The aim of this study is to evaluate the safety and effectiveness results of sleeve gastrectomy as a bariatric technique.
MethodsObservational follow-up study of a cohort of patients who underwent sleeve gastrectomy in our center between 2008 and 2017. A total of 223 patients were included: 166 as a primary technique (group 1) and 57 as a hypothetical first stage (group 2).
ResultsIn group 1, the postoperative morbidity is 12.6%, with a fistula rate of 4.2%; 5.4% required reoperation and mortality was 0.6%. In group 2, postoperative morbidity is 14%, with a fistula rate of 5.3%; 10.5% required reoperation and mortality was 5.3%. In group 1, 79.6% and 62.5% of patients at 2 and 5 years respectively managed to achieve a % EBMIL > 50%. In group 2, the second stage was completed only in 8 patients (14.0%). Of the patients who did not complete the second stage, 32.2% and 5.9% achieved a % EEBMIL > 100% at 2 and 5 years. Analyzing those who completed the second stage, the mean EEBMIL% was 90.5% and 93.4% at 2 and 5 years.
ConclusionsSleeve gastrectomy is a safe technique in patients with BMI < 45 and effective in terms of weight loss in the short-medium term. In patients with BMI > 55, a preoperative optimization aimed at reducing morbidity and mortality is necessary, as well as adequately planning the second stage, without which it is clearly insufficient.
El objetivo de este trabajo es evaluar los resultados de seguridad y efectividad de la gastrectomía vertical como técnica bariátrica.
MétodosEstudio observacional de seguimiento de una cohorte de Patients intervenidos de gastrectomía vertical en nuestro centro entre los years 2008 y 2017. Se incluyen en total de 223 Patients: 166 como técnica primaria (grupo 1) y 57 como teórico primer tiempo (grupo 2).
ResultadosEn el grupo 1, la morbilidad postoperatoria es del 12,6%, siendo la tasa de fístula del 4,2%; un 5,4% precisó reintervención quirúrgica, y la mortalidad es del 0,6%. En el grupo 2, la morbilidad postoperatoria es del 14%, con una tasa de fístula del 5,3%; un 10,5% precisó reintervención quirúrgica y la mortalidad es del 5,3%. En el grupo 1, un 79,6 y un 62,5% de los Patients a los 2 y 5 years, respectivamente, consiguen alcanzar un %EBMIP > 50%. En el grupo 2, el segundo tiempo se completó únicamente en 8 Patients (14,0%). De los Patients que no completaron el segundo tiempo, el 32,2 y el 5,9% alcanzan un %EBMIPE > 100% a 2 y 5 years. Analizando los Patients que completaron el segundo tiempo, el %EBMIPE medio fue de 90,5 y 93,4% a los 2 y 5 years del mismo.
ConclusionesLa gastrectomía vertical es una técnica segura en Patients con BMI < 45 y efectiva en cuanto a la pérdida de peso a corto-medio plazo. En Patients con BMI > 55 es necesario una optimización preoperatoria encaminada a reducir la morbimortalidad, así como planificar adecuadamente el segundo tiempo, sin el cual resulta claramente insuficiente.
In patients with morbid obesity, surgery is the only treatment that has demonstrated sufficient, sustained weight loss over time, while also reducing morbidity and mortality rates.1 However, one of the main problems when determining the best bariatric technique is the lack of long-term data. In this context, in 2014 the IFSO established the IFSO Global Registry, a single international registry in which all bariatric surgery patients could be included. According to the most recent report, sleeve gastrectomy (SG) is the most frequently performed surgical intervention worldwide (46%),2 and, together with gastric bypass, it represents >80% of bariatric procedures performed.3
SG was initially proposed by Regan et al.4 in 2003 as the first stage in super-obese patients, with the aim to reduce surgical risk. Later, given the good results obtained, it was established as an independent technique. Its main advantage is that it is a purely restrictive technique that does not require anastomosis, which theoretically reduces the risk of postoperative complications.5 However, its indiscriminate use, together with a certain laxity in the indications, can lead to the loss of this benefit. The primary objective of this study is to evaluate the results of SG as a surgical technique in bariatric surgery.
MethodsDesign and objectivesThis is an observational follow-up study of a cohort of patients who underwent SG as the primary technique at the Hospital Universitario Basurto (Vizcaya, Spain) between 2008 and 2017. Data were obtained retrospectively from the patient medical records.
The objective of the study was to evaluate perioperative complications, weight loss and the resolution of comorbidities after SG used as a single technique (group 1) and as a theoretical first stage (group 2) in the medium and long term.
Inclusion and exclusion criteriaAll patients who underwent SG at the HU Basurto between January 2008 and December 2017 were included in the study. Patients with previous bariatric surgery were excluded. The technique was initially indicated in all patients with a body mass index (BMI) under 50, although it was later limited as a primary technique for patients with a BMI between 35 and 45 who did not have gastroesophageal reflux disease (GERD) or type 2 diabetes mellitus (T2DM). Due to the high risk and potential technical limitations, it was performed as a theoretical first stage in patients with BMI > 55.
Variables under studyFor both groups, we have analyzed the baseline anthropometric data and their evolution during follow-up, demographic data (age and gender), comorbidities (T2DM, GERD, dyslipidemia, hypertension [HTN]) and their response to surgery. Other variables analyzed included hospital stay, postoperative morbidity (<30 days) according to the Clavien-Dindo Classification, specifically fistulae and the need for reoperation or revision surgery.
To evaluate weight loss, we calculated the percentage of total weight lost (%TWL), the percentage of excess BMI lost (%EBMIL) for group 1, and the percentage of expected excess BMI lost (%EEBMIL) for group 2. This latter variable was proposed by Baltasar et al.,6 as reaching a BMI of 25 is not very realistic in super-obese patients.
To define the resolution of T2DM, we followed the criteria of the American Diabetes Association (ADA).7 We also used the standards published by the American Society for Bariatric and Metabolic Surgery (ASMBS)8 to assess the evolution of hypertension and dyslipidemia.
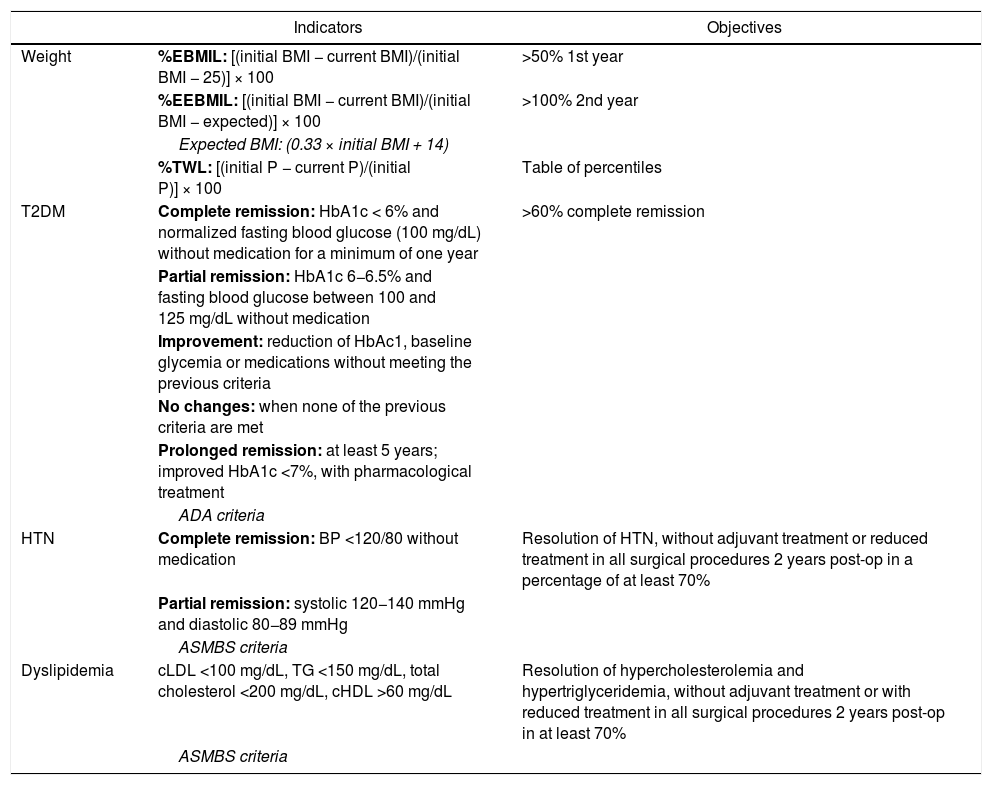
Table 1 shows the formulas for calculating these variables and their objectives.
Main indicators with their objectives and recommendations.
| Indicators | Objectives | |
|---|---|---|
| Weight | %EBMIL: [(initial BMI − current BMI)/(initial BMI − 25)] × 100 | >50% 1st year |
| %EEBMIL: [(initial BMI − current BMI)/(initial BMI − expected)] × 100 | >100% 2nd year | |
| Expected BMI: (0.33 × initial BMI + 14) | ||
| %TWL: [(initial P − current P)/(initial P)] × 100 | Table of percentiles | |
| T2DM | Complete remission: HbA1c < 6% and normalized fasting blood glucose (100 mg/dL) without medication for a minimum of one year | >60% complete remission |
| Partial remission: HbA1c 6−6.5% and fasting blood glucose between 100 and 125 mg/dL without medication | ||
| Improvement: reduction of HbAc1, baseline glycemia or medications without meeting the previous criteria | ||
| No changes: when none of the previous criteria are met | ||
| Prolonged remission: at least 5 years; improved HbA1c <7%, with pharmacological treatment | ||
| ADA criteria | ||
| HTN | Complete remission: BP <120/80 without medication | Resolution of HTN, without adjuvant treatment or reduced treatment in all surgical procedures 2 years post-op in a percentage of at least 70% |
| Partial remission: systolic 120−140 mmHg and diastolic 80−89 mmHg | ||
| ASMBS criteria | ||
| Dyslipidemia | cLDL <100 mg/dL, TG <150 mg/dL, total cholesterol <200 mg/dL, cHDL >60 mg/dL | Resolution of hypercholesterolemia and hypertriglyceridemia, without adjuvant treatment or with reduced treatment in all surgical procedures 2 years post-op in at least 70% |
| ASMBS criteria |
ADA: American Diabetes Association; ASMBS: American Society for Bariatric and Metabolic Surgery; T2DM; type 2 diabetes mellitus; HbA1c: glycosylated hemoglobin; HTN: hypertension; BMI: body mass index; %EBMIL: percent excess BMI lost; %EEBMIL: percent expected excess BMI lost; %TWL: percent total weight lost.
SG was performed laparoscopically, following the technical recommendations established and published by Rosenthal et al.9 in 2012. We began the technique with the devascularization of the greater curvature up to about 4−5 cm from the pylorus using a 36 F Faucher catheter as a guide. The staple line was reinforced, usually with a running non-invaginating suture, in order to reduce the risk of bleeding. Finally, we checked for leaks by insufflation of the gastric tube immersed in serum.
In the 2nd surgical stage, our technique of choice was the distal gastric bypass, with an alimentary limb of about 250 cm and a common limb of 100 cm.
Statistical analysisSPSS® v.25 software was used for the statistical analysis. Categorical variables are described in absolute frequencies and percentages. To describe the quantitative variables, the mean and standard deviation were used if the normality criteria were met, and the mean and interquartile range were used if these criteria were not met. We also conducted an intention-to-treat analysis. Categorical variables were compared with Fisher’s exact test and quantitative variables with the ANOVA test or Mann–Whitney test, as appropriate. P < .05 was considered significant.
ResultsBaseline characteristics and comorbidities223 sleeve gastrectomies were included in the study, 166 of which were as a single technique (group 1) and 57 as a theoretical first-stage (group 2).
Both groups included a higher proportion of women (more than 60%), and the mean age of the sample was 45.8 ± 11.3 years. The mean initial weight in group 1 was 122.9 ± 15.0 kg (BMI: 44.2 ± 3.8 kg/m2), and in group 2 169.7 ± 27.9 kg (BMI: 62.1 ± 6.9 kg/m2). The most frequent comorbidities in group 1 were hypertension (42.7%), hyperlipidemia (33.1%), arthropathy (32.5%) and DM 2 (28.9%); 7.2% of the patients in this group had GERD. In group 2, the most frequent comorbidities were hypertension (49.1%), obstructive sleep apnea/hypopnea syndrome (OSAHS) (43.8%), cardiopathy (43.8%) and T2DM (24.6%).
Complications of surgeryIn group 1, 21 patients (12.6%) presented complications within the first 30 days (11 minor, or Clavien-Dindo III). The most frequent were: 7 fistulae (4.2%, 6 of which required reoperation), 5 hemoperitoneum (3%, with 3 reoperations) and 3 pleural effusion (1.8%). Nine patients (5.4%) required reoperation in the immediate postoperative period. Two patients required conversion to gastric bypass within the management of the fistula. Only one death was recorded (0.6%).
In group 2, 8 patients (14%) suffered complications, including 3 fistulae (5.3%) and 2 acute respiratory distress syndrome (ARDS) (3.5%). Six patients required reoperation (10.5%), and 3 died (5.3%).
The mean hospital stay in both groups was 5 days, with an interquartile range between 4 and 6.
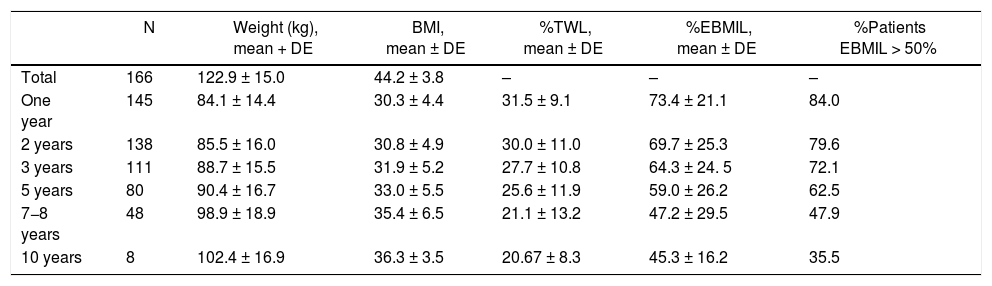
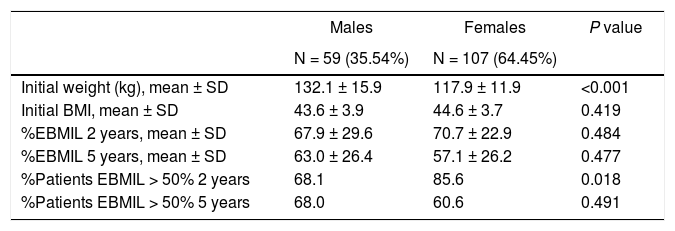
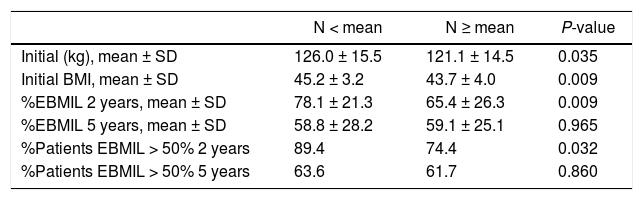
Evolution of weight lossThe weight loss results of the patients in group 1 are shown in Table 2. A total of 16 patients (9.6%) required revision surgery in subsequent years. The rates of 2-year and 5-year follow-up were 96% and 87.9%, respectively. The greatest weight loss occurred during the first 2 years after surgery, followed by progressive weight regain. The percentage of patients with a %EBMIL > 50% was 62.5% after 5 years, which decreased to 47.9% after 7−8 years. Tables 3–5 show the differences in weight loss based on gender, age and BMI after 2 and 5 years in group 1 patients, with no statistically significant differences being observed after 5 years, except for women and younger patients after 2 years.
Weight evolution of patients who treated with SG as a primary technique (group 1).
| N | Weight (kg), mean + DE | BMI, mean ± DE | %TWL, mean ± DE | %EBMIL, mean ± DE | %Patients EBMIL > 50% | |
|---|---|---|---|---|---|---|
| Total | 166 | 122.9 ± 15.0 | 44.2 ± 3.8 | – | – | – |
| One year | 145 | 84.1 ± 14.4 | 30.3 ± 4.4 | 31.5 ± 9.1 | 73.4 ± 21.1 | 84.0 |
| 2 years | 138 | 85.5 ± 16.0 | 30.8 ± 4.9 | 30.0 ± 11.0 | 69.7 ± 25.3 | 79.6 |
| 3 years | 111 | 88.7 ± 15.5 | 31.9 ± 5.2 | 27.7 ± 10.8 | 64.3 ± 24. 5 | 72.1 |
| 5 years | 80 | 90.4 ± 16.7 | 33.0 ± 5.5 | 25.6 ± 11.9 | 59.0 ± 26.2 | 62.5 |
| 7−8 years | 48 | 98.9 ± 18.9 | 35.4 ± 6.5 | 21.1 ± 13.2 | 47.2 ± 29.5 | 47.9 |
| 10 years | 8 | 102.4 ± 16.9 | 36.3 ± 3.5 | 20.67 ± 8.3 | 45.3 ± 16.2 | 35.5 |
SG: sleeve gastrectomy; BMI: body mass index; N: number of patients; %EBMIL; percentage of excess BMI lost; %TWL: percent total weight lost.
Evolution of weight according to sex (group 1).
| Males | Females | P value | |
|---|---|---|---|
| N = 59 (35.54%) | N = 107 (64.45%) | ||
| Initial weight (kg), mean ± SD | 132.1 ± 15.9 | 117.9 ± 11.9 | <0.001 |
| Initial BMI, mean ± SD | 43.6 ± 3.9 | 44.6 ± 3.7 | 0.419 |
| %EBMIL 2 years, mean ± SD | 67.9 ± 29.6 | 70.7 ± 22.9 | 0.484 |
| %EBMIL 5 years, mean ± SD | 63.0 ± 26.4 | 57.1 ± 26.2 | 0.477 |
| %Patients EBMIL > 50% 2 years | 68.1 | 85.6 | 0.018 |
| %Patients EBMIL > 50% 5 years | 68.0 | 60.6 | 0.491 |
BMI: body mass index; %EBMIL: percentage excess BMI lost.
Evolution of weight according to age (group 1).
| N < mean | N ≥ mean | P-value | |
|---|---|---|---|
| Initial (kg), mean ± SD | 126.0 ± 15.5 | 121.1 ± 14.5 | 0.035 |
| Initial BMI, mean ± SD | 45.2 ± 3.2 | 43.7 ± 4.0 | 0.009 |
| %EBMIL 2 years, mean ± SD | 78.1 ± 21.3 | 65.4 ± 26.3 | 0.009 |
| %EBMIL 5 years, mean ± SD | 58.8 ± 28.2 | 59.1 ± 25.1 | 0.965 |
| %Patients EBMIL > 50% 2 years | 89.4 | 74.4 | 0.032 |
| %Patients EBMIL > 50% 5 years | 63.6 | 61.7 | 0.860 |
BMI: body mass index; %EBMIL: percentage excess BMI lost.
Mean = 47 years.
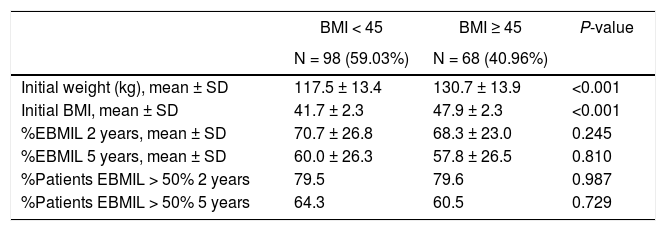
Evolution of weight according to BMI (group 1).
| BMI < 45 | BMI ≥ 45 | P-value | |
|---|---|---|---|
| N = 98 (59.03%) | N = 68 (40.96%) | ||
| Initial weight (kg), mean ± SD | 117.5 ± 13.4 | 130.7 ± 13.9 | <0.001 |
| Initial BMI, mean ± SD | 41.7 ± 2.3 | 47.9 ± 2.3 | <0.001 |
| %EBMIL 2 years, mean ± SD | 70.7 ± 26.8 | 68.3 ± 23.0 | 0.245 |
| %EBMIL 5 years, mean ± SD | 60.0 ± 26.3 | 57.8 ± 26.5 | 0.810 |
| %Patients EBMIL > 50% 2 years | 79.5 | 79.6 | 0.987 |
| %Patients EBMIL > 50% 5 years | 64.3 | 60.5 | 0.729 |
BMI: body mass index; %EBMIL: percentage excess BMI lost.
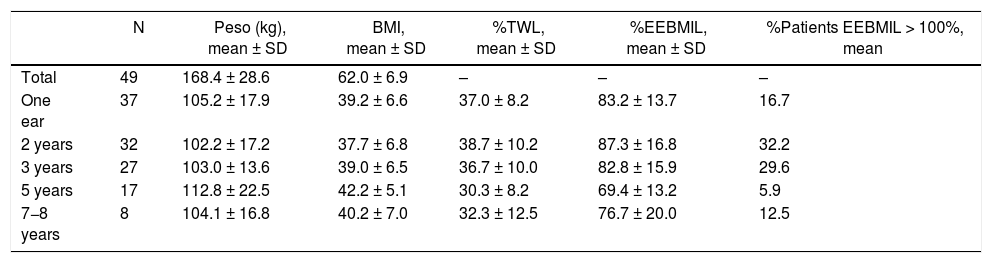
Out of the 57 patients who underwent SG as a 1st stage, the 2nd stage was only completed in 8 (14.0%). Out of the 46 (excluding the 3 deceased) in whom it was not completed, 2 required conversion to proximal gastric bypass due to persistent postoperative fistula. In this group, the 2-year and 5-year follow-up rates were 90.5% and 62.1%, respectively. The weight results in which the second half was not completed are shown in Table 6. After 7−8 years, the percentage of patients with an %EEBMIL > 100% was 12.5%.
Weight evolution of SG (group 2).a
| N | Peso (kg), mean ± SD | BMI, mean ± SD | %TWL, mean ± SD | %EEBMIL, mean ± SD | %Patients EEBMIL > 100%, mean | |
|---|---|---|---|---|---|---|
| Total | 49 | 168.4 ± 28.6 | 62.0 ± 6.9 | – | – | – |
| One ear | 37 | 105.2 ± 17.9 | 39.2 ± 6.6 | 37.0 ± 8.2 | 83.2 ± 13.7 | 16.7 |
| 2 years | 32 | 102.2 ± 17.2 | 37.7 ± 6.8 | 38.7 ± 10.2 | 87.3 ± 16.8 | 32.2 |
| 3 years | 27 | 103.0 ± 13.6 | 39.0 ± 6.5 | 36.7 ± 10.0 | 82.8 ± 15.9 | 29.6 |
| 5 years | 17 | 112.8 ± 22.5 | 42.2 ± 5.1 | 30.3 ± 8.2 | 69.4 ± 13.2 | 5.9 |
| 7−8 years | 8 | 104.1 ± 16.8 | 40.2 ± 7.0 | 32.3 ± 12.5 | 76.7 ± 20.0 | 12.5 |
Three patients died during the postoperative period.
SG: sleeve gastrectomy; BMI: body mass index; N: number of patients; %EBMIL; percentage excess BMI lost; %TWL: percent total weight lost.
Out of the 8 patients in whom a second stage was performed, in 7 this was a distal gastric bypass, while in one case a proximal bypass was conducted due to technical difficulties. The time elapsed from the performance of the SG to the second stage ranged from 20 to 60 months (mean 34 months). With an initial BMI of 63.41 kg/m2, the mean BMI reached at 2 and 5 years of the 2nd stage were 37 and 39.1 kg/m2. The mean %EEBMIL was 90.5% after 2 years and 93.4% after 5 years.
Resolution of comorbiditiesIn group 2, this data has not been analyzed, because the indication was based mainly on the degree of obesity and the consequent surgical risk, so we have focused on group 1.
The complete or partial remission of T2DM and HTN 2 years after surgery was 69.4% (38.9% complete) and 26.7%, respectively. In this same period, 61.3% of patients presented resolution of dyslipidemia.
DiscussionIn our hospital, we currently perform more than 100 procedures a year, approximately one-third of which are SG. It is striking that the BMI of group 1 was at the upper limit of the indication for SG as a single technique, because in the initial years of the study this surgery was indicated for a BMI of up to 50. It should also be noted that, out of the total number of surgically treated patients in this group, a considerable percentage presented T2DM or GERD, conditions that currently contraindicate performing the technique. In patients with T2DM, we preferred initially performing gastric bypass due to the higher rate of remission in the long term, as shown by recent studies.10,11 In contrast, in elderly patients or when T2DM has a very long evolution and any expectation of its resolution is limited, SG is sometimes chosen due to its apparent simplicity. Likewise, we consider GERD a contraindication for SG, since it can worsen the preexisting disease or even cause the appearance of de novo reflux, with rates reported of up to 23% according to recent publications.12 However, its correlation with technical factors such as gastric tube size or hiatal hernia repair remains to be determined. The objective of our study was not to analyze the correlation of the technique with reflux; these data are collected in a multicenter study coordinated by SECO.
In terms of postoperative mortality rate, the results of group 1 agree with the established standards.13 However, in light of the mortality observed in group 2, in the last 5 years we have implemented a preoperative optimization program that includes hospitalization for patients at higher risk (no patient has died in this period).
The early morbidity in our series is higher than the established limit (7%), probably due in part to the learning curve. The fistula rate is very close to the quality standards established in the study by Gero et al.,14 which includes the results of 19 high-volume hospitals worldwide (≥200 cases). However, 6.7% of the patients required reoperation, which is higher than the 2.5% established in that study.14
Similar to several studies,15,16 weight loss is greater during the first 12 months after SG. In our series, both groups presented an increase in weight after the 3rd or 5th year, which is similar to data obtained by Himpens et al.17 In group 1, our %TWL results coincide with the P50 of the multicenter study published by Sabench et al.,18 where more favorable weight loss was reported for males 6 months after surgery. In our series, greater weight loss was observed in the youngest patients and in females 2 years after surgery. We have also analyzed the difference in weight loss as a function of BMI, establishing the cut-off point at 45, since it is the current limit for indicating the technique. However, we have not found statistically significant differences. Only 8 patients in our series completed 10 years of follow-up, which may be due in part to the fact that many were discharged from the clinic after 5 years.
Regarding the patients in group 2, it should be noted that only 14% completed the second stage of surgery, and the 5-year follow-up of the patients who did not complete it is only 62.1%. This may indicate that patients with a BMI > 55 are satisfied with the weight loss they have achieved and do not wish to undergo a second surgery. However, only 32.2% and 5.9% reached a %EEBMIL > 100% after 2 and 5 years, which is far from the goal. In patients who completed the 2nd stage, the mean 2-year and 5-year %EEBMIL were 90.5% and 93.4%, respectively. Due to this, and after analyzing the results, it is essential to improve the follow-up rate and perform the second stage in most patients.
For the 2nd surgical phase, our technique of choice is the distal bypass due to greater familiarity with the technique and because we consider the duodenal-ileal anastomosis of the duodenal switch or SADI-S to be of greater risk. In addition, the SADI-S was not approved by the IFSO as a bariatric technique until 2018. With the intention of avoiding malnutrition in patients due to the small reservoir, we performed the distal bypass with an alimentary limb of about 250 cm and a common limb of 100 cm,
In recent studies, complete remission of T2DM 5 years after the intervention occurs in approximately 80% of patients.15,19 With regard to HTN, and according to the findings of Gadiot et al.,20 the remission and improvement rates are 53% and 33%, respectively. The variability in the remission rates published by various studies and the poor results obtained in our series may be due to the different remission criteria used, the absence of data in the history to be able to evaluate it properly, or the selection of patients, particularly the elderly or with long-standing disease, whose possible response is globally smaller.
Limitations of this study include the biases inherent to the type of study and data collection. It is essential to standardize a series of indicators and remission criteria to be able to evaluate weight loss and the evolution of comorbidities. This would make it possible to establish quality criteria that define good clinical practice, while being able to compare the results published in the different studies and between different surgical techniques. The main strength of our study is its homogeneity, with a standardized technique, a large volume and a long follow-up, which make it a benchmark for comparisons.
In conclusion, we can affirm that SG is a safe technique in experienced medical centers, while emphasizing the need for preoperative optimization in patients with BMI > 55. In addition, in patients with a BMI < 45 it is an effective technique in the short term in terms of weight loss, although in the long term its effectiveness seems to decrease. In patients with a BMI > 55, it is a clearly insufficient technique, so we must rethink the need to complete the second stage in a larger number of patients.
FundingThis study has received no specific funding from public, commercial or non-profit organizations.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Castro Vázquez J, Saravia Barahona F, Loureiro González C, Leturio Fernández S, García Fernández M, Moro Delgado A, et al. Gastrectomía vertical como técnica quirúrgica en cirugía bariátrica: análisis de resultados de seguridad y efectividad. Cir Esp. 2022;100:88–94.