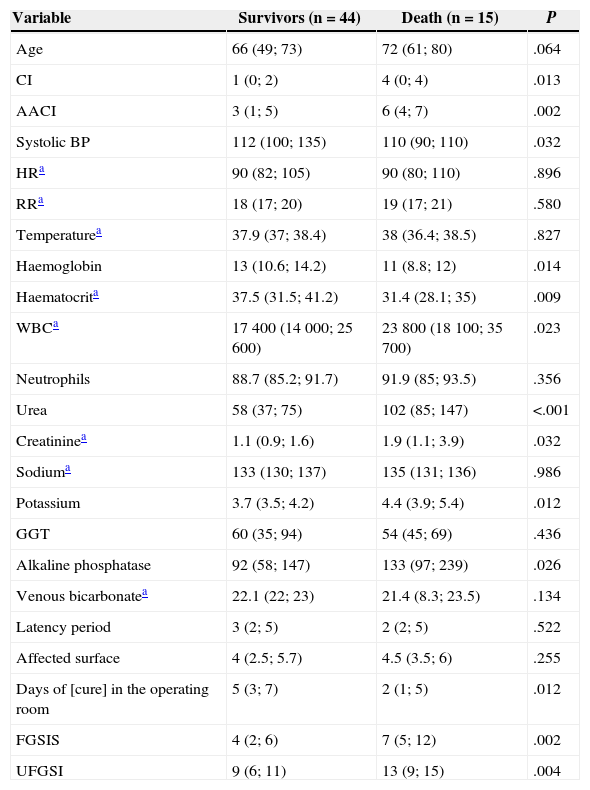
Univariate Analysis of Mortality-predictive Quantitative Variables.
| Variable | Survivors (n=44) | Death (n=15) | P |
|---|---|---|---|
| Age | 66 (49; 73) | 72 (61; 80) | .064 |
| CI | 1 (0; 2) | 4 (0; 4) | .013 |
| AACI | 3 (1; 5) | 6 (4; 7) | .002 |
| Systolic BP | 112 (100; 135) | 110 (90; 110) | .032 |
| HRa | 90 (82; 105) | 90 (80; 110) | .896 |
| RRa | 18 (17; 20) | 19 (17; 21) | .580 |
| Temperaturea | 37.9 (37; 38.4) | 38 (36.4; 38.5) | .827 |
| Haemoglobin | 13 (10.6; 14.2) | 11 (8.8; 12) | .014 |
| Haematocrita | 37.5 (31.5; 41.2) | 31.4 (28.1; 35) | .009 |
| WBCa | 17400 (14000; 25600) | 23800 (18100; 35700) | .023 |
| Neutrophils | 88.7 (85.2; 91.7) | 91.9 (85; 93.5) | .356 |
| Urea | 58 (37; 75) | 102 (85; 147) | <.001 |
| Creatininea | 1.1 (0.9; 1.6) | 1.9 (1.1; 3.9) | .032 |
| Sodiuma | 133 (130; 137) | 135 (131; 136) | .986 |
| Potassium | 3.7 (3.5; 4.2) | 4.4 (3.9; 5.4) | .012 |
| GGT | 60 (35; 94) | 54 (45; 69) | .436 |
| Alkaline phosphatase | 92 (58; 147) | 133 (97; 239) | .026 |
| Venous bicarbonatea | 22.1 (22; 23) | 21.4 (8.3; 23.5) | .134 |
| Latency period | 3 (2; 5) | 2 (2; 5) | .522 |
| Affected surface | 4 (2.5; 5.7) | 4.5 (3.5; 6) | .255 |
| Days of [cure] in the operating room | 5 (3; 7) | 2 (1; 5) | .012 |
| FGSIS | 4 (2; 6) | 7 (5; 12) | .002 |
| UFGSI | 9 (6; 11) | 13 (9; 15) | .004 |
HR, heart rate; FGSIS, Fournier's Gangrene Severity Index Score; RR, respiratory rate; GGT, gamma-glutamyl transpeptidase; CI, Charlson index; AACI, age-adjusted Charlson index; UFGSI, Uludag Fournier's Gangrene Severity Index.
Mortality Predictive Factors in Logistic Regression.
| Predictive variables | Beta coefficient | Beta TE | P | OR (95% CI) |
|---|---|---|---|---|
| Peripheral vascular disease | 2.598 | 0.960 | .007 | 13.433 (2.046–88.193) |
| Severe sepsis | 2.297 | 0.971 | .018 | 9.947 (1.483–66.737) |
| Potassium | 1.092 | 0.515 | .034 | 2.980 (1.086–8.175) |
TE, typical error; CI, confidence interval; OR, odds ratio.
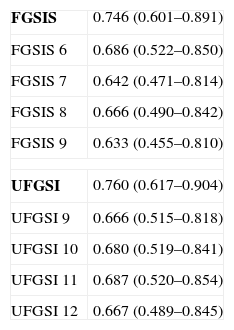
The discriminatory capacity of the established scales is shown in Table 5 and the comparison between the different models can be found in Fig. 1.
Discriminatory Capacity of the FGSIS and UFGSI and Their Cut-off Points.
| FGSIS | 0.746 (0.601–0.891) |
| FGSIS 6 | 0.686 (0.522–0.850) |
| FGSIS 7 | 0.642 (0.471–0.814) |
| FGSIS 8 | 0.666 (0.490–0.842) |
| FGSIS 9 | 0.633 (0.455–0.810) |
| UFGSI | 0.760 (0.617–0.904) |
| UFGSI 9 | 0.666 (0.515–0.818) |
| UFGSI 10 | 0.680 (0.519–0.841) |
| UFGSI 11 | 0.687 (0.520–0.854) |
| UFGSI 12 | 0.667 (0.489–0.845) |
FGSIS, Fournier's Gangrene Severity Index Score; UFGSI, Uludag Fournier's Gangrene Severity Index.
FG has a high mortality rate, from 7%–75% in classic series to 10%–40% in more recent series; in our case the mortality rate was 25% and its independent predictive factors were the presence of peripheral vascular disease, high serum potassium levels, and severe sepsis criteria.1,7–10
Multiple predictive factors have been described. Age has been rising progressively in the published series, and there are a growing number of studies showing that the patients who die are older, as in our case, although statistical significance was not achieved.1–3,7–13
The most frequent comorbidities described have been chronic renal failure and neoplasms, while in our series the only frequent comorbidity was peripheral vascular disease. DM, despite being a common predisposing factor and being related to a reduced function of defence cells, has not been associated with a higher mortality rate in most series or in ours.9,12,14–18 The CCI and AACCI have been recently described as mortality predictive factors, as it occurred in our series.10,18
With respect to the aetiology, most series do not show differences among the various aetiologic groups (colorectal, genitourinary, traumatic and idiopathic); in this study, mortality was higher in the colorectal and traumatic cases but failed to reach a statistical significance.2,8,9,19
The laboratory parameters most frequently seen are low levels of haematocrit, bicarbonate and albumin as well as elevated levels of urea, creatinine, WBCs, sodium, potassium, alkaline phosphatase and lactate.9–13,19 In our series, mortality was significantly higher in patients with low levels of haemoglobin (P=.014) and haematocrit (P=.009) as well as elevated levels of alkaline phosphatase (P=.026), urea (P<.001), creatinine (P=.032) and potassium (P=.012), which reflects the important role that renal failure plays in the prediction of mortality.
A delay in treatment should result in a higher mortality rate. However, different series have shown that the latency period and a cut-off point of two days as the difference between early and late treatment proved to be predictive of mortality, whereas in other series, as in ours, this did not occur.1,9,11,13,19
Performing a derivative colostomy or cystostomy, just as in our series, has not shown any prognostic value.1,2,7,9,12,19,20
In the microbiological cultures, the different groups (gram-positive, gram-negative aerobes and anaerobes) have not been correlated to mortality, just as in our series.21,22
Regarding the ABS, its prognostic value is unclear, even though more and more series are showing a larger ABS in the deceased patients, like this series, although no significant differences are found.1,7–11,15,19
Some authors have described a higher mortality rate as the number of debridement procedures increases, arguing disease progression with a larger affected surface and sepsis; however, these data have not been corroborated in most series, similar to our series.3,7,11,12,15,19
Every prognostic scale should predict a certain event in the best and easiest way possible. In the case of FG, the primary event is mortality, for which there are two validated scales so far: FGSIS and UFGSI.2
Laor created the FGSIS in 1995, modifying the Acute Physiology and Chronic Health Evaluation II (APACHE-II) scale; he established a cut-off point so that, with FGSIS >9, the likelihood of mortality was 75%, and with FGSIS ≤9, the likelihood of survival was 78%.23 Its predictive value and the cut-off point have been confirmed in most series.2,9–11,15,19 In our case, the FGSIS score was significantly higher in the patients who died, with an acceptable discriminatory capacity; however, even though the differences existing with the cut-off point of 9 were significant, the discriminatory capacity was better with a cut-off point of 6. In 2010, Yilmazlar created a new score (UFGSI) by adding two parameters (age and disease extension), improving the discriminatory capacity in his series but also making it more complex compared to FGSIS. Yilmazlar used a cut-off point of 9 according to which, when UFGSI was >9, the likelihood of mortality was 94%, and when UFGSI was ≤9, the likelihood of survival was 81%.2 In our series, the discriminatory capacity of UFGSI slightly improved the discriminatory capacity of FGSIS; nevertheless, the cut-off point with the best discriminatory capacity was 11.
The use of prognostic scales is important for various reasons:
- (a)
Modification of therapeutic behaviours: Yilmazlar suggested that when the UFGSI is >9, given that the likelihood of mortality was high, the case should be managed in the ICU whereas when the UFGSI was ≤9, the likelihood of survival was high and the ICU was rarely required, so unnecessary costs could be avoided, as well as the morbidity related to a stay in the ICU.2
- (b)
Feedback and correction of both diagnostic and therapeutic errors in all patients, particularly in the patients who died even though their scale-based likelihood was low.
The main problem with these scales lies in that their discriminatory capacity is reduced. Consequently, as some studies have shown, the scales could potentially be improved because they are made up of variables that fail to show significant differences between the patients who survive and those who die. This was similar to what happened in our series, where 5 out of 8 in the FGSIS and 7 out of 10 in the UFGSI were not significant.9,10,15,19 The discriminatory capacity in our model was higher than in the FGSIS and UFGSI because, unlike them, all its parameters presented significant differences between the patients who survived and those who died.
We consider that our study's main limitation is its retrospective nature and the small sample size, something that occurs in most of these studies due to the low incidence of FG.
In conclusion, FG is a disease with low incidence and a high mortality rate, without an ideal mortality prediction model. We have created a predictive model whose factors were peripheral vascular disease, high serum potassium levels and severe sepsis criteria, with higher discriminatory capacity than in the models previously described. The purpose of this model would be to understand this disease better, to be able to identify mistakes in case of unexpected mortality and to improve survival; however, the model still needs to be validated in longer series.
Contribution from the AuthorsAndrés García Marín: study design, data acquisition and collection, analysis and interpretation of results, article writing, critical review and approval of the final version.
Fernando Turégano Fuentes: study design, analysis and interpretation of results, article writing, critical review and approval of the final version.
Marta Cuadrado Ayuso: data acquisition and collection, article writing, critical review and approval of the final version.
Juan Antonio Andueza Lillo, Juan Carlos Cano Ballesteros and Mercedes Pérez López: article writing, critical review and approval of the final version.
Conflicts of InterestThe authors declare that they do not have any conflicts of interest.
Please cite this article as: García Marín A, Turégano Fuentes F, Cuadrado Ayuso M, Andueza Lillo JA, Cano Ballesteros JC, Pérez López M. Factores predictivos de mortalidad en la gangrena de Fournier: serie de 59 casos. Cir Esp. 2015;93:12–17.
Partial presentation at the XIX Reunión Nacional de Cirugía [XIX National Surgery Meeting] (Burgos, 23–25 October 2013).