Laparoscopic sleeve gastrectomy (LSG) has been providing very good results for weight loss as well as improvement in comorbidities.1–3 The fact it has been mistakenly considered a simple, easy-to-reproduce technique has led to its being performed by a large number of surgeons. When compared with gastric bypass and biliopancreatic diversions, it may seem a more manageable surgery from a laparoscopic standpoint, but its complications may be even more serious than those of other techniques. This is especially true for gastric fistulas. We report the case of a patient who was hospitalized for more than 15 months due to a gastric fistula after LSG.
The patient had a BMI of 61.3 and OSAS that was being treated with cPAP. LSG was performed. Gastric dissection was performed using a 34F gastric tube. Afterwards, the staple line was invaginated with a continuous monofilament 2-0 suture. 48h later, a methylene blue water tightness test performed was normal and after 72h the patient was discharged with a liquid diet.
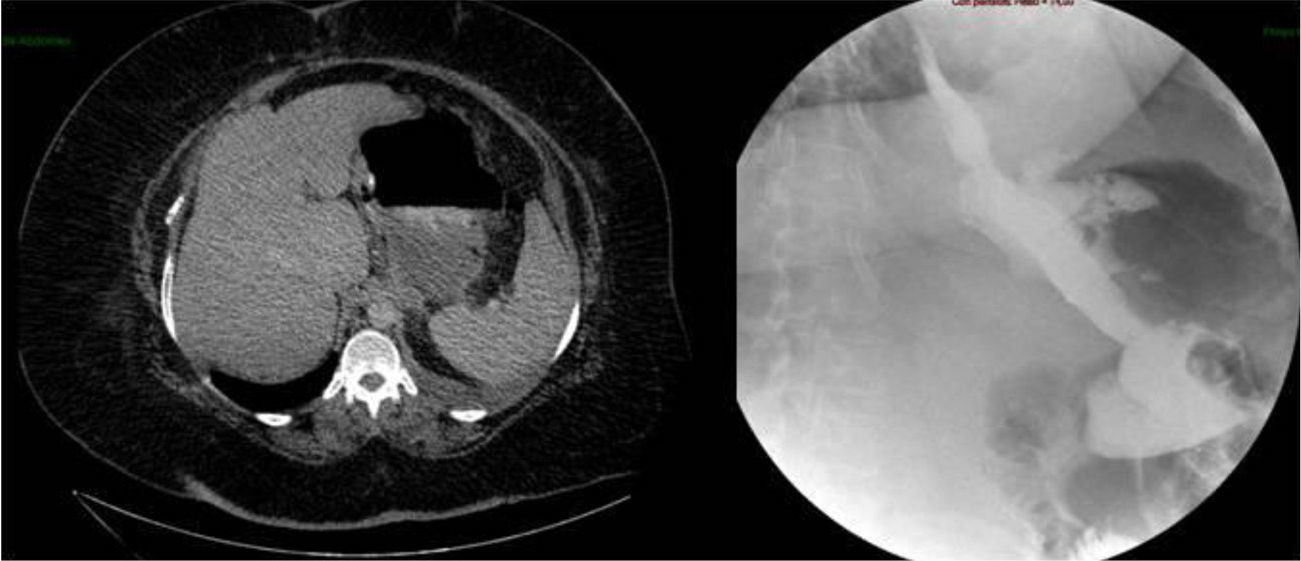
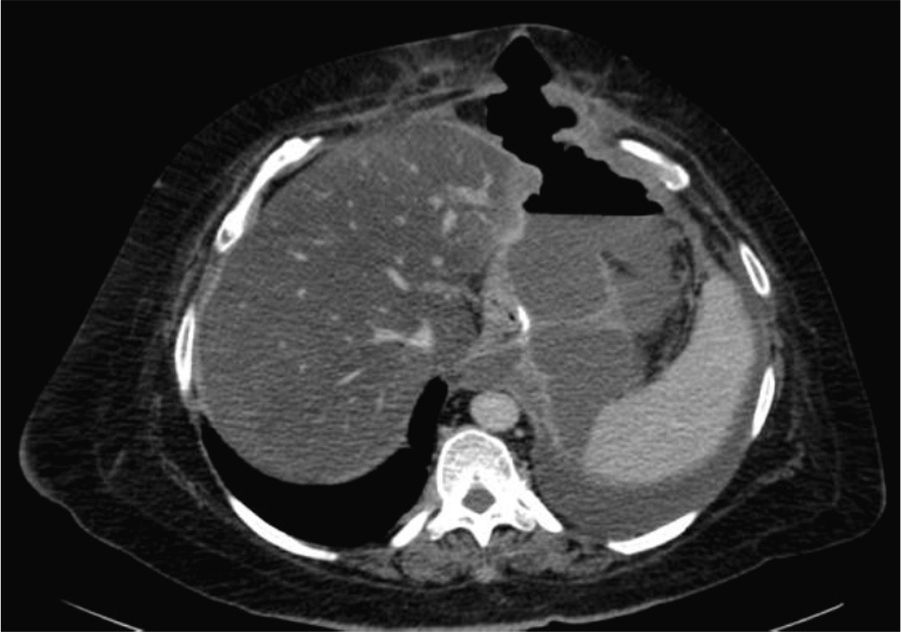
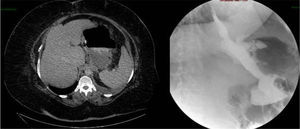
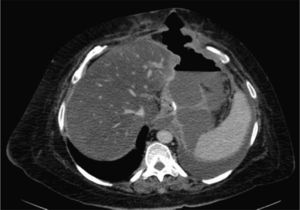
Two weeks later, the patient was hospitalized due to fever with no abdominal pain. Emergency CT revealed a large abdominal collection secondary to a gastric fistula (Fig. 1). Given the situation of the patient, we decided to operate in order to drain the abscess, wash the cavity and place a drain tube (intraoperatively, it was impossible to observe the fistula since the tests done with air and methylene blue were negative). Three days after the surgery, an upper gastrointestinal series showed a leak in the proximal third of the gastrectomy with no associated distal stenosis (Fig. 1). Conservative treatment was begun (no oral intake, parenteral nutrition and jejunostomy feeding catheter). After 6 weeks, the discharge through the drain tube was largely reduced, but the signs of the fistula still remained on the imaging tests. We implanted a covered stent, but it had to be withdrawn 4 days later due to poor tolerance by the patient. After 5 whole months of conservative treatment, and given the demands of the patient, an operation was decided to try to resolve the problem surgically. During the procedure, we observed a pointed fistula centered on healthy tissue, so we decided on a simple closure and invagination (double suture). During the 2 weeks after the surgery, the water-tightness tests were negative, and the patient was discharged. Two months later, the patient was re-hospitalized due to fever and dyspnea, with CT results that demonstrated a large abdominal abscess and left pleural effusion secondary to gastric fistula (Fig. 2). The abscess was drained with endoscopic needle aspiration and the fistula continued to have a daily discharge of 20 cc for 2 months. We decided to operate once again with the intention to reduce the intraluminal pressure, and a gastric bypass was performed. Since this last surgery, the patient has been asymptomatic, has tolerated oral intake and has had negative radiological tests.
LSG is an apparently simple technique for the treatment of morbid obesity. Nevertheless, its complications, although uncommon, may put patients’ lives at risk. The main complications with this technique are hemorrhage (0%–6.4%) and gastric fistula in the proximal third (0%–20%), with a mortality rate that ranges between 0 and 3.2%.3–5 The management of fistulas after LSG is difficult because conservative treatment does not always provide good results and can become chronic for months.4–8 When conservative treatment is not successful, most authors argue that the next step should be the use of flexible covered stents5,9,10 in an attempt at creating a “temporary bypass” of the fistula. Other proposed treatments include the endoscopic use of adhesives (surgical glues),5,7 which has provided controversial results. In cases that do not respond to any treatment, the possibility of treating the distal stenosis (if there is any) with endoscopic dilatations should be assessed. If there is no other option left except for surgery, simple fistula closure should be avoided and gastric intraluminal pressure should be reduced by converting the technique to gastric bypass.
Please cite this article as: Ferrer Márquez M, Belda Lozano R, Solvas Salmerón MJ, Ferrer Ayza M. Fístula crónica refractaria a tratamiento tras gastrectomía vertical laparoscópica. Cir Esp. 2014;92:365–366.