The laparoscopic approach in colorectal complications is controversial because of its difficulty. However, it has been proven that it can provide advantages over open surgery. The aim of this study is to compare laparoscopic approach in reoperations for complications after colorectal surgery with the open approach taking into account the severity of the patient prior to reoperation.
MethodsPatients who underwent laparoscopic colorectal surgery from January 2006 to December 2015 were retrospectively reviewed. Patients requiring urgent surgical procedures for complications in the postoperative period were divided in two groups: laparoscopic surgery (LS) and open surgery (OS). To control clinical severity prior to reoperation, The Mannheim Peritonitis Index (MPI) was calculated.
ResultsA total of 763 patients were studied, 40 required urgent surgery (24 OS/16 LS). More ileostomies were performed in the LS group (68.7% vs 29.2%) and more colostomies in the OS group (37.5% vs 6.2%), P<.05. MPI was higher in OS group (27.31±6.47 [19–35] vs 18.36±7.16 [11–24], P<.001). Hospital stay after re-intervention, oral tolerance and surgical wound infection, were favorable in LS (P<.05 in all cases). In patients with MPI score ≤26, laparoscopic approach showed shorter hospital stay after re-intervention, less stay in the critical care unit after re-intervention, earlier start of oral tolerance and less surgical wound infection (P<.05).
ConclusionsA laparoscopic approach in re-intervention for complications after laparoscopic colorectal surgery associates a faster recovery reflected in a shorter hospital stay, earlier start of oral tolerance and a lower abdominal wall complication rate in patients with low severity index.
El abordaje laparoscópico en la cirugía por complicaciones colorrectales es controvertido. Sin embargo, puede proporcionar ventajas sobre la cirugía abierta. El objetivo del estudio es comparar el abordaje laparoscópico vs el abordaje abierto en la reintervención por complicaciones tras cirugía colorrectal.
MétodosSe han analizado de forma retrospectiva, sobre una base de datos prospectiva, los pacientes intervenidos mediante cirugía laparoscópica colorrectal desde enero de 2006 a diciembre de 2015. Los pacientes que requirieron reintervenciones urgentes por complicaciones en el postoperatorio se dividieron según el abordaje (cirugía laparoscópica [CL] y cirugía abierta [CA]) y según su gravedad clínica (en función del índice de peritonitis de Mannheim [IPM]).
ResultadosDe 763 pacientes, 40 requirieron cirugía urgente (24 CA/16 CL). Se realizaron más ileostomías en el grupo CL (68,7% vs 29,2%) y más colostomías en el grupo CA (37,5% vs 6,2%), p<0,05. El IPM fue mayor en el grupo CA (27,31±6,47 [19-35] vs 18,4±7,2 [11-24], p<0,001). La estancia hospitalaria tras la reintervención, tolerancia oral e infección de herida quirúrgica fueron favorables en CL (p<0,05). En pacientes con un IPM≤26, el abordaje laparoscópico mostró menor estancia hospitalaria, menor permanencia en unidad de críticos, tolerancia oral más temprana y menor infección de herida quirúrgica (p<0,05).
ConclusionesEl abordaje laparoscópico en la reintervención por complicaciones tras cirugía colorrectal laparoscópica asocia una recuperación más rápida objetivada en un inicio precoz de tolerancia oral, menor estancia hospitalaria y menor tasa de hernia incisional en pacientes con bajo índice de gravedad.
Laparoscopic approaches have demonstrated oncological and functional results similar to open procedures, while providing clear advantages in terms of postoperative recovery in comparison.1–8 The short- and long-term benefits of these minimally invasive treatments have been described in a large number of publications: less intraoperative blood loss, less postoperative pain, earlier tolerance of oral intake, shorter hospital stay, faster return to daily activities, less infection of the surgical site and lower rates of incisional hernia.2,5,9,10
Laparoscopy has become the standard treatment for colorectal surgery. However, the use of this approach to manage complications occurring after colorectal surgery, such as hemoperitoneum, intestinal obstruction or anastomotic leakage, remains controversial.11 Some studies highlight the difficulties that may arise during the use of laparoscopy to manage distended small bowel loops and the possibility of not achieving an optimal view of the abdominal cavity. Other arguments against the laparoscopic approach include the difficulties to find the point of bleeding in cases of hemoperitoneum or to perform an adequate abdominal lavage in cases of purulent or fecaloid peritonitis.12,13
The advantages of the laparoscopic approach over open surgery in reoperations after previous laparoscopic abdominal procedures, such as bariatric surgery, acute cholecystitis or acute appendicitis, have already been discussed in other studies.10,11,14,15 However, very little has been published on the usefulness of re-laparoscopy in the treatment of colorectal complications.16–19
One of the most important prognostic factors before reoperation is the patient's initial clinical situation.11,17,20 Classically, open surgery has been preferred by most surgeons in hemodynamically unstable patients or those with advanced peritonitis. However, there are reports that the laparoscopic approach can also provide benefits in the reoperation of this group of patients. The minimally invasive approach can also reduce wound infections, hospital stay and incisional hernia rates in this population group.13,21
The objective of this study is to compare the safety and viability of the laparoscopic approach compared to open surgery for reoperation due to complications after colorectal surgery, while taking into account the patient severity.
MethodsStudy design. All patients who had undergone scheduled laparoscopic colorectal surgery between January 2006 and December 2015 at the Virgen de la Arrixaca University Hospital (Murcia, Spain) were included in a prospective database for retrospective analysis.
Exclusion criteria included the conversion to open surgery and the performance of any other non-laparoscopic procedures, such as extracorporeal anastomosis. Out of a total of 763 patients with an entirely laparoscopic approach, patients whose complications were treated with radiology-guided percutaneous drainage or transanal repair were excluded. In the end, a sample of 40 patients was obtained, divided into two groups: laparoscopic surgery (LS) and open surgery (OS). The decision of the approach used in each case was left to the discretion of the surgeon.
Surgical technique. The initial surgery in all cases was a scheduled laparoscopic colorectal procedure performed by the same surgical team of colorectal surgeons with extensive experience in laparoscopic techniques. The reoperations were performed urgently by the same team following the same criteria. For the laparoscopic approach, the patient was placed in the Lloyd-Davies position. The trocars were introduced through the previous insertion ports. The first trocar was introduced using the periumbilical port under direct vision. In the open procedures, the patient's position was the same, performing midline laparotomy. Closure of the abdominal wall was performed with continuous polydioxanone suture (PDS®).
In the cases of anastomotic dehiscence, according to the intraoperative findings and the clinical severity of the patient, primary repair or anastomotic resection and new anastomosis were performed. The creation of a diverting stoma was left to the discretion of the surgeon. In the cases of fecal peritonitis due to dehiscence of the colorectal anastomosis, a Hartmann procedure was done. In all patients, peritoneal lavage was conducted with at least 3L of saline solution. A drainage catheter was placed adjacent to the anastomosis.
In the case of hemoperitoneum, peritoneal lavage was performed until adequate vision was achieved of the entire abdominal cavity. Hemostasis was performed with clips, sutures or bipolar coagulation. In these cases, a drain tube was placed in the pelvis.
Study variables. We analyzed the demographic and baseline variables related to the first surgery: age (years), sex, body mass index (BMI), classification of the American Society of Anesthesiologists (ASA), surgery and previous comorbidities, etiology (benign or malignant) and type of intervention. Regarding the surgical reoperation, we evaluated the interval until reoperation (time in days from scheduled surgery to reoperation), severity of organ failure before reoperation, number of transfusions and operative time. After the reoperation, data were collected for total hospital stay, intensive care unit stay, initiation of oral intake, abdominal wall complications, additional procedures required (radiological, surgical invasive technique or both), death and complications during follow-up.
To establish comparable groups in terms of clinical severity prior to reoperation, the Mannheim peritonitis index (MPI) was calculated.20 This index predicts mortality rates due to peritonitis by globally evaluating different patient parameters (including age, sex, existence of organ failure, neoplastic origin, existence of sepsis originating in the colon and duration of peritonitis) and intraoperative data, such as type and extension of peritonitis, providing an overview of the patient's state of severity as well as origin and duration. Patients with an MPI score equal to or less than 26 were analyzed (cut-off point predicted as a mortality predictor). No patients with MPI greater than 26 were analyzed because of the small sample size.
Statistical AnalysisThe data was analyzed using SPSS Statistics 22.0® (IBM systems, Chicago, USA). For the comparative analysis, the Student's t test was used for quantitative variables or the Mann–Whitney U when necessary. Chi-squared and Fisher's exact tests were used for categorical variables. Patients who required conversion from laparoscopic to open surgery were included in the analysis with their original (laparoscopic) group, conducting an “intention to treat” analysis even though it was a non-randomized study. A P level <.05 was considered statistically significant. For the data analysis, informed consent was obtained from patients, and hospital confidentiality guidelines were followed during patient treatment.
ResultsA total of 763 patients underwent laparoscopic colorectal surgery, 40 of whom (5.2%) required urgent surgery due to complications (24 OS and 16 LS). Two patients were converted to laparotomy (12.5%) because of small bowel loop dilatation.
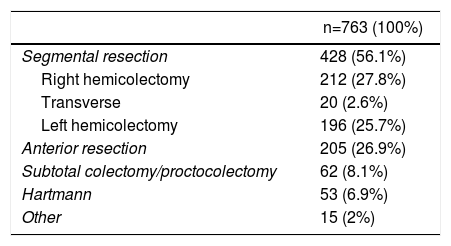
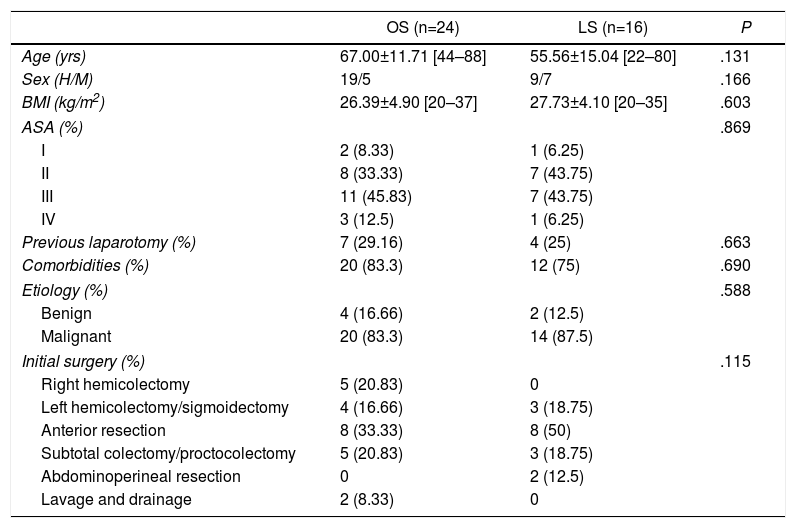
Tables 1 and 2 show the surgical procedures performed and the patients’ baseline variables. No differences were found between the two groups.
Surgeries and Indications.
| n=763 (100%) | |
|---|---|
| Segmental resection | 428 (56.1%) |
| Right hemicolectomy | 212 (27.8%) |
| Transverse | 20 (2.6%) |
| Left hemicolectomy | 196 (25.7%) |
| Anterior resection | 205 (26.9%) |
| Subtotal colectomy/proctocolectomy | 62 (8.1%) |
| Hartmann | 53 (6.9%) |
| Other | 15 (2%) |
| n=763 (100%) | |
|---|---|
| Colon cancer | 390 (51.1%) |
| Rectal cancer | 241 (31.6%) |
| Inflammatory disease | 73 (9.6%) |
| Familial adenomatous polyposis | 38 (4.9%) |
| Other | 21 (2.8%) |
Baseline and Demographic Variables.
| OS (n=24) | LS (n=16) | P | |
|---|---|---|---|
| Age (yrs) | 67.00±11.71 [44–88] | 55.56±15.04 [22–80] | .131 |
| Sex (H/M) | 19/5 | 9/7 | .166 |
| BMI (kg/m2) | 26.39±4.90 [20–37] | 27.73±4.10 [20–35] | .603 |
| ASA (%) | .869 | ||
| I | 2 (8.33) | 1 (6.25) | |
| II | 8 (33.33) | 7 (43.75) | |
| III | 11 (45.83) | 7 (43.75) | |
| IV | 3 (12.5) | 1 (6.25) | |
| Previous laparotomy (%) | 7 (29.16) | 4 (25) | .663 |
| Comorbidities (%) | 20 (83.3) | 12 (75) | .690 |
| Etiology (%) | .588 | ||
| Benign | 4 (16.66) | 2 (12.5) | |
| Malignant | 20 (83.3) | 14 (87.5) | |
| Initial surgery (%) | .115 | ||
| Right hemicolectomy | 5 (20.83) | 0 | |
| Left hemicolectomy/sigmoidectomy | 4 (16.66) | 3 (18.75) | |
| Anterior resection | 8 (33.33) | 8 (50) | |
| Subtotal colectomy/proctocolectomy | 5 (20.83) | 3 (18.75) | |
| Abdominoperineal resection | 0 | 2 (12.5) | |
| Lavage and drainage | 2 (8.33) | 0 | |
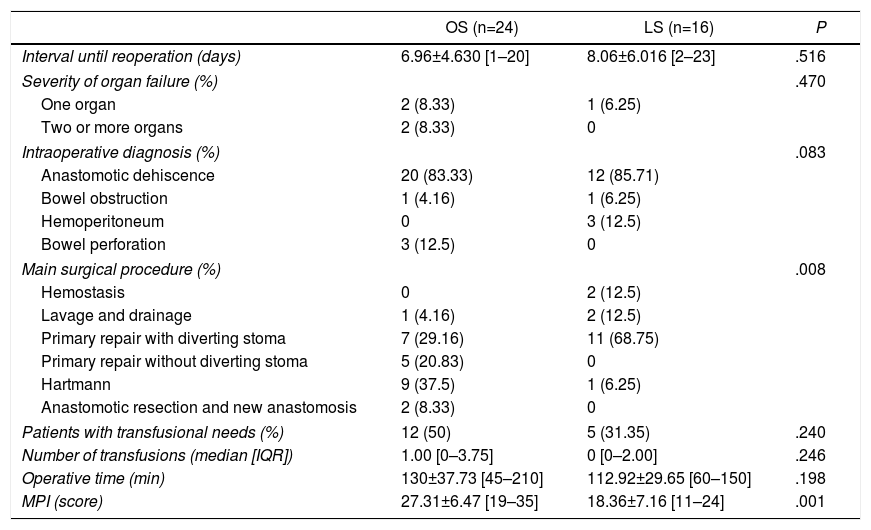
According to the results after reoperation (Table 3), although no differences were found in the intraoperative diagnosis between both groups (P=.08), there were differences in the procedures performed (P=.008). In cases of anastomotic leak, more patients underwent primary anastomosis repair without a diverting stoma in the OS group (20.83%). In addition, in this same group, more Hartmann procedures were performed (9 versus 1 in LS). The operative time was similar in both groups (130.00±37.73 [45–210] minutes in OS compared to 112.92±29.25 [60–150] minutes in LS, P=.19). When the severity of the patients was analyzed, the MPI was different in both groups (P=.001) and the patients in the OS group had a higher score.
Results During Reoperation.
| OS (n=24) | LS (n=16) | P | |
|---|---|---|---|
| Interval until reoperation (days) | 6.96±4.630 [1–20] | 8.06±6.016 [2–23] | .516 |
| Severity of organ failure (%) | .470 | ||
| One organ | 2 (8.33) | 1 (6.25) | |
| Two or more organs | 2 (8.33) | 0 | |
| Intraoperative diagnosis (%) | .083 | ||
| Anastomotic dehiscence | 20 (83.33) | 12 (85.71) | |
| Bowel obstruction | 1 (4.16) | 1 (6.25) | |
| Hemoperitoneum | 0 | 3 (12.5) | |
| Bowel perforation | 3 (12.5) | 0 | |
| Main surgical procedure (%) | .008 | ||
| Hemostasis | 0 | 2 (12.5) | |
| Lavage and drainage | 1 (4.16) | 2 (12.5) | |
| Primary repair with diverting stoma | 7 (29.16) | 11 (68.75) | |
| Primary repair without diverting stoma | 5 (20.83) | 0 | |
| Hartmann | 9 (37.5) | 1 (6.25) | |
| Anastomotic resection and new anastomosis | 2 (8.33) | 0 | |
| Patients with transfusional needs (%) | 12 (50) | 5 (31.35) | .240 |
| Number of transfusions (median [IQR]) | 1.00 [0–3.75] | 0 [0–2.00] | .246 |
| Operative time (min) | 130±37.73 [45–210] | 112.92±29.65 [60–150] | .198 |
| MPI (score) | 27.31±6.47 [19–35] | 18.36±7.16 [11–24] | .001 |
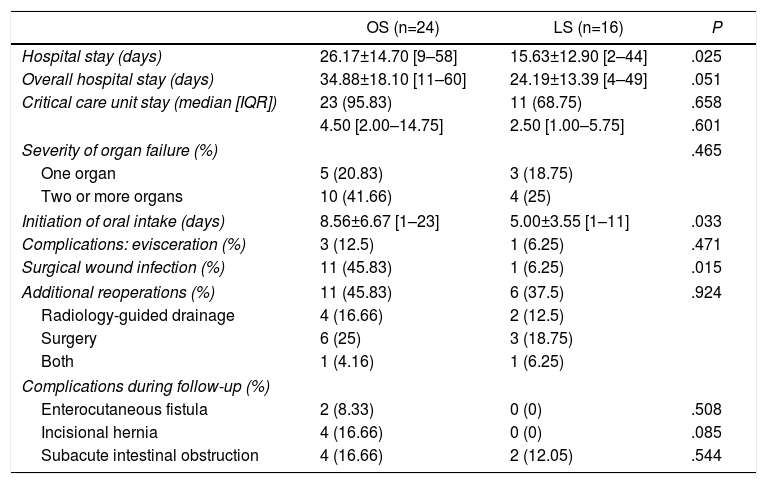
Regarding postoperative results (Table 4), patients in the LS group had a shorter mean hospital stay after reoperation (26.27±14.70 [9–58] days in the OS group versus 15.63±12.90 [2–44] days in LS, P=.02), shorter overall hospital stay (34.88±18.10 [11–60] days in the OS group vs 24.19±13.39 [4–49] days in the LS group, P=.05), less time transpired until the initiation of oral intake (8.56±6.67 [1–23] days in OS versus 5.00±3.55 [1–11] days in the LS group, P=.03), and lower rates of surgical wound infection (45.83% in OS vs 6.25% in LS, P=.01). One patient in the LS group with conversion to laparotomy had a severe surgical wound infection with evisceration and need for reoperation.
Postoperative Results.
| OS (n=24) | LS (n=16) | P | |
|---|---|---|---|
| Hospital stay (days) | 26.17±14.70 [9–58] | 15.63±12.90 [2–44] | .025 |
| Overall hospital stay (days) | 34.88±18.10 [11–60] | 24.19±13.39 [4–49] | .051 |
| Critical care unit stay (median [IQR]) | 23 (95.83) | 11 (68.75) | .658 |
| 4.50 [2.00–14.75] | 2.50 [1.00–5.75] | .601 | |
| Severity of organ failure (%) | .465 | ||
| One organ | 5 (20.83) | 3 (18.75) | |
| Two or more organs | 10 (41.66) | 4 (25) | |
| Initiation of oral intake (days) | 8.56±6.67 [1–23] | 5.00±3.55 [1–11] | .033 |
| Complications: evisceration (%) | 3 (12.5) | 1 (6.25) | .471 |
| Surgical wound infection (%) | 11 (45.83) | 1 (6.25) | .015 |
| Additional reoperations (%) | 11 (45.83) | 6 (37.5) | .924 |
| Radiology-guided drainage | 4 (16.66) | 2 (12.5) | |
| Surgery | 6 (25) | 3 (18.75) | |
| Both | 1 (4.16) | 1 (6.25) | |
| Complications during follow-up (%) | |||
| Enterocutaneous fistula | 2 (8.33) | 0 (0) | .508 |
| Incisional hernia | 4 (16.66) | 0 (0) | .085 |
| Subacute intestinal obstruction | 4 (16.66) | 2 (12.05) | .544 |
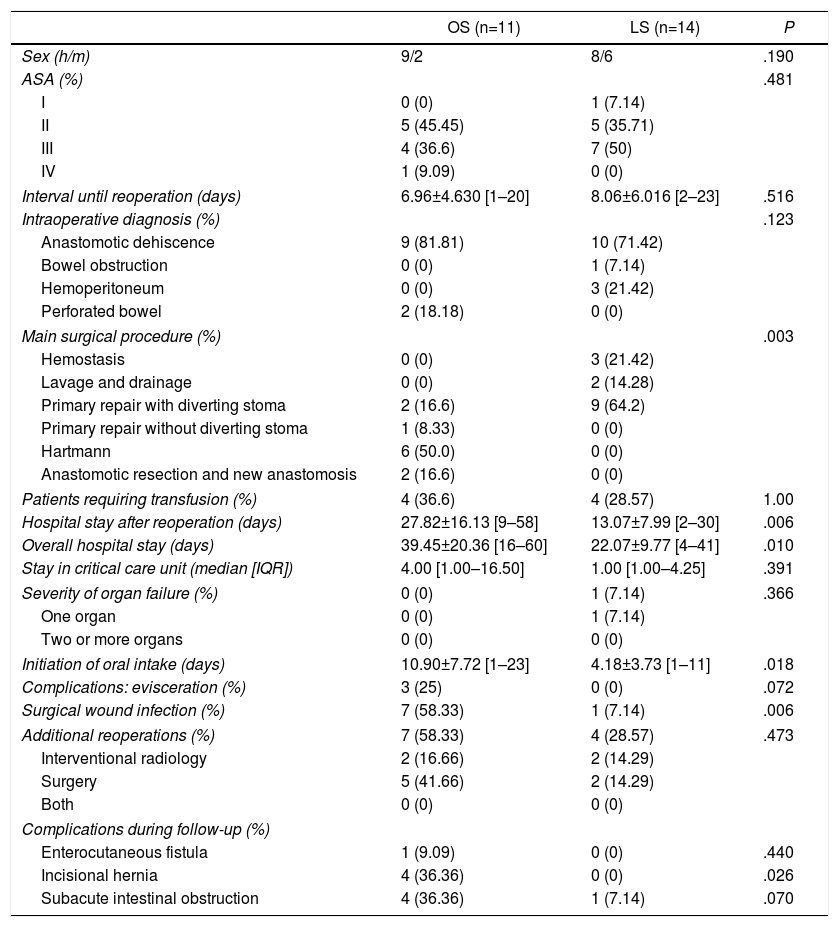
Table 5 shows the results of patients with an MPI score equal to or less than 26. No significant differences were found in the baseline variables. Two patients in the LS group (14.28%) were converted to open surgery due to adhesions and dilated intestinal loops. As in the first group, patients with MPI <26 and the laparoscopic approach presented shorter hospital stay after reoperation, shorter general hospital stay, earlier initiation of oral intake and lower rates of wound infection (P=.006, P=.01, P=.01 and P=.006, respectively). Although none of the patients in the LS group presented abdominal wall complications, no differences were found in the evisceration rates between the two groups. One patient of the LS group died. The mean follow-up of both groups was of 35.91±12.91 [12–62] months in OS versus 31.00±27.21 [2–84] months in the LS group, P=.59.
Postoperative Results in Patients With MPI Lower Than 26.
| OS (n=11) | LS (n=14) | P | |
|---|---|---|---|
| Sex (h/m) | 9/2 | 8/6 | .190 |
| ASA (%) | .481 | ||
| I | 0 (0) | 1 (7.14) | |
| II | 5 (45.45) | 5 (35.71) | |
| III | 4 (36.6) | 7 (50) | |
| IV | 1 (9.09) | 0 (0) | |
| Interval until reoperation (days) | 6.96±4.630 [1–20] | 8.06±6.016 [2–23] | .516 |
| Intraoperative diagnosis (%) | .123 | ||
| Anastomotic dehiscence | 9 (81.81) | 10 (71.42) | |
| Bowel obstruction | 0 (0) | 1 (7.14) | |
| Hemoperitoneum | 0 (0) | 3 (21.42) | |
| Perforated bowel | 2 (18.18) | 0 (0) | |
| Main surgical procedure (%) | .003 | ||
| Hemostasis | 0 (0) | 3 (21.42) | |
| Lavage and drainage | 0 (0) | 2 (14.28) | |
| Primary repair with diverting stoma | 2 (16.6) | 9 (64.2) | |
| Primary repair without diverting stoma | 1 (8.33) | 0 (0) | |
| Hartmann | 6 (50.0) | 0 (0) | |
| Anastomotic resection and new anastomosis | 2 (16.6) | 0 (0) | |
| Patients requiring transfusion (%) | 4 (36.6) | 4 (28.57) | 1.00 |
| Hospital stay after reoperation (days) | 27.82±16.13 [9–58] | 13.07±7.99 [2–30] | .006 |
| Overall hospital stay (days) | 39.45±20.36 [16–60] | 22.07±9.77 [4–41] | .010 |
| Stay in critical care unit (median [IQR]) | 4.00 [1.00–16.50] | 1.00 [1.00–4.25] | .391 |
| Severity of organ failure (%) | 0 (0) | 1 (7.14) | .366 |
| One organ | 0 (0) | 1 (7.14) | |
| Two or more organs | 0 (0) | 0 (0) | |
| Initiation of oral intake (days) | 10.90±7.72 [1–23] | 4.18±3.73 [1–11] | .018 |
| Complications: evisceration (%) | 3 (25) | 0 (0) | .072 |
| Surgical wound infection (%) | 7 (58.33) | 1 (7.14) | .006 |
| Additional reoperations (%) | 7 (58.33) | 4 (28.57) | .473 |
| Interventional radiology | 2 (16.66) | 2 (14.29) | |
| Surgery | 5 (41.66) | 2 (14.29) | |
| Both | 0 (0) | 0 (0) | |
| Complications during follow-up (%) | |||
| Enterocutaneous fistula | 1 (9.09) | 0 (0) | .440 |
| Incisional hernia | 4 (36.36) | 0 (0) | .026 |
| Subacute intestinal obstruction | 4 (36.36) | 1 (7.14) | .070 |
Laparoscopic colorectal surgery is a routine practice for many surgeons. However, the approach currently recommended for reoperation is open surgery due to the scarce quantity and quality of scientific evidence available in favor of laparoscopy.13,17,18 Technological advances as well as the increasing experience with this approach may tempt some surgeons to perform this type of reoperations laparoscopically. In urgent surgery, either due to the clinical severity of the patient or to the anatomical anomalies characteristic of the peritonitic or hemorrhagic abdomen, the laparoscopic approach can be very difficult and technically demanding. Although some studies have questioned the impact of pneumoperitoneum on a septic abdomen, arguing that an increase in intra-abdominal pressure could favor the onset of endotoxemia,22,23 recent studies have shown that the laparoscopic approach is safe in cases of peritonitis24 and can even reduce the potential trauma and systemic stress caused by open surgery.21–25 In 2006, the European Association for Endoscopic Surgery concluded that laparoscopy in emergency surgery and in expert hands can reach diagnostic and therapeutic levels over 90%.11
The most frequent cause of reoperation in colorectal surgery is anastomotic leak, with an incidence of 1%–30% according to different series and optimal values from 2% to 5%.26,27 In our study, the overall reoperation rate was 5.2% and 4.6% due to anastomotic dehiscence (n=35). Similar to Kwak et al.,28 in our study the LS group showed a lower incidence of wound infection and incisional hernia. This supports our theory that laparoscopic reoperation allows the patient to enjoy the benefits of a minimally invasive approach13 despite needing a second intervention.
In the case of anastomotic dehiscence, regardless of the group and the procedure, bypass surgery with a diverting stoma is an accepted option in patients with dehiscence of less than 50% of the circumference and absence of diffuse peritonitis due to its lower morbidity and mortality.19,29–31 In our study, the LS group had a higher percentage of diverting ileostomy.
The repair of the anastomosis with peritoneal cavity lavage was the most frequent procedure in our series (49.99% in the OS group versus 68.75% in the LS group). In the cases of diverting ileostomy, this was performed through one of the trocars used during the reoperation. The advantages of managing anastomotic dehiscence with the laparoscopic approach have already been described by numerous authors.13,19,32 In our case, this strategy was successful in 14 out of 16 patients (87.5%), which is similar to previous studies.32
However, our conversion rate is twice that of other studies, such as the Cuccurrullo et al. (6%) study.33 This may be due to the fact that, in our opinion, early conversion is an appropriate decision when there is a possible risk for causing iatrogenic injuries. In our series, two cases were converted to open surgery due to difficulty involved in the management of dilated small bowel loops, which is, in our opinion, one of the main disadvantages of this approach.
Regarding the evaluation of the patient's clinical status before the reoperation, Vennix et al.17 has indicated that there is a lack of such data in the series published and warn of probable selection bias in the surgical approach. In their article, they suggest applying a scoring system for the severity of peritonitis to control homogeneity between groups. In the study published recently by Lee et al.,18 the preoperative clinical differences in their patients were not analyzed, nor was the presence of purulent or fecaloid peritonitis reported; we believe that these data have an important prognostic value, which could significantly influence their conclusions.20,34,35
In the first analysis of our study, the type of approach is clearly influenced by the patient's clinical severity. In these circumstances, laparotomy is preferred, as in previous studies.13,16,17 We are aware that, in view of the results, there is selection bias in the choice of approach due to clinical severity. Therefore, to determine whether clinical severity could be a confounding factor in our study, homogenous groups were established and a new analysis was conducted.
Although there are many scales to predict the severity of patients and the risk of postoperative death,36 we chose MPI because it takes into account indicators such as the origin and extension of the peritonitis, is easy to apply and has been validated previously.37,38 Significant differences have been found in favor of the LS group, confirming its advantages as indicated by Vennix et al.17 On the other hand, their conclusions support our results, as they found statistically significant differences in the degree of peritoneal contamination, with a higher percentage of fecaloid peritonitis in the OS group, which is an indirect high severity indicator of great importance in the MPI scale (12 points). Therefore, patients in the OS group will have a worse prognosis, as described with the MPI results of our series, with higher rates of postoperative morbidity and mortality regardless of the surgical approach.
In the second analysis, a cut-off point of 26 points was used in the MPI following the study by Biondo et al.,34 in which this score was set as a predictor for low mortality risk. Taking into account only patients with MPI≤26, this study attempted to provide a comparison between homogeneous groups in terms of clinical severity. Once again, our results ratify those of previous studies,13,18,21 however, this time in patients with a similar state of severity.
Although there are no statistically significant differences in the additional procedures that were required after the reoperation in the overall analysis and in the MPI group <26 points, it does highlight the fact that in both instances there is a greater need for additional procedures in the OS group. Even though in the overall analysis this can be considered due to the difference in the clinical severity of the patients between both groups, after adjusting for MPI <26 in homogeneous groups, we consider that it supports our hypothesis that the group reoperated by laparoscopic surgery presents a lower rate of postoperative complications.
However, we are aware of the small sample size, the absence of randomization and the retrospective nature of the study. Furthermore, our surgical team consists of surgeons with extensive experience in laparoscopic surgery, which we consider to be an important factor in this type of surgery since it can be very complex and technically demanding. Therefore, despite the favorable results for the LS group and the recent appearance of two systematic reviews39,40 on this subject whose conclusions are comparable to ours, we feel that prospective randomized studies should be conducted in order to consolidate these hypotheses since the majority of published studies to date have been descriptive and/or retrospective.
Finally, in our opinion, the laparoscopic approach is useful as a diagnostic-therapeutic tool for reoperation due to complications after laparoscopic colorectal surgery. This approach has a faster associated recovery time, resulting in shorter hospital stays, earlier initiation of oral intake and a lower rate of abdominal wall complications in patients with a low severity index. Despite this, these reoperations are technically demanding and early conversion should be considered to avoid iatrogenic injuries.
Authors’ ContributionData analysis and interpretation: Ibañez N., Sánchez P., Soriano M.T.
Composition: Sánchez P., Ibañez N, Abrisqueta J., Arevalo-Pérez J., Parrilla P.
Revision: Ibañez N., Abrisqueta J., Arevalo-Pérez J., Lujan J., Parrilla P.
Approval of the final version: Lujan J., Parrilla P.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Ibáñez N, Abrisqueta J, Luján J, Sánchez P, Soriano MT, Arevalo-Pérez J, et al. Reintervención tras complicaciones en cirugía laparoscópica colorrectal. ¿Aporta ventajas el abordaje laparoscópico? Cir Esp. 2018;96:109–116.