The association of a loop ileostomy decreases the severity of complications after rectal surgery but can increase the postoperative stay. The aim of this study is to investigate if a diverting ileostomy influences the postoperative outcomes in a series of patients included in a multimodal rehabilitation program (MMRP).
MethodsWe analysed a series of 104 patients that underwent elective surgery with primary anastomosis for rectal adenocarcinoma using a MMRP: 66 men and 38 women, with a median age of 64 (IQR: 55–75) years. Group A included patients with an associated loop ileostomy, and Group B, those without a protective stoma.
ResultsGroup A=58, group B=46 patients with neither differences in age, ASA, BMI and other risk factors nor in the surgical approach (laparoscopic in 34%), although there were more neoadjuvant treatments in group A: 77.5 vs 36.9%; P=.001. In group A, the most common operation was total mesorectal excision (96%) and in the group B, a subtotal mesorectal excision (90%). There were no differences in postoperative complications (Group A 34.4 vs group B 28.2%; P=.322), anastomotic leaks (8.3 vs 10.8%; P=.475), or postoperative ileus (20.7 vs 10.9%; P=.140), neither in postoperative stay (7.9 vs 6.9 days; P=.058), readmissions (7 vs 13.6%; P=.22) nor postoperative stay including readmissions (8.4 vs 9.1 days; P=.49).
ConclusionsThe association of a loop ileostomy does not extend the length of stay nor increases the rate of complications in patients that underwent a rectal resection with anastomosis included in a MMRP.
La asociación de ileostomía disminuye la gravedad de las complicaciones tras anastomosis rectal baja pero puede alargar la estancia postoperatoria. El objetivo del presente estudio es averiguar si un estoma derivativo modifica la estancia postoperatoria o las complicaciones, en pacientes intervenidos bajo un régimen de rehabilitación multimodal perioperatoria (RHMM).
MétodosAnalizamos a 104 pacientes intervenidos de resección con anastomosis por adenocarcinoma rectal con cuidados de RHMM: 66 varones y 38 mujeres, mediana de edad de 64 años (RIQ: 55–75). En el grupo A, se incluyó a los que se asoció ileostomía derivativa y en el B a aquellos sin ileostomía.
ResultadosGrupo A = 58, grupo B = 46 pacientes sin diferencias en edad, ASA, IMC, factores de riesgo, ni en el tipo de abordaje, laparoscópico en un 34%, si bien hubo más neoadyuvancia en el grupo A: 77,5 frente a 36,9%. En este grupo, la intervención habitual fue la exéresis total del mesorrecto (96%) y en el B la subtotal (90%). No hubo diferencias en las complicaciones postoperatorias (34,4 frente a 28,2%; p = 0,322) ni en la de dehiscencias anastomóticas (8,3 frente a 10,8%; p = 0,475), o íleo prolongado (20,7 frente a 10,9%; p = 0,140). Tampoco las hubo en la estancia postoperatoria (7,9 frente a 6,9 días; p = 0,058), reingresos (7 frente a 13,6%; p = 0,22), o en la estancia total incluyendo reingresos (8,4 frente a 9,1 días; p = 0,49).
ConclusionesLa asociación de una ileostomía no alarga la EP ni incrementa las complicaciones en pacientes intervenidos de resección rectal en régimen de RHMM.
Resection with anastomosis is currently the most widely used technique in the surgical treatment of rectal cancer, and it achieves the preservation of anal sphincter function in more than 70% of cases.1 However, the lower the anastomosis, the higher the anastomotic leak rate is, and performing a diverting stoma (generally an ileostomy) reduces morbidity,2–4 although it has been reported to increase postoperative stay.5 Given that colorectal surgery has been amongst the first to implement perioperative care encouraged in a standardised way, with the consequent reduction of complications and length of stay,6–9 one should question if performing a diverting stoma influences the postoperative progress and stay in patients who receive this care after having undergone rectal cancer surgery, which is the aim of our study.
MethodsWe retrospectively analysed a prospective database of patients who had undergone elective surgery involving primary colorectal or coloanal anastomosis due to rectal adenocarcinoma from January 2007 to November 2011. All patients underwent the surgery in two centres of reference (one of them a university hospital), by the same surgical team. Patients were divided into group A, including those patients who had an associated stoma, and group B, which included those without stoma.
We estimated sample size assuming that patients with an associated stoma would have a mean stay two days longer than those without stoma. Thus, considering a 5% statistical significance with an 80% power, 50 patients were required per arm.
All patients followed a perioperative multimodal rehabilitation programme (MMRP) protocol and a clinical pathway previously approved by the local Clinical Research Ethics Committee (Table 1), and the specific information regarding the chances of performing a stoma and its marking was provided by a colorectal surgeon and a stomatherapist, respectively. Surgical procedures were performed or supervised directly by surgeons devoted specifically to coloproctology (European Board). Patients who underwent an abdominoperineal amputation, a Hartmann's procedure or transanal endoscopic microsurgery were excluded. The stoma was constructed when a total mesorectal excision (TME) was performed and, at the surgeon's discretion, when an increased risk of anastomotic leak was estimated.
Components of MMRP Protocol.
| • Preoperative information of perioperative care and stoma-specific information |
| • Anterograde preparation of the colon only if a diverting stoma is planned. In the rest of the cases, cleansing enema |
| • Antibiotic and thromboembolic prophylaxis |
| • Decrease in the impact of general anaesthesia and prevention of surgical stress |
| • Preoperative intake of glucose (non-constant) |
| • Anaesthetics with minimum postoperative residual effect |
| • Optimisation of IV fluid therapy (1500mL/24h in the postoperative period) |
| • Maintenance of normal temperature |
| • Minimally invasive techniques (small incisions, laparoscopy) |
| • No use of nasogastric tube |
| • Multimodal perioperative analgesia |
| • No use of opioids |
| • Epidural analgesia (except in laparoscopic approach) |
| • Resumption of oral intake within the first 12h |
| • Respiratory incentive |
| • Early mobilisation |
| • Daily control of stoma by stomatherapist |
MMRP, multimodal rehabilitation programme.
Prospective general data on demographics, comorbidities, tumour-related variables, operating parameters, morbidity and length of stay were obtained. Such data were collected and analysed using the statistical programme SPSS (version 20) for Windows (SPSS, Inc., Chicago, IL, U.S.A.). The statistical analysis was performed using the Student's t-test for independent data or Mann–Whitney U test for numerical variables, as appropriate, and the chi-square test or the Fisher's exact test for qualitative variables. A P-value<.05 was considered statistically significant.
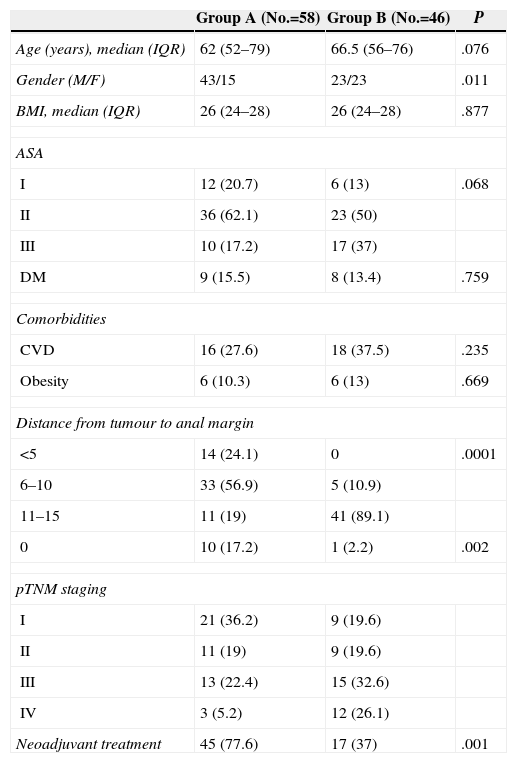
ResultsA total of 104 patients were included, 66 (66.3%) men and 38 (37%) women, with a median age of 64 (IQR: 55–75) years. We performed an associated ileostomy in 58 (65%) cases (Table 2). There were significantly more men in group A and in this group anastomosis was significantly lower. There were more patients with metastatic disease in the group without stoma (B), and this group also received less neoadjuvant therapy.
Characteristics of Patients and Tumours.
| Group A (No.=58) | Group B (No.=46) | P | |
|---|---|---|---|
| Age (years), median (IQR) | 62 (52–79) | 66.5 (56–76) | .076 |
| Gender (M/F) | 43/15 | 23/23 | .011 |
| BMI, median (IQR) | 26 (24–28) | 26 (24–28) | .877 |
| ASA | |||
| I | 12 (20.7) | 6 (13) | .068 |
| II | 36 (62.1) | 23 (50) | |
| III | 10 (17.2) | 17 (37) | |
| DM | 9 (15.5) | 8 (13.4) | .759 |
| Comorbidities | |||
| CVD | 16 (27.6) | 18 (37.5) | .235 |
| Obesity | 6 (10.3) | 6 (13) | .669 |
| Distance from tumour to anal margin | |||
| <5 | 14 (24.1) | 0 | .0001 |
| 6–10 | 33 (56.9) | 5 (10.9) | |
| 11–15 | 11 (19) | 41 (89.1) | |
| 0 | 10 (17.2) | 1 (2.2) | .002 |
| pTNM staging | |||
| I | 21 (36.2) | 9 (19.6) | |
| II | 11 (19) | 9 (19.6) | |
| III | 13 (22.4) | 15 (32.6) | |
| IV | 3 (5.2) | 12 (26.1) | |
| Neoadjuvant treatment | 45 (77.6) | 17 (37) | .001 |
Data are expressed in numbers with percentages between brackets, except as indicated.
BMI, body mass index; CVD, cardiovascular diseases; DM, diabetes mellitus; F, female; IQR, interquartile range; M, male.
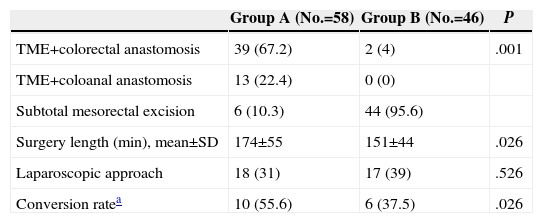
TME was performed in 52 (89.6%) patients in group A vs 2 (4.3%) in group B; P<.001. A laparoscopic approach was performed in 35 (34%) patients, with no differences among groups in relation to the conversion rate. Procedure duration was slightly shorter in the group without diverting ileostomy (Table 3).
Surgical Variables.
| Group A (No.=58) | Group B (No.=46) | P | |
|---|---|---|---|
| TME+colorectal anastomosis | 39 (67.2) | 2 (4) | .001 |
| TME+coloanal anastomosis | 13 (22.4) | 0 (0) | |
| Subtotal mesorectal excision | 6 (10.3) | 44 (95.6) | |
| Surgery length (min), mean±SD | 174±55 | 151±44 | .026 |
| Laparoscopic approach | 18 (31) | 17 (39) | .526 |
| Conversion ratea | 10 (55.6) | 6 (37.5) | .026 |
Data are expressed in numbers with percentages between brackets.
SD, standard deviation; TME, total mesorectal excision.
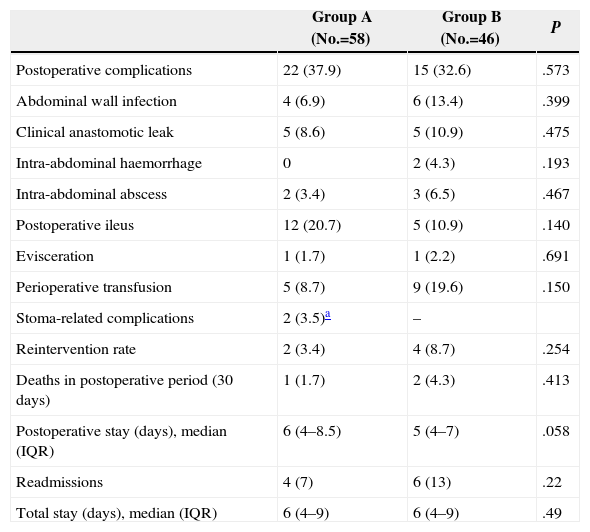
No statistically significant differences were seen among groups regarding perioperative mortality, overall complications, anastomotic leak rate, intra-abdominal or abdominal wall infections, postoperative ileus or further surgery. Median postoperative stay was six days in group A compared to five in group B, with no significant differences (Table 4). There were also no significant differences in relation to the readmission rate or overall postoperative stay, including such readmissions. The most common causes of readmission were pelvic abscesses, intestinal obstruction and abdominal wall infection.
Perioperative Outcomes.
| Group A (No.=58) | Group B (No.=46) | P | |
|---|---|---|---|
| Postoperative complications | 22 (37.9) | 15 (32.6) | .573 |
| Abdominal wall infection | 4 (6.9) | 6 (13.4) | .399 |
| Clinical anastomotic leak | 5 (8.6) | 5 (10.9) | .475 |
| Intra-abdominal haemorrhage | 0 | 2 (4.3) | .193 |
| Intra-abdominal abscess | 2 (3.4) | 3 (6.5) | .467 |
| Postoperative ileus | 12 (20.7) | 5 (10.9) | .140 |
| Evisceration | 1 (1.7) | 1 (2.2) | .691 |
| Perioperative transfusion | 5 (8.7) | 9 (19.6) | .150 |
| Stoma-related complications | 2 (3.5)a | – | |
| Reintervention rate | 2 (3.4) | 4 (8.7) | .254 |
| Deaths in postoperative period (30 days) | 1 (1.7) | 2 (4.3) | .413 |
| Postoperative stay (days), median (IQR) | 6 (4–8.5) | 5 (4–7) | .058 |
| Readmissions | 4 (7) | 6 (13) | .22 |
| Total stay (days), median (IQR) | 6 (4–9) | 6 (4–9) | .49 |
Data are expressed in numbers with percentages between brackets.
IQR, interquartile range.
Encouraged perioperative care, MMRP or fast-track programmes, is a set of measures aimed at reducing morbidity and hospital stay.7 In fact, they may notably improve outcomes, thus shaking up many attitudes based on surgical tradition.8–10
Colorectal surgery has been one of the surgical areas where this kind of care has been implemented, due to its prevalence, repercussion on costs and stay, and the potential ability to reduce complications in this field.11–13 In fact, rectal cancer surgery is also the model of change due to both training and specialisation of surgeons and the importance of a multidisciplinary team during treatment.14
The risk of anastomotic leak and fistula is known in cases where the colorectal anastomosis is more distal; for such reason, there is a body of doctrine that supports protection of these anastomosis with a diverting stoma, which does not reduce leak rate, but decreases its associated morbidity.2–4 However, performing a stoma is not exempt from specific complications that may increase hospital stay and overall complications.5
However, we have the working hypothesis that with the association of a MMRP programme, and in the setting of a multidisciplinary team composed of especially devoted surgeons and stomatherapists, morbidity and hospital stay are not influenced by surgery including a stoma.
In order to analyse this, we studied two cohorts of patients, obtained from a prospective database, who underwent anterior rectal resection and anastomosis performed by surgeons specialised in colorectal surgery. In patients of the first group, a diverting ileostomy was performed and in the second group, it was not. This implied a bias inherent to the higher potential severity of surgery in patients of the first group, given that, per protocol, a stoma was associated in the cases where a TME was performed, and thus, a lower anastomosis, and also when there were doubts about integrity if mesorectal excision was subtotal; but this group of patients also received neoadjuvant chemoradiotherapy in a much higher rate, since one of the criteria to administer it is that the location of the tumour should be below the peritoneal reflection. However, the fact that there were no differences in the surgical risk (ASA) between both groups, regarding body mass index or the percentage of laparoscopic approach, makes the samples comparable.
Cartmell et al.5 were not the only one who showed an increase in length of stay using a diverting stoma. In 2006, King et al. compared open colorectal surgery to laparoscopic surgery and the impact of the creation of a stoma on hospital stay, concluding that it extended the postoperative period by three to four days, regardless of the technique used or whether a MMRP protocol was used or not, in spite of the fact that this paper did not specifically analyse patients with rectal cancer. Both in our study and in the one recently published by Wignett et al.16 the use of a stoma did not influence the length of stay or complications, readmissions or anastomotic leak rates. Perhaps, the combination of MMRP with specific and continued care by the stomatherapist as it happens in our practice may lead to a reduced stay in this group as reported by the Cleveland Clinic17 or more recently by Younis et al.18 concluding that the delay in hospital discharge secondary to stoma management may be significantly reduced through a MMRP. Chaudhri et al. also compared conventional information to preoperative and postoperative specific information regarding the stoma provided by a specialised professional and concluded that it reduced length of stay by two days.19
Readmission rate has been a topic under debate, given that it could be increased by early discharge. However, both several systematic reviews8–12 and in our own study, no differences were seen among groups regarding this topic, even though there is a bias of higher potential risk of anastomotic leak in the group subject to ileostomy since they are patients more frequently subjected to chemoradiotherapy and since it necessarily involves lower anastomosis.2–4,20 Evidently, patients with stoma shall be readmitted later for its closure, which causes an additional hospital stay, particular risks and additional costs. However, a decrease in the severity of anastomotic complications compensates for these disadvantages.21 Thus, only 3.4% of patients in the group with stoma required further surgery in the postoperative period compared to 8.7% of patients who did not have an ileostomy.
Our mortality rate, anastomotic leaks and overall mortality are similar to the ones stated in other literature series15–17,22–25 and the current use of fast-track protocols is more than justified, taking into account the clinical advantages offered and, as evidenced by our series, the overall stay of patients, with a median of six days, which is practically half of that shown in a recent study conducted by the Asociación Española de Cirujanos26 [Spanish Association of Surgeons].
In conclusion, the addition of a protective stoma in the cases of colorectal anastomosis carrying the highest risk does not involve an increase in operative stay or complications when it is performed in the setting of a MMRP.
Conflicts of InterestThe authors declare that they do not have any conflicts of interest.
Please cite this article as: Gumbau V, García-Armengol J, Salvador-Martínez A, Ivorra P, García-Coret MJ, García-Rodríguez V, et al. Impacto del estoma derivativo en un protocolo de rehabilitación multimodal en cirugía de recto. Cir Esp. 2015;93:18–22.