A controversial aspect of breast cancer management is the use of sentinel lymph node biopsy (SLNB) in patients requiring neoadjuvant chemotherapy (NCT). This paper discusses the detection rate (DT) and false negatives (FN) of SLNB after NCT to investigate the influence of initial nodal disease and the protocols applied.
MethodsProspective observational multicenter study in women with breast cancer, treated with NCT and SLNB post-NCT with subsequent lymphadenectomy. DT and FN rates were calculated, both overall and depending on the initial nodal status or the use of diagnostic protocols pre-SLNB.
ResultsNo differences in DT between initial node-negative cases and positive cases were found (89.8% vs 84.4%, P=.437). Significant differences were found (94.1% vs 56.5%, P=.002) in the negative predictive value, which was lower when there was initial lymph node positivity, and a higher rate of FN, not significant (18.2% vs 43.5%, P=.252) in the same cases. The axillary study before SLNB and after the NCT, significantly decreased the rate of FN in patients with initial involvement (55.6 vs 12.5, P=.009).
ConclusionsNCT means less DT and a higher rate of FN in subsequent SLNB, especially if there is initial nodal involvement. The use of protocols in axillary evaluation after administering the NCT and before BSGC decreases the FN rate in these patients.
La utilidad de la biopsia selectiva del ganglio centinela (BSGC) en pacientes con cáncer de mama que precisan quimioterapia neoadyuvante (QTN) es controvertida. Nuestro objetivo es analizar la tasa de detección (TD) y de falsos negativos (FN) de la BSGC tras QTN así como la influencia de la afectación ganglionar inicial y de los protocolos aplicados.
MétodosEstudio prospectivo observacional multicéntrico con mujeres con cáncer de mama tratadas con QTN y a las que se les realizó BSGC tras recibir la QTN y linfadenectomía posterior. Se calcularon las TD y las tasas de FN, tanto globales como dependientes de la afectación ganglionar inicial o del uso de protocolos de diagnóstico pre-BSGC.
ResultadosNo se demostraron diferencias en la TD entre los casos sin afectación ganglionar inicial y los que sí la tuvieron (89,8 vs. 84,4%; p=0,437). Sí se encontraron diferencias significativas (94,1 vs. 56,5%; p=0,002) en el valor predictivo negativo, menor cuando existía afectación ganglionar inicial, y mayor tasa de FN, aunque no de forma significativa (18,2 vs. 43,5%; p=0,252) en ese mismo supuesto. Un estudio de la axila antes de indicar la BSGC y tras la QTN disminuyó significativamente la tasa de FN en los casos en los que existía afectación inicial (55,6 vs. 12,5; p=0,009).
ConclusionesLa QTN da lugar a una menor TD y a una mayor tasa de FN en la BSGC posterior, sobre todo si hay afectación ganglionar inicial. Los protocolos para la evaluación axilar después de administrar la QTN y antes de la BSGC disminuyen la tasa de FN en estas pacientes.
In extension studies of breast cancer, sentinel lymph node biopsy (SLNB) is the current standard diagnostic technique used. Through this procedure, it is possible to demonstrate lymph node involvement and therefore avoid the morbidity associated with axillary lymphadenectomies, which used to be performed systematically. At present, even the possibility of not performing this type of lymphadenectomy in selected cases with limited lymph node involvement is being considered.1,2
However, in all these studies, it is considered contraindicated to perform an SLNB when primary systemic therapy (PST) has been administered. Chemotherapy has been seen as a factor that can sometimes interfere in the detection and correct identification of the sentinel node due to the changes that it produces in the structure of the lymphatic drainage system, in particular when it generates an effective response.3,4
Currently, one controversial aspect is whether the growing number of patients who are receiving PST would benefit from an SLNB and what moment would be best to perform the biopsy.5–8
This study has a double objective: (1) to analyse the detection rate (DR) and false negatives (FN) of SLNB performed after administration of neoadjuvant chemotherapy (NCT) and (2) to establish whether results are affected by the detection of initial nodal disease and by the application of care protocols.
Patients and MethodsProspective observational multicentric clinical study (GEICAM 2005–07) that included patients with invasive breast cancer for whom PST was indicated and on whom an SLNB was performed after administering PST, with subsequent axillary lymphadenectomy.
Patients with a history of previous axillary surgery, an inflammatory carcinoma or for whom SLNB was contraindicated were excluded from the study. Patients on whom, for whatever reason, the SLNB was not performed in the standard manner according to normal care protocols were also excluded. The study was approved by the Research Ethics Committee of the participating centres. All patients were informed and specific informed consent was obtained in order to include the patient in the study.
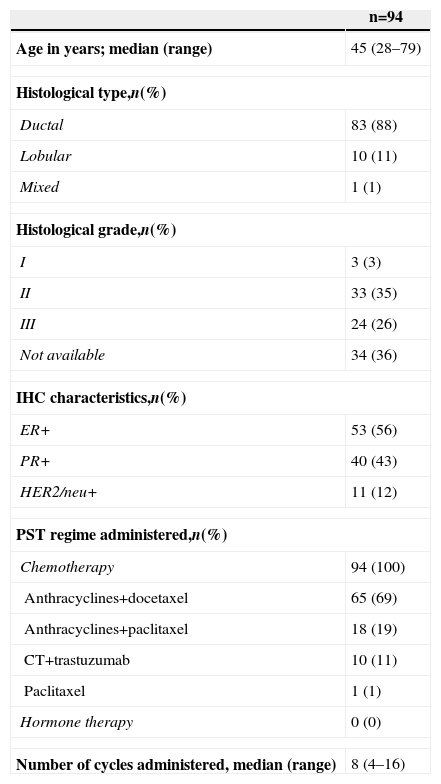
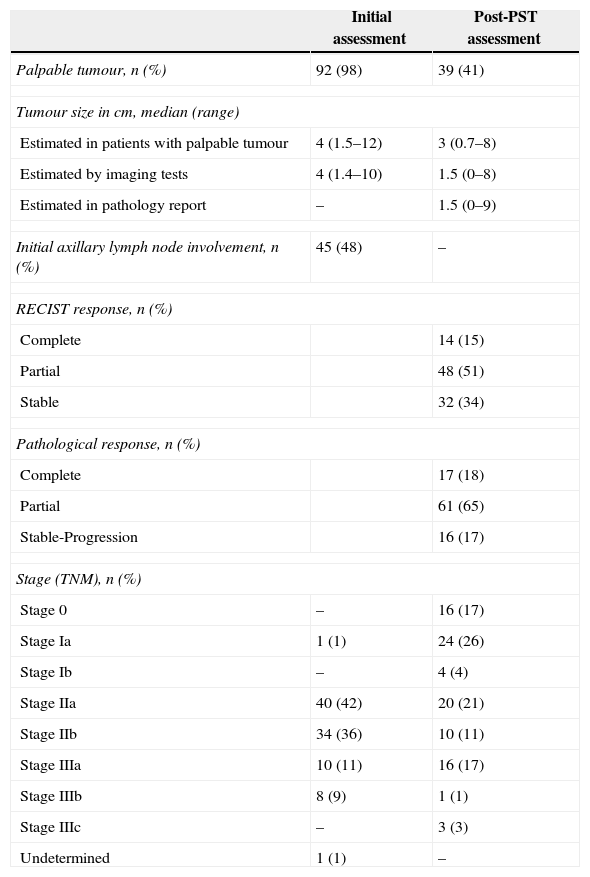
The general characteristics of the study are shown in Table 1, and Table 2 details the principal changes pre- and post-PST.
General Characteristics of the Study.
| n=94 | |
|---|---|
| Age in years; median (range) | 45 (28–79) |
| Histological type,n(%) | |
| Ductal | 83 (88) |
| Lobular | 10 (11) |
| Mixed | 1 (1) |
| Histological grade,n(%) | |
| I | 3 (3) |
| II | 33 (35) |
| III | 24 (26) |
| Not available | 34 (36) |
| IHC characteristics,n(%) | |
| ER+ | 53 (56) |
| PR+ | 40 (43) |
| HER2/neu+ | 11 (12) |
| PST regime administered,n(%) | |
| Chemotherapy | 94 (100) |
| Anthracyclines+docetaxel | 65 (69) |
| Anthracyclines+paclitaxel | 18 (19) |
| CT+trastuzumab | 10 (11) |
| Paclitaxel | 1 (1) |
| Hormone therapy | 0 (0) |
| Number of cycles administered, median (range) | 8 (4–16) |
IHC: immunohistochemistry; ER: oestrogen receptors; PR: progesterone receptors; PST: primary systemic therapy.
Characteristics Before and After Primary Systemic Therapy.
| Initial assessment | Post-PST assessment | |
|---|---|---|
| Palpable tumour, n (%) | 92 (98) | 39 (41) |
| Tumour size in cm, median (range) | ||
| Estimated in patients with palpable tumour | 4 (1.5–12) | 3 (0.7–8) |
| Estimated by imaging tests | 4 (1.4–10) | 1.5 (0–8) |
| Estimated in pathology report | – | 1.5 (0–9) |
| Initial axillary lymph node involvement, n (%) | 45 (48) | – |
| RECIST response, n (%) | ||
| Complete | 14 (15) | |
| Partial | 48 (51) | |
| Stable | 32 (34) | |
| Pathological response, n (%) | ||
| Complete | 17 (18) | |
| Partial | 61 (65) | |
| Stable-Progression | 16 (17) | |
| Stage (TNM), n (%) | ||
| Stage 0 | – | 16 (17) |
| Stage Ia | 1 (1) | 24 (26) |
| Stage Ib | – | 4 (4) |
| Stage IIa | 40 (42) | 20 (21) |
| Stage IIb | 34 (36) | 10 (11) |
| Stage IIIa | 10 (11) | 16 (17) |
| Stage IIIb | 8 (9) | 1 (1) |
| Stage IIIc | – | 3 (3) |
| Undetermined | 1 (1) | – |
PST: primary systemic therapy.
The pre-PST evaluation of the patients included an axillary assessment using clinical ultrasonography that was accompanied by fine needle aspiration (FNAB) when indicated in order to confirm the presence of nodal disease and its classification as N+pre-PST. Where these results were not found, patients were classified as N0 pre-PST. Health care centres were classified according to the type of treatment performed after PST, taking into account whether a specific protocol was applied that included a reassessment with ultrasonography, and with FNAB or core needle biopsy (CNB) where necessary, after delivering PST and before performing the SLNB.
The SLNB was performed in accordance with the methodology included in the protocols of the Spanish Society of Senology and Breast Pathology (Sociedad Española de Senología y Patología Mamaria).9 In all cases, an axillary lymphadenectomy was performed and both the number of isolated lymph nodes and the number of affected lymph nodes were recorded.
The detection rate (DR) or positive identification was considered when at least one sentinel node was detected during surgical intervention, either with an isotopic tracer or with a mixed method using an isotope and dye. If during the pre-operative scan or during surgery nothing was detected, it was considered to be a negative result.
Cases with positive SLNB and with involvement of non-sentinel nodes isolated during the lymphadenectomy were defined as true positives (TP), and cases with negative sentinel nodes without involvement of any of the nodes isolated during the lymphadenectomy were defined as true negatives (TN). Cases where the sentinel node was negative and yet involved non-sentinel nodes were identified during the lymphadenectomy were defined as false negatives (FN). The false negative rate (FNR) was calculated by dividing the number of false negatives by the sum of false negatives and true positives.
The global DR and FNR were studied, as well as those in cases that demonstrated initial lymph node involvement (cN+) and those that did not (cN0), and the DR and FNR of these groups were compared with one another.
An initially unplanned sub-analysis was carried out, which evaluated the health care centres according to the protocols that were applied before the SLNB was performed. A comparison was made between centres in which an axillary examination using ultrasonography was systematically performed by specialist radiologists dedicated exclusively to breast health after neoadjuvant chemotherapy was delivered and before the SLNB was performed, which was excluded in cases where there was manifest involvement, and health care centres in which this examination was not performed or the radiological examination was performed by non-specialist radiologists.
Statistical AnalysisA descriptive study was carried out of the demographic and anatomopathological variables and of the variables related to clinical and radiological evolution, both before and after PST. For the qualitative variables, the distribution of frequencies was calculated, whereas for the quantitative variables, measures of central tendency were used, such as the median and the mean. Both the point estimators of the DR, PPV, NPV and FNR and their confidence intervals (CI) at 95% were used. When dealing with small sample sizes, the CI was calculated using the formula proposed by Clopper and Pearson.10
The rates (DR, PPV, NPV, FNR) for the different subsets of patients were compared. To do so, either a chi-square test was used, or if the expected frequency was less than 5, Fisher's exact test was used.
The evaluation of the validity of classifications made using the sentinel node technique compared with those using a lymphadenectomy (the gold standard technique) was performed using ROC curves. To do so, the sensitivity, specificity and area under the curve were calculated. These calculations were carried out both globally and by separating cases with initial axillary involvement from those without.
The statistical calculations were performed using PASW v18 software. A level of P<.05 was considered to be significant.
ResultsOut of a total of 100 cases, 94 were ultimately studied. One case that eventually proved to be an inflammatory carcinoma was excluded, along with another five in which an SLNB was ultimately not performed, for various reasons.
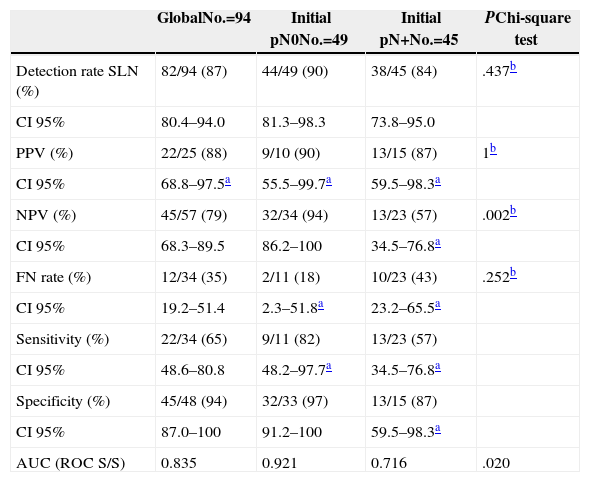
Of the 94 SLNB performed, at least one sentinel node was detected in 82 cases (87%), and no difference was shown between cases with initial lymph node involvement and those with confirmed involvement (90% vs 84%; P=.437). Table 3 shows the sensitivity values, specificity values, predicted values and the FNR both for the study overall and for the groups with and without initial lymph node involvement. It can be seen that there are significant differences (94% vs 57%; P=.002) in the NPV, which is lower where initial lymph node involvement is present, as well as a greater FNR, although it is not significant (18% vs 44%; P=.252) in this case.
Results of the Study According to Initial Lymph Node Involvement.
| GlobalNo.=94 | Initial pN0No.=49 | Initial pN+No.=45 | PChi-square test | |
|---|---|---|---|---|
| Detection rate SLN (%) | 82/94 (87) | 44/49 (90) | 38/45 (84) | .437b |
| CI 95% | 80.4–94.0 | 81.3–98.3 | 73.8–95.0 | |
| PPV (%) | 22/25 (88) | 9/10 (90) | 13/15 (87) | 1b |
| CI 95% | 68.8–97.5a | 55.5–99.7a | 59.5–98.3a | |
| NPV (%) | 45/57 (79) | 32/34 (94) | 13/23 (57) | .002b |
| CI 95% | 68.3–89.5 | 86.2–100 | 34.5–76.8a | |
| FN rate (%) | 12/34 (35) | 2/11 (18) | 10/23 (43) | .252b |
| CI 95% | 19.2–51.4 | 2.3–51.8a | 23.2–65.5a | |
| Sensitivity (%) | 22/34 (65) | 9/11 (82) | 13/23 (57) | |
| CI 95% | 48.6–80.8 | 48.2–97.7a | 34.5–76.8a | |
| Specificity (%) | 45/48 (94) | 32/33 (97) | 13/15 (87) | |
| CI 95% | 87.0–100 | 91.2–100 | 59.5–98.3a | |
| AUC (ROC S/S) | 0.835 | 0.921 | 0.716 | .020 |
AUC (ROC S/S): area under the ROC curve of sensitivity/specificity; FN: false negatives; SLN: sentinel lymph node; CI: confidence interval; NPV: negative predictive value; PPV: positive predictive value.
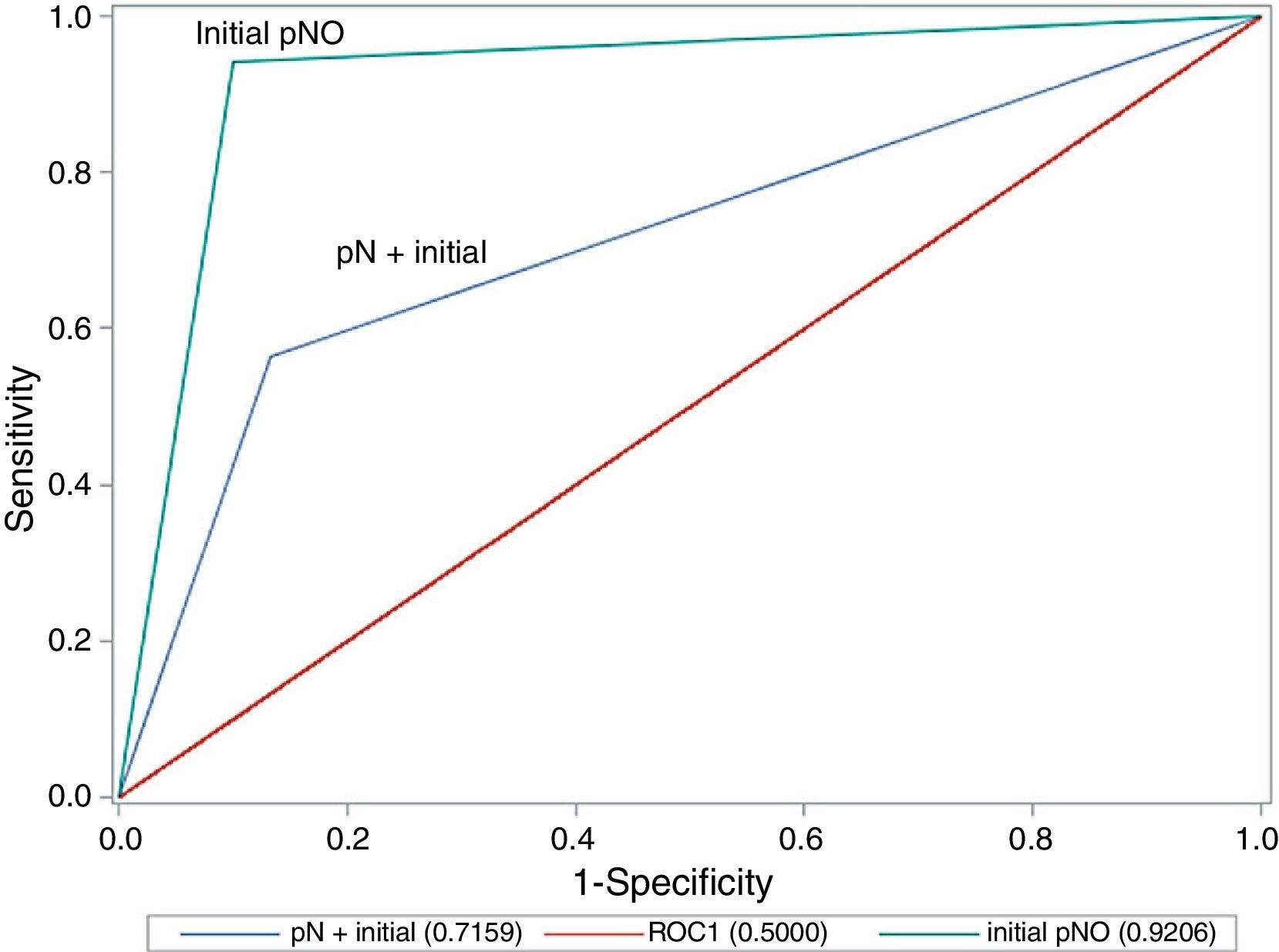
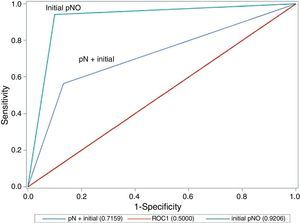
A comparison of the area under the ROC curve between cases with lymph node involvement and those without (Fig. 1) shows that there are significant differences between the curves, as precision is much greater where there is no initial lymph node involvement (0.921 in cN0 vs 0.716 in cN+; P=.020; chi-square test).
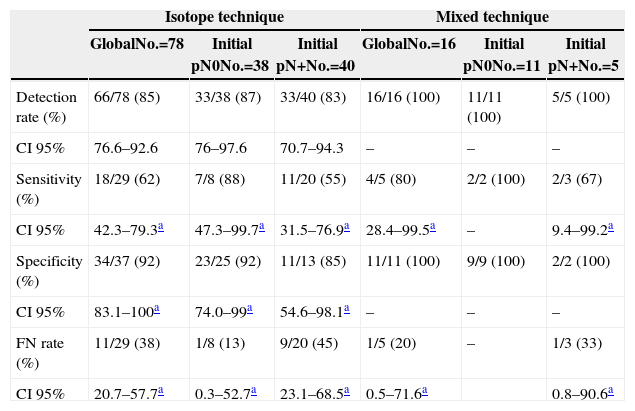
Regarding the techniques used, lymphatic mapping was performed using an isotopic tracer in 78 cases (83%) and with the mixed technique using dye plus isotopic tracer in the remaining 16 cases (17%). If we consider the technique used to perform the SLNB (Table 4), we can see that, although the use of the mixed technique improves the FNR values in comparison with the isotope-only technique (33% vs 45%), the figures remain elevated when compared with the group without lymph node involvement before PST was administered; however, the sample size must be taken into account, in particular within the mixed technique group.
Results According to Sentinel Node Biopsy Technique.
| Isotope technique | Mixed technique | |||||
|---|---|---|---|---|---|---|
| GlobalNo.=78 | Initial pN0No.=38 | Initial pN+No.=40 | GlobalNo.=16 | Initial pN0No.=11 | Initial pN+No.=5 | |
| Detection rate (%) | 66/78 (85) | 33/38 (87) | 33/40 (83) | 16/16 (100) | 11/11 (100) | 5/5 (100) |
| CI 95% | 76.6–92.6 | 76–97.6 | 70.7–94.3 | – | – | – |
| Sensitivity (%) | 18/29 (62) | 7/8 (88) | 11/20 (55) | 4/5 (80) | 2/2 (100) | 2/3 (67) |
| CI 95% | 42.3–79.3a | 47.3–99.7a | 31.5–76.9a | 28.4–99.5a | – | 9.4–99.2a |
| Specificity (%) | 34/37 (92) | 23/25 (92) | 11/13 (85) | 11/11 (100) | 9/9 (100) | 2/2 (100) |
| CI 95% | 83.1–100a | 74.0–99a | 54.6–98.1a | – | – | – |
| FN rate (%) | 11/29 (38) | 1/8 (13) | 9/20 (45) | 1/5 (20) | – | 1/3 (33) |
| CI 95% | 20.7–57.7a | 0.3–52.7a | 23.1–68.5a | 0.5–71.6a | 0.8–90.6a | |
FN: false negative; CI: confidence interval.
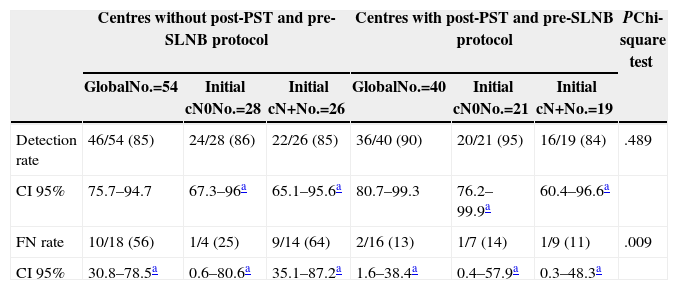
Lastly, Table 5 shows the results when comparing the use of a protocol that examines the axilla using ultrasonography before the SLNB is indicated and after PST is delivered, and which, based on the findings, manages to rule out lymph node involvement through either FNAB or CNB of the suspect lymph nodes. In Table 5, we highlight how the application of this type of protocol significantly reduces the FN rate in cases with initial involvement (56% vs 13%; P=.009), although at the expense of a lower detection rate in this patient group.
Results According to Existence of Axillary Examination After Primary Systemic Therapy.
| Centres without post-PST and pre-SLNB protocol | Centres with post-PST and pre-SLNB protocol | PChi-square test | |||||
|---|---|---|---|---|---|---|---|
| GlobalNo.=54 | Initial cN0No.=28 | Initial cN+No.=26 | GlobalNo.=40 | Initial cN0No.=21 | Initial cN+No.=19 | ||
| Detection rate | 46/54 (85) | 24/28 (86) | 22/26 (85) | 36/40 (90) | 20/21 (95) | 16/19 (84) | .489 |
| CI 95% | 75.7–94.7 | 67.3–96a | 65.1–95.6a | 80.7–99.3 | 76.2–99.9a | 60.4–96.6a | |
| FN rate | 10/18 (56) | 1/4 (25) | 9/14 (64) | 2/16 (13) | 1/7 (14) | 1/9 (11) | .009 |
| CI 95% | 30.8–78.5a | 0.6–80.6a | 35.1–87.2a | 1.6–38.4a | 0.4–57.9a | 0.3–48.3a | |
SLNB: sentinel lymph node biopsy; FN: false negatives; CI: confidence interval; PST: primary systemic therapy.
The results demonstrate that SLNB performed after neoadjuvant therapy presents a lower DR than that described in the literature after primary surgery or when performed before neoadjuvant therapy, as well as a significantly higher FNR. As a further point of relevance to this study, it was also found that these figures depend not only on the presence of initial lymph node involvement, but also on the care protocols applied by the different health care centres.
In this respect, FN figures are greatly reduced by the application of clinical pathways involving an assessment of the axillary lymph node after primary chemotherapy using ultrasonography and, where necessary, through an FNAB or CNB guided by ultrasonography, as well as by the specialisation of the radiologists in this field; however, FN figures still do not reach those achieved when the SLNB is performed before chemotherapy or in the context of primary surgery.
In a recent retrospective study of 178 patients with lymph node involvement, who were treated using neoadjuvant chemotherapy and on whom an SLNB was performed,11 acceptable detection rates of 94.9% were achieved, albeit with an FNR of 22%, and therefore its use is clearly not advisable. In any case, this study describes immunophenotypic subgroups, specifically the triple-negative subgroup, which reached 7% of the total and in which this diagnostic procedure could be considered in cases where there is a lymph node response to primary chemotherapy.
In the meta-analysis carried out by Tan et al.12 of 10 studies published in English between 2000 and 2008, which examined 499 cases of breast cancer with clinically negative axilla after receiving neoadjuvant chemotherapy and in which an SLNB was performed, it was concluded that this is a suitable tool for extension studies, as it achieves a very good identification rate of up to 94.3% and an FNR of 7.4%. Unfortunately, although a subanalysis was performed of SLNB methodology both in terms of the tracers used and whether immunohistochemistry was used, no analysis was carried out on the difference between the studies that included cases with initial lymph node involvement and those that did not. In fact, in 5 of the 10 studies examined, the inclusion criteria were N0–2, without knowing the real percentage of involvement, and in one study it was not specified at all.
Another systematic review13 analysed 40 studies that included 3328 patients and in which a distinction was made between cases with initial lymph node involvement and those without. They describe axillary understaging following neoadjuvant therapy at a rate of between 20% and 44%, and in general, N0 procedures performed before neoadjuvant therapy achieved a detection rate of 95% with an FNR of 11.4%, whereas those that included N+pre-PST patients showed a detection rate of 86.5% and an FNR of 10.3%. However, the authors conclude that indications of lymphadenectomy must be adjusted according to the results of the SLNB and the response to neoadjuvant chemotherapy in order to benefit patients in whom there is a lymph node response by avoiding the need to perform a lymphadenectomy.
The results of the SENTINA trial were published recently14; this was a four-arm German cohort study which considered both the presence of lymph node involvement before chemotherapy as well as the optimal moment for performing the SLNB. In cases where the biopsy was performed after a clinical sentinel node response was detected, the FNR was unacceptably high. The use of this procedure is therefore not recommended for breast cancer extension studies. The significant differences between the AUC that we found when comparing the procedure in cases with and without initial lymph node involvement concur with the differences found between the corresponding arms of the German study.
Another relevant study is ACOSOG Z1071,15 in which the usefulness of SLNB was analysed in cases where lymph node disease had been detected before chemotherapy. The sentinel node response rate was 40% and, in these cases, an FNR of more than 12% was found.
When accepting or justifying the figures found in these studies for their application in clinical practice, it should be taken into consideration that in the validation criteria for SLNB in the various consensus groups9,16 for staging cases of primary surgery in early breast cancer, DR values over 95% and FNR values below 5% were defined as acceptable; these are greater and lower, respectively, than those found when initial lymph node involvement is present.
The other point worth noting is that greater efficiency and effectiveness could be achieved in this method by the improved selection of cases that do not actually present with lymph node disease after treatment, as determined by an adequate examination following neoadjuvant chemotherapy, using ultrasonographic assessment and a biopsy where necessary, performed by radiologists who specialise primarily or exclusively in the field.17
As a conclusion to the present study, it may be said that the administration of PST supposes a lower DR and a greater FNR in cases with initial lymph node involvement, with lower and higher figures respectively than those recommended in the approval of SLNB for use in early-stage breast cancer treated with primary surgery. Furthermore, the application of protocols during the axillary assessment, using a clinical and ultrasonographic examination, after administering chemotherapy and before performing the SLNB, reduces the FNR in these patients.
Conflict of InterestThe authors declare that they have no conflict of interest.
Please cite this article as: Piñero-Madrona A, Escudero-Barea MJ, Fernández-Robayna F, Alberro-Adúriz JA, García-Fernández A, Vicente-García F, et al. Biopsia selectiva del ganglio centinela tras quimioterapia neoadyuvante en el cáncer de mama: resultados del estudio GEICAM 2005-07. Cir Esp. 2015;93:23–29.