Liver resection is the only curative treatment for colorectal liver metastasis. The identification of predictive factors leads to personalize patient management to enhance their long-term outcomes. This population-based study aimed to characterize factors associated with, and survival impact of patients who received hepatectomy for colorectal liver metastasis.
MethodsA retrospective cohort study of all the hepatectomies for colorectal liver metastasis performed at third-level hospital of Spain (2010–2018) was conducted. The Kaplan–Meier method was used for survival analyses. Multivariable Cox and regression models were used to determine prognostic factors associated with overall survival.
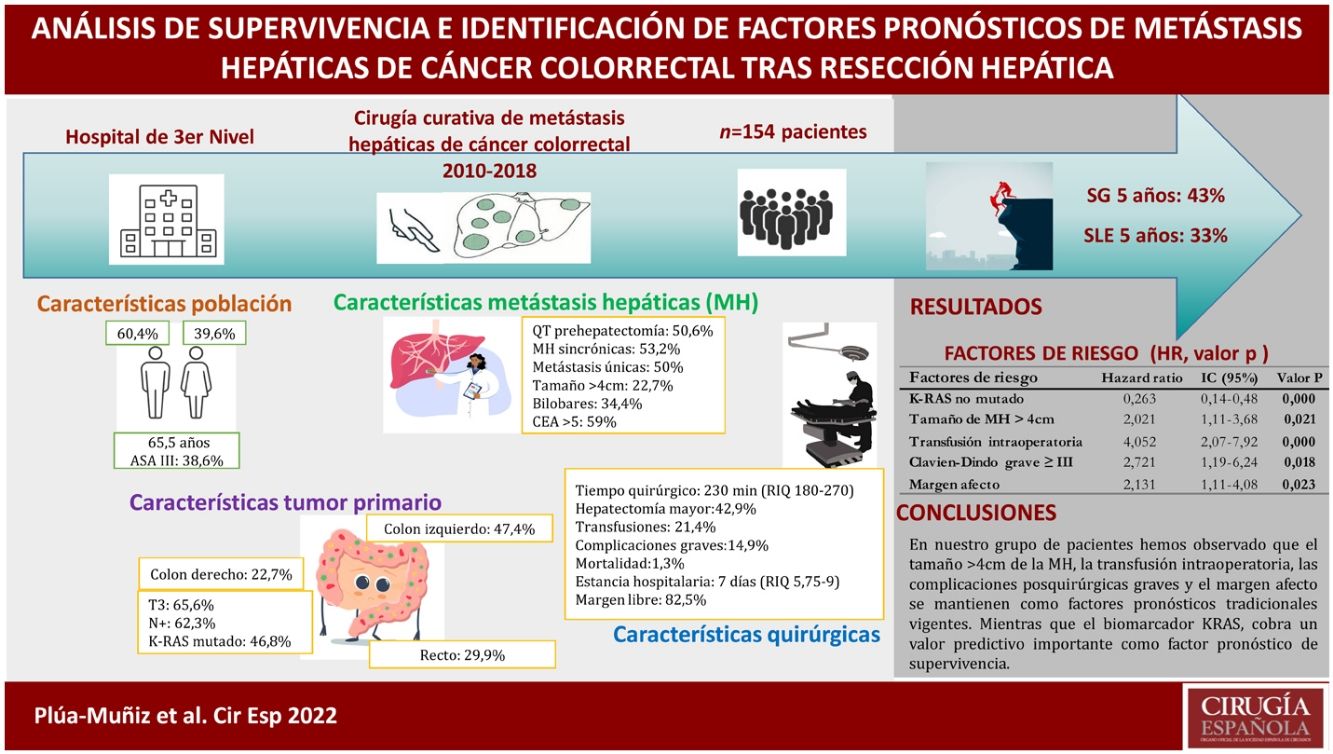
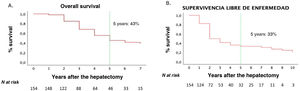
ResultsThe 5-year overall survival and disease-free survival were 42 and 33%, respectively. Survival analysis showed that metastasis features (number, largest size, distribution, and extrahepatic disease) and postsurgical factors (transfusion, major complications, and positive margin resection), as well as non-mutated KRAS, showed a significant association with survival. Otherwise, on multivariate analysis, only 5 independent risk factors were identified: major size metastasis >4 cm, RAS mutation, positive margin resection, intraoperative transfusion, and major complications.
ConclusionsAccording to our findings, major size metastasis >4 cm, intraoperative transfusion, and major postoperative complications continue to be traditional prognostic factors. Meanwhile, the KRAS biomarker has a powerful impact as a survival prognostic factor.
El único tratamiento curativo para las metástasis hepáticas de cáncer colorrectal es la cirugía. La identificación de factores pronósticos nos permite individualizar el tratamiento de estos pacientes, mejorando sus resultados oncológicos a largo plazo. El objetivo de nuestro estudio es identificar la supervivencia y los factores pronósticos de pacientes sometidos a tratamiento quirúrgico de metástasis hepáticas de cáncer colorrectal.
MétodosSe trata de un estudio retrospectivo de todos los casos de hepatectomías por metástasis hepáticas de cáncer colorrectal, operadas en un hospital de tercer nivel en España, entre los años 2010 y 2018. Se realizó análisis de supervivencia univariante con las curvas de supervivencia Kaplan-Meier. Mediante el modelo multivariante regresión de Cox se determinaron los factores pronósticos asociados a la supervivencia global.
ResultadosLa supervivencia global y supervivencia libre de enfermedad de nuestra serie, a los 5 años fue del 43% y del 33%, respectivamente. En el análisis de supervivencia, las características de las metástasis hepáticas (números, tamaño mayor, distribución bilobar y enfermedad extrahepática) y postoperatorias (transfusión sanguínea, complicaciones graves y margen afecto), así como el KRAS no mutado resultaron estadísticamente significativos. No obstante, después de aplicar la regresión de Cox, solo se identificaron 5 factores de riesgo independientes: mutación del KRAS, metástasis hepáticas >4 cm, transfusión intraoperatoria, complicaciones graves y margen afecto.
ConclusionesEn nuestro grupo de pacientes hemos observado que el tamaño >4 cm de la metástasis hepáticas, la transfusión intraoperatoria, las complicaciones posquirúrgicas graves y el margen afecto se mantienen como factores pronósticos tradicionales vigentes. Mientras que el biomarcador KRAS cobra un valor predictivo importante como factor pronóstico de supervivencia.
Colorectal cancer (CRC) is a disease associated with considerable incidence and mortality worldwide. In 2020, it was the most frequently diagnosed malignant pathology in Spain, with an estimated mortality rate of 14.6%.1 By the end of 2021, 43 581 new cases are expected to be diagnosed.1 The liver is the most common location of CRC metastases; some 40%–50% of patients diagnosed with CRC will develop liver metastasis during the first 3 years,2 while 20%–25% of patients present liver metastases at the time of diagnosis.3
Currently, the only curative treatment for liver metastases (LM) is surgery, with a 5-year survival rate of 36%–58%.4 However, only 15% of patients diagnosed with colorectal cancer liver metastases (CLM) are candidates for curative surgical treatment, while 35% are potentially resectable and 50% unresectable. Without treatment, these patients have a life expectancy of 5–6 months.5
It has been shown that aggressive surgical treatment of CLM can improve survival in properly selected patients,6 and prognostic factors play an important role in this selection process. The first published prognostic factors on survival in patients operated on for CLM date back to 1996 by Nordlinger et al.7 However, since then, many factors involved in the treatment and management of CLM have changed radically.
The main objective of our study is to identify which prognostic factors influence the postoperative survival of patients treated for CLM. In addition, we will discuss whether the prognostic factors obtained with this study are similar to those identified previously.
MethodsThis is a retrospective observational study, including all hepatectomies for CLM performed in a tertiary care hospital in Spain between January 2010 and August 2018.
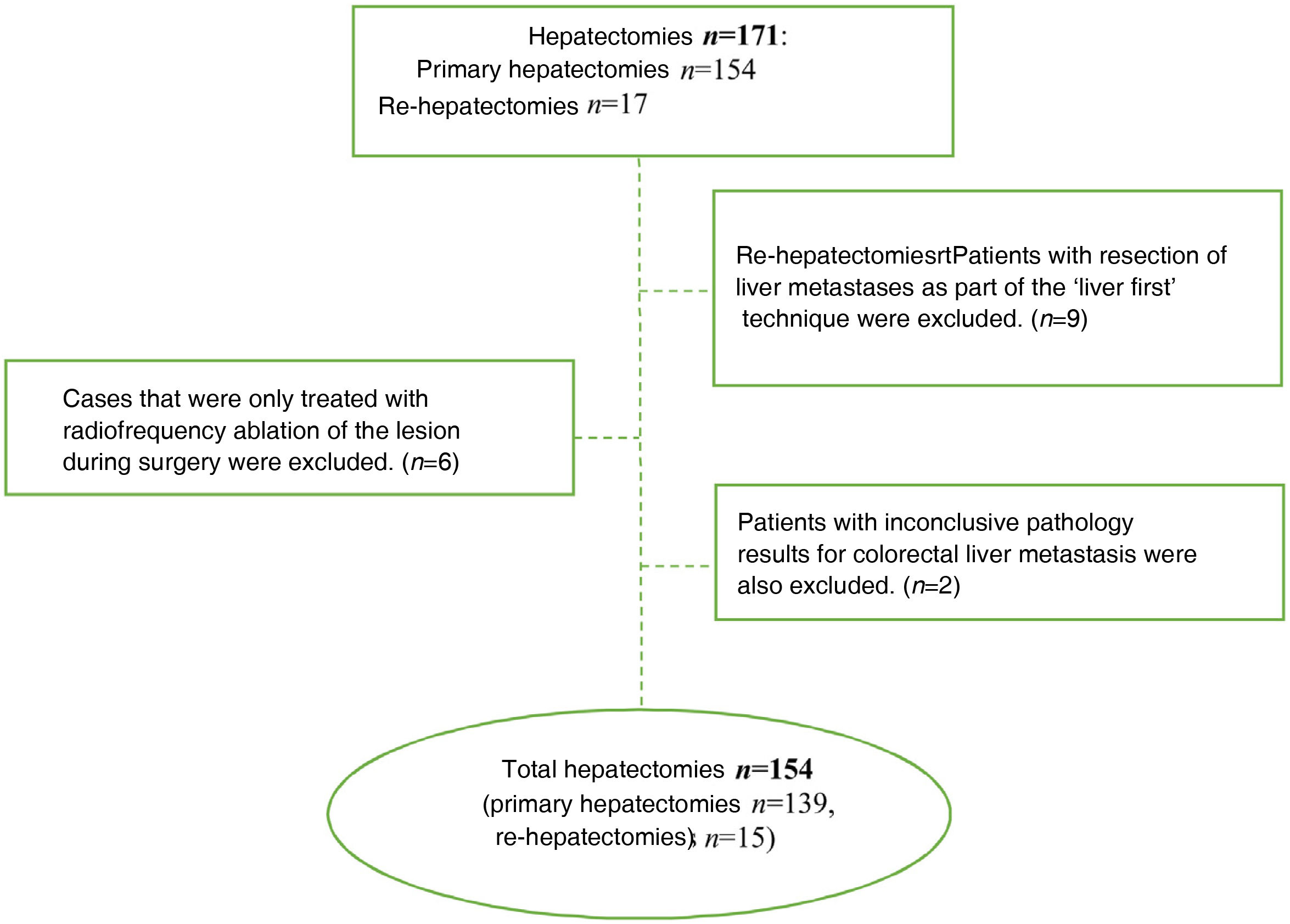
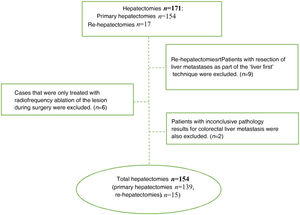
The inclusion criteria were: patients treated with curative hepatic resection due to CLM, which was confirmed by the pathological study of the surgical specimen. We excluded patients in whom curative surgery was not possible (including those who received thermal ablation treatment exclusively), when the histological study of the specimen did not confirm the diagnosis of CLM, and cases treated with the ‘liver first’ technique because the colon resection was not completed in several patients due to disease progression, as well as because of the small number of cases. Re-hepatectomies were considered new cases.
All patients underwent an extension study with thoracoabdominal computed tomography, which ruled out metastatic disease other than the liver involvement. Hepatic magnetic resonance imaging was subsequently indicated for the detailed study of LM. In cases of diagnostic uncertainties regarding the presence of extrahepatic metastases that could not be confirmed with computed tomography or magnetic resonance imaging, a positron emission tomography study was requested. Unresectable extrahepatic disease was considered a contraindication for CLM resection surgery.
Synchronous LM were defined as LM present at the time of diagnosis of the primary tumor, and simultaneous surgery was only indicated in cases where factors such as age, characteristics of the primary tumor, number and size of the metastases, and future liver remnant were optimal.
We defined borderline metastases as those potentially modifiable for curative resection, including: metastases >5 cm, presence of 4 or more metastases, or patients in whom the future liver remnant was expected to be less than 30%. In these cases, neoadjuvant chemotherapy (CTx) with dual therapy (fluorouracil with oxaliplatin and/or irinotecan) associated with monoclonal antibodies was indicated: cetuximab and/or panitumumab for non-mutated KRAS cases or bevacizumab if they were mutated KRAS. Neoadjuvant CTx is not performed in our hospital in patients with surgically resectable metastases from the onset. All cases were evaluated by a multidisciplinary team to define the appropriate and individualized management that each case warranted.
The study variables included: age, sex, ASA grade, primary tumor type, primary tumor stage, LM type, neoadjuvant CTx, KRAS type, preoperative carcinoembryonic antigen, type of hepatectomy, intraoperative transfusion, resection margins, postoperative morbidity and mortality, and long-term postoperative follow-up.
Morbidity and mortality occurring in the following 30 days after surgery were evaluated. Types III, IV and V of the Clavien-Dindo classification8 were considered major complications.
The resection margin was classified as free if there were no tumor cells in 1 mm; a margin less than 1 mm was considered margin involvement. The anatomical distribution of liver lesions was defined according to Couinaud’s nomenclature.9 Brisbane 200010 terminology was used for liver resections.
This study was duly approved by the Ethics Committee for Drug Research (CEIm) at our hospital.
Before proceeding with liver resection, we carefully reviewed the entire abdominal cavity of each patient by means of laparotomy. Then, after liver mobilization, we proceeded with palpation and intraoperative ultrasound of the liver. Subsequently, we performed hepatic vascular exclusion maneuvers according to the needs of each procedure, not systematically. When required, we used the intermittent Pringle maneuver for 10 min, followed by 5 min of reperfusion.
Similarly, liver transection was performed using the Cavi-Pulse Ultrasonic Surgical Aspirator (CUSA) together with conventional electrocoagulation. Resections of fewer than 2 liver segments were considered minor hepatectomies, while resections of 3 or more segments were considered major hepatectomies.
Patients were followed-up every 3–6 months after surgery for the first 3 years, and every 6 months thereafter. Follow-up studies included thoracoabdominal computed tomography and tumor marker levels. Patient follow-up was extended until November 2020 in order to have the maximum possible number of patients with long-term follow-up. In cases of recurrence, the patients were treated following the same initial scheme: direct surgery if the LM was resectable, provided that unresectable extrahepatic disease was ruled out; in unresectable cases, either conversion or palliative CTx was chosen, depending on the individual.
Statistical analysisThe baseline characteristics of the population were described using absolute and relative frequencies (percentages) of their categories. We calculated the 95% confidence intervals (95% CI) of the parameters. The normality of the quantitative variables was verified for their expression as arithmetic mean with standard deviation or as median and interquartile range (IQR).
Survival rates were calculated using the Kaplan-Meier estimation method, and the survival curves obtained were compared with the log-rank test. Variables that showed a P < 0.10 were selected for the multivariate analysis.
In the multivariate analysis, the Cox regression model was used to identify independent risk factors associated with overall survival (OS) and disease-free survival (DFS). Variables with P < 0.05 were considered significant.
The statistical analysis was performed with the statistical package SPSS® version 25 (IBM Corporation®, Armonk, New York, USA).
ResultsDuring the study period, 171 hepatectomies were performed for CLM (154 primary and 17 re-hepatectomies), and 17 cases were excluded for not meeting the inclusion criteria (Fig. 1). Thus, 154 cases were analyzed, 61 of which were women and 93 men. Median age was 65.5 years (IQR: 57−72.3). Sequential colon-liver surgery was performed in 132 (85.7%) cases and simultaneous surgery in 22 (14.3%). In 53 cases, the metastases were bilobar. In 35 cases (22.7%), the size of the metastasis was >4 cm. All the clinical and pathological characteristics of our series are described in Table 1.
Demographic and clinical-pathological characteristics of patients treated with liver resection due to CLM (n = 154).
| Characteristics | Value | % |
|---|---|---|
| Age, median (IQR) | 65.50 (57−72) | |
| <70 years | 102 | 66.2 |
| Males | 93 | 60.4 |
| High ASA risk (iii) | 60 | 38.9 |
| Primary tumor | ||
| Right colon | 35 | 22.7 |
| Left colon | 73 | 47.4 |
| Rectum | 46 | 29.9 |
| T type: | ||
| T1 | 3 | 1.9 |
| T2 | 17 | 11.0 |
| T3 | 101 | 65.6 |
| T4 | 32 | 20.8 |
| Lymph node metastases of the primary tumor | 96 | 62.3 |
| Primary tumor with lymphovascular involvement | 67/144 | 43.5 |
| Primary tumor with perineural involvement | 27/144 | 17.5 |
| Hepatic metastases | ||
| Synchronous LM | 72 | 46.8 |
| Metachronous LM | 82 | 53.2 |
| Colon-liver surgery | 132 | 85.7 |
| Simultaneous surgery | 22 | 14.3 |
| Size of the largest metastasis (>4 cm) | 35 | 22.7 |
| Solitary metastasis | 77 | 50.0 |
| Unilobar metastases | 101 | 65.6 |
| Multilobar metastases | 53 | 34.4 |
| Mutated KRAS | 51/109 | 46.8 |
| CEA >5 ng/mL | 85/144 | 59.0 |
| Neoadjuvant chemotherapy LM | 78 | 50.6 |
| Surgical time (min), median (IQR) | 230 (180−270) | |
| Intraoperative transfusion | 33 | 21.4 |
| Major hepatectomy | 66 | 42.9 |
| Extrahepatic disease | 10 | 6.5 |
| Free margin | 127 | 82.5 |
| Mild complications (Clavien-Dindo I and II) | 16 | 39.6 |
| Severe complications (Clavien-Dindo ≥III) | 23 | 14.9 |
| Mortality | 2 | 1.3 |
| Postoperative stay (days, IQR) | 7 (5.75–9) | |
| Prolonged postoperative time (>7 days) | 63 | 40.9 |
| Post-hepatectomy adjuvant therapy | 107 | 69.9 |
| Recurrence at the end of follow-up: | 103 | 66.9 |
| Specific recurrence | 23 | 14.94 |
| Hepatic and extrahepatic recurrence | 58 | 37.67 |
| Exclusive extrahepatic recurrence | 22 | 14.29 |
CEA: carcinoembryonic antigen; LM: liver metastases; CLM: colorectal cancer liver metastases; IQR: interquartile range.
50.6% of the cases received neoadjuvant CTx, as described in Table 2.
Clinical characteristics of the patients who received neoadjuvant chemotherapy versus those who did not.
| Characteristics | neoCTx (n = 78) | No neoCTx (n = 76) |
|---|---|---|
| n (%) | n (%) | |
| Adjuvant treatment primary tumor | 73 (93) | 41 (53.9) |
| Lymph node metastases of the primary tumor | 38 (53.5) | 29 (39.7) |
| Synchronous LM | 51 (65.4) | 21 (27.6) |
| Solitary metastasis | 31 (39.7) | 46 (60.5) |
| Size of largest metastasis (>4 cm) | 22 (28.2) | 13 (17.1) |
| Multilobar metastasis | 35 (44.9) | 18 (23.7) |
| Post-hepatectomy adjuvant therapy | 59 (75.6) | 48 (64) |
LM: liver metastasis; neoCTx: neoadjuvant chemotherapy.
The median surgical time was 230 min (IQR: 180−270). The median hospital stay was 7 days (IQR: 5.8–9), which was prolonged (≥8 days) in 40.9% of cases. The intraoperative transfusion rate was 21.4%, and the morbidity rate was 14.9%. The postoperative mortality rate was 1.3%. The maximum follow-up time was 155 months, with a median of 38 months (IQR: 21–65).
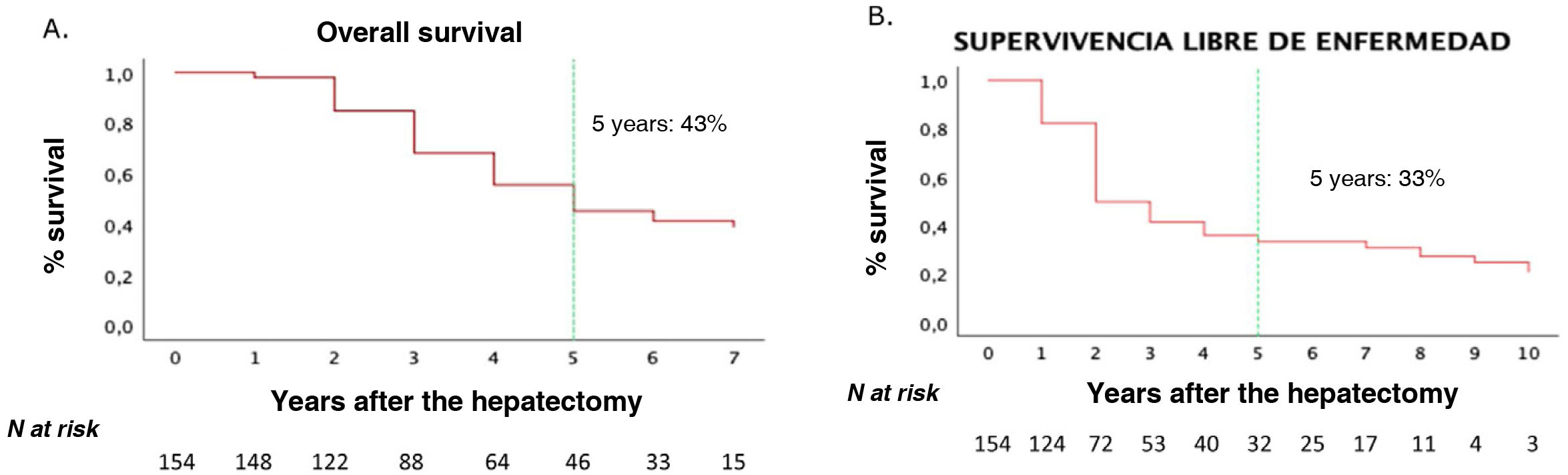
One-, 3- and 5-year OS rates for our series were 90%, 62% and 43%, respectively. One-year, 3-year and 5-year DFS rates were 58%, 40% and 33%, respectively (Fig. 2).
Neither age, nor sex, nor preoperative comorbidity (ASA type) were associated with worse OS or DFS outcomes (Table 3).
Univariate analysis of 5-year overall survival and 5-year disease-free survival.
| N patients | 5-year OS (%) | P-value | 5-year DFS (%) | P-value | |
|---|---|---|---|---|---|
| Clinical-pathological factors | |||||
| Age | |||||
| <70 | 102 | 46.0 | 35.2 | ||
| >70 | 50 | 34.5 | NS | 24.9 | NS |
| Sex | |||||
| Female | 61 | 43.3 | 34.8 | ||
| Male | 91 | 43.0 | NS | 30.9 | NS |
| ASA | |||||
| ASA I-II | 93 | 43.2 | 31.6 | ||
| ASA III | 59 | 40.2 | NS | 33.8 | NS |
| Factors depending on the primary tumor | |||||
| Location of the primary tumor | |||||
| Colon | 108 | 45.2 | 35.8 | ||
| Rectum | 44 | 37.6 | NS | 25.2 | NS |
| Primary tumor embryological origin | |||||
| Right colon | 35 | 44.1 | 25.1 | ||
| Left colon | 117 | 41.6 | NS | 34.6 | NS |
| T stage | |||||
| T1-T2 | 20 | 37.1 | 27.4 | ||
| T3-T4 | 132 | 44.0 | NS | 33.4 | NS |
| N stage | |||||
| N0 | 57 | 52.7 | 40.8 | ||
| N+ | 95 | 37.2 | 0.011 | 25.4 | 0.008 |
| Lymphovascular involvement | |||||
| Yes | 67 | 40 | 22.4 | ||
| No | 76 | 42.3 | NS | 38.4 | NS |
| Perineural involvement | |||||
| Yes | 27 | 31.5 | 20 | ||
| No | 116 | 44 | NS | 35.2 | NS |
| Metastasis-related factors | |||||
| Type of metastasis | |||||
| Metachronous | 81 | 46.8 | 34 | ||
| Synchronous | 71 | 36.7 | NS | 27.9 | 0.040 |
| Number of metastases | |||||
| One metastasis | 77 | 48.7 | 39.2 | ||
| More than one metastasis | 75 | 34.7 | 0.040 | 25.8 | 0.011 |
| Greater size of LM | |||||
| >4 cm | 34 | 25.2 | 22.7 | ||
| <4 cm | 118 | 48.2 | 0.001 | 35.3 | 0.017 |
| Distribution of metastases | |||||
| Unilobar | 100 | 48.9 | 38.5 | ||
| Bilobular | 52 | 32.4 | 0.019 | 20.8 | 0.001 |
| Neoadjuvant CTx | |||||
| Yes | 77 | 32.9 | 21.6 | ||
| No | 75 | 51.8 | 0.011 | 41.3 | 0.001 |
| Extrahepatic metastases | |||||
| Yes | 10 | 15.6 | 15 | ||
| No | 142 | 44.9 | 0.039 | 34.4 | 0.046 |
| Wild-type KRAS | |||||
| Yes | 57 | 48.5 | 36.5 | ||
| No | 51 | 20.0 | 0.000 | 7.8 | 0.001 |
| Pre-hepatectomy CEA | |||||
| CEA < 5 | 59 | 52.0 | 43.7 | ||
| CEA > 5 | 84 | 38.7 | 0.008 | 28.4 | 0.007 |
| Surgical variables | |||||
| Intraoperative transfusion | |||||
| Yes | 32 | 29.5 | 20.8 | ||
| No | 120 | 46.8 | 0.038 | 36 | 0.033 |
| Type of hepatectomy | |||||
| Minor hepatectomy | 88 | 47.3 | 34.6 | ||
| Major hepatectomy | 64 | 36.7 | NS | 27 | NS |
| Postoperative variables | |||||
| Long postoperative stay | |||||
| Yes | 61 | 30.8 | 26.4 | ||
| No | 91 | 50.4 | 0.045 | 34.7 | 0.036 |
| Overall complications | |||||
| Yes | 82 | 34.4 | 28.3 | ||
| No | 70 | 51 | 0.025 | 37.6 | NS |
| Severe Clavien ≥ iii | |||||
| Yes | 21 | 45.4 | 28.6 | ||
| No | 131 | 26.9 | 0.011 | 33.3 | NS |
| Margin involvement | |||||
| Yes | 26 | 26 | 13.2 | ||
| No | 126 | 46.6 | 0.003 | 36.7 | 0.004 |
OS: global survival; SLE: disease-free survival.
Only lymph node stage was significantly related to OS and DFS. N0 type tumors had a 5-year OS of 52.7% compared to N + tumors, which achieved a 5-year OS of 37.2% (P = 0.011). The T type did not significantly influence survival.
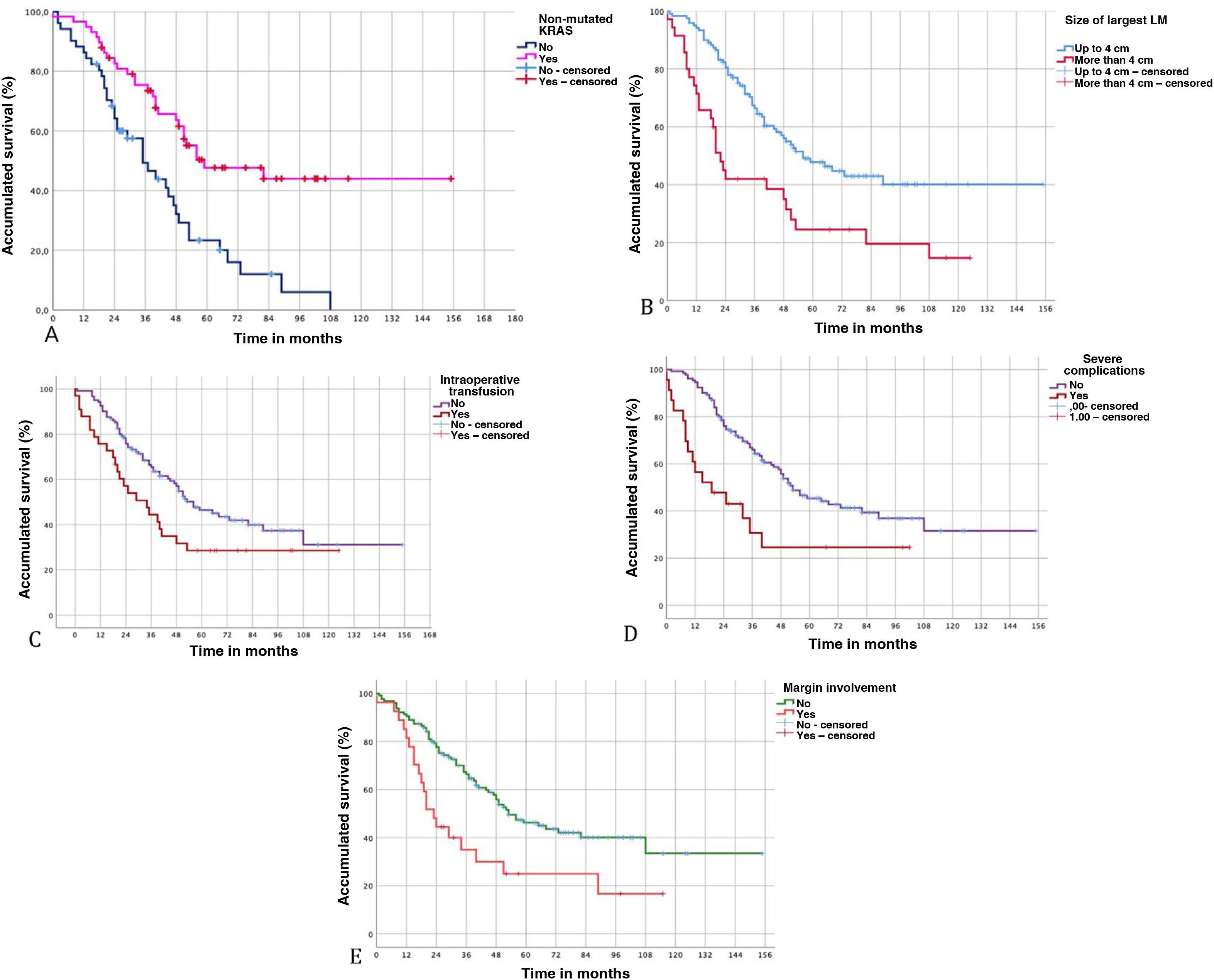
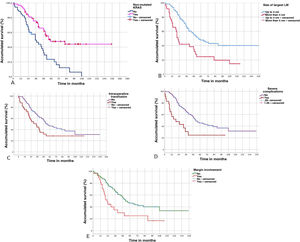
Characteristics of liver metastasesA number >1 of LM was shown to decrease OS (P = 0.04) and DFS (P = 0.011). LM size >4 cm also significantly affected OS (P = 0.001) and DFS (P = 0.017). Similarly, the bilobar distribution of LM and the presence of extrahepatic metastatic disease at the time of liver resection were associated with worse OS and DFS (Table 3).
Neoadjuvant CTx turned out to be a negative determinant of OS (P = 0.011) and DFS (P = 0.001) in our series.
Biomolecular and surgical characteristicsThe presence of wild-type KRAS together with carcinoembryonic antigen ≤5 mg/mL was associated with a significant increase in OS and DFS.
The transfusion of packed red blood cells was associated with a significant decrease in OS (P = 0.038) and DFS (P = 0.033).
Patients with serious complications had worse OS (P = 0.011) and worse DFS (P = 0.104), although the latter was not significant. Prolonged postoperative stay was also associated with worse OS (P = 0.045).
Lastly, among the variables related to the histopathological study of LM, the presence of an involved margin (<1 mm) also showed worse OS (P = 0.003) and DFS (P = 0.004) (Fig. 3).
Multivariate analysis of survival predictorsApplying Cox regression, we found 5 risk factors associated with OS. Wild-type KRAS was identified an independent factor for a good prognosis, while LM > 4 cm, intraoperative transfusion, serious Clavien-Dindo complications, and the presence of margin involvement were identified as independent prognostic factors for a poor prognosis (Table 4).
Results of Cox regression, with the identification of independent predictive factors for survival.
| Risk factors | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Non-mutated KRAS | 0.26 | 0.14−0.48 | 0.000 |
| Size of LM > 4 cm | 2.02 | 1.11−3.68 | 0.021 |
| Intraoperative transfusion | 4.05 | 2.07−7.92 | 0.000 |
| Severe Clavien-Dindo ≥iii | 2.72 | 1.19−6.24 | 0.018 |
| Margin involvement | 2.13 | 1.11−4.08 | 0.023 |
95% CI: 95% confidence interval; LM: liver metastasis.
In the literature, various publications provide multiple prognostic factors, from which different prognostic scales have been developed to improve the selection of patients who are candidates for liver surgery. The Fong criteria11 represent one of the most widely used scales; however, there are also many discordant data, leading to modifications over the years.12 It is currently very difficult to contraindicate surgery in patients with minimal possibilities for obtaining R0 resection or in patients with resectable extrahepatic disease.13
In our series, baseline factors such as sex, age, or preoperative comorbidities had no statistical relationship with patient survival. Therefore, supporting the results obtained in other studies and ours, neither age nor sex should be factors that strongly contraindicate LM surgery.14–16
Many studies have found a significant correlation between the characteristics of the primary tumor and the prognosis of LM.7,11,17 In our series, this premise has not been confirmed. Engstrand et al18 found worse survival for LM originating in the right colon compared to in the left colon. Although our study did not show differences according to the location of the primary tumor, we observed that, as in the Engstrand study, we have a greater number of patients with LM of the left colon than the right colon (73 vs 35), which could indicate that patients with LM of the right colon have fewer options for liver surgery.
The TN status of the primary tumor has also been associated with a worse survival outcome for patients with LM. In our case, the different types of T did not influence survival in our series. N is a strong predictor of survival in CRC, and some studies also suggest its importance in the prognosis of LM.19,20 In our case, it correlated with lower survival in the univariate analysis, but not in the multivariate analysis.
In our series, mutated KRAS was a constant determinant for worse survival. Several studies in the last decade have suggested that the KRAS mutation is an important predictive biomarker in colorectal liver metastatic disease,21–23 and its presence is indicative of worse tumor biology with more aggressive LM behavior.24,25 Passiglia et al22 found that mutated KRAS was a negative prognostic biomarker in patients treated with surgical resection of CLM, recommending the evaluation of this biomarker together with clinicopathological factors to more precisely determine the risk and survival of patients who are candidates for liver surgery, thereby improving their selection.
The objective of neoadjuvant CTx in CLM is to increase survival, favoring complete surgical resection; however, the role of neoadjuvant CTx is different depending on whether we are talking about resectable or unresectable disease.26 In resectable LM at the outset, neoadjuvant CTx would allow us to determine the pathological response to treatment as well as the tumor biology, meaning that an individualized therapeutic plan could be developed for each patient; however, we still do not have enough scientific evidence in this regard.27,28 For unresectable or borderline LM, the goal is to make the disease resectable (conversion therapy),29 as published studies show a significant increase in survival, with 5-year OS rates between 25% and 58%. In our case, neoadjuvant CTx was indicated in borderline cases and was identified as a negative factor for survival in the univariate analysis, while showing no significance in the multivariate analysis. This datum is important to compare our results with those that exist in the literature, since OS does improve when comparing initially unresectable patients who are resected after chemotherapy versus patients who are unresectable with exclusive CT treatment. However, our results have also shown that OS worsens when patients with conversion therapy are compared to initially resectable patients.30–32
Both the size and number of metastases are significant indicators of survival according to several published prognostic scales.7,11,33 Currently, due to the introduction of new biological agents, the predictive value of the morphological characteristics of LM (number and size) has been modified, becoming inconclusive in other studies.34,35 In our case, only the maximum size >4 cm was an independent negative predictive factor.
As in most studies, margin involvement was an independent negative predictive factor. Much has been discussed to establish the ideal size of the affected margin, and some studies have suggested that a margin >1 cm is optimal and an independent factor for survival. However, margins <1 cm also have associated favorable results and, therefore, should not contraindicate surgery.36 In 2015, the research group Experts in Onco-Surgical Management of Hepatic Metastasis (Expertos en Manejo Onco-Quirúrgico de Metástasis Hepáticas, EGOSLIM) suggested that a free margin of 1 mm is sufficient in CLM surgery.37
Our results also show that intraoperative blood transfusion is an independent predictor for survival. An immunological interaction related to transfusions has been observed, which in turn negatively influences oncological results. It could be argued that the greater the disease, the greater the surgical difficulty, with a high risk of bleeding and therefore a greater need for transfusion, as well as the consequent and inherent risk of recurrence. However, studies have shown that, after eliminating all confounding factors (tumor burden, length of surgery, preoperative anemia, etc), transfusion alone leads to lower cancer-specific survival, and this risk increases as the number of transfused units increases.38
Serious surgical complications were also an independent factor for worse survival in our study. Postoperative complications are known to produce a prolonged period of immunosuppression, which leads to the proliferation and survival of residual tumor cells and is associated with greater neoplastic proliferation and worse survival.39,40
The selection of candidate patients for CLM surgery has traditionally been based on clinicopathological prognostic factors; however, the fact that most patients undergoing surgery subsequently experience recurrence reinforces the need to continue searching for more effective predictive markers when deciding on the treatment to follow. Our study has demonstrated the important predictive value of KRAS, which supports emerging evidence that biomolecular markers may play an important role in better patient selection for surgical treatment.41 The limitations of our study are that the sample is from a single center, with no external validation, therefore the outcome cannot be generalized. As it is a retrospective study, there is also possible selection error, especially in patients who underwent neoadjuvant CTx who had worse tumor biology and were not randomized.
In conclusion, our study identifies 5 prognostic factors for survival: larger size of LM > 4 cm, intraoperative transfusions, serious postoperative complications, margin involvement, and mutated KRAS. Among these, 4 factors are well identified in the literature and apparently continue to have significant predictive value. The KRAS mutation has been shown to be a consistent negative predictor for prognosis in patients with CLM. However, more multicenter studies are needed to strengthen the prognostic weight of our results.
Conflict of interestsThe authors have no conflict of interests to declare.