The purpose of this study was to evaluate the accuracy of multidetector computed tomography (MDCT) for locating the site of gastrointestinal tract perforations and to determine the most predictive signs in this diagnosis.
Material and methodsA total of 98 patients with pneumoperitoneum on MDCT were retrospectively analyzed. Two experienced radiologists reviewed the presence or absence of direct signs (extravasation of oral contrast, focal defect in the bowel wall, focal defect with multiplanar reformations images), and indirect signs (free air in supramesocolic, inframesocolic, supramesocolic, and inframesocolic compartments, concentration of extraluminal air bubbles adjacent to the bowel wall, extraluminal fluid, segmental bowel-wall thickening, perivisceral fat stranding, abscess) to identify the site of the perforation. The Kappa index was evaluated between radiologists to determine the site of perforation and for each predictive sign, as well as Kappa index between the site of perforation detected with MDCT and the site proven at surgery. The frequency, sensitivity, specificity, and positive and negative predictive value (PPV and NPV, respectively) were calculated.
ResultsThe perforation site was identified correctly in 80.4% of cases. Kappa index between radiologists to identify the site was excellent (0.919), varying between 0.5 and 1.0 for each radiological sign. The most frequent site of perforation at surgery (33.7%) and in MDCT (40.82%) was the sigmoid colon/rectum. Concentration of extraluminal air bubbles adjacent to the bowel wall was the most sensitive (91%) sign and “segmental bowel-wall thickening” had the highest PPV (90%).
ConclusionMDCT is useful for locating the site of GI perforation, with a high sensitivity (80%) and an excellent agreement between radiologists.
Valorar la capacidad de la tomografia computarizada multidetector (TCMD) para identificar la localización de la perforación gastrointestinal (GI).
Material y métodosAnálisis retrospectivo de 98 pacientes con neumoperitoneo en la TCMD. Dos radiólogos expertos evaluaron la presencia o ausencia de signos radiológicos directos (extravasación del contraste oral; defecto focal de la pared; defecto focal en reconstrucciones multiplanares) e indirectos (aire libre supramesocólico; inframesocólico; supra- e inframesocólico; burbujas de gas adyacentes a la pared; líquido libre; engrosamiento parietal segmentario; trabeculación de la grasa; abscesos) de perforación para identificar su ubicación. Se determinó la concordancia kappa entre los radiólogos para identificar el lugar de la perforación y la presencia o ausencia de cada uno de los signos radiológicos; así como la correlación kappa de la localización detectada mediante TCMD y su confirmación o no en la intervención quirúrgica. Se calculó para cada signo radiológico su frecuencia, sensibilidad, especificidad, valor predictivo positivo (VPP) y negativo (VPN).
ResultadosSe diagnóstico correctamente el sitio de la perforación en un 80% de los casos. El índice kappa entre radiólogos para la localización fue excelente (0,919), variando para cada signo radiológico entre 0,5 y 1. La localización más frecuente de la perforación en la intervención quirúrgica (33,7%) y en la TCMD (40,8%) fue colon sigmoideo/recto. “Burbujas de gas adyacentes a la pared” fue el signo con mayor S (91%) y el “engrosamiento parietal segmentario” el que tuvo un mayor VPP (90%).
ConclusiónLa TCMD permite localizar las perforaciones gastrointestinales con una alta sensibilidad (80%) y excelente correlación interobservador.
The finding of pneumoperitoneum in a patient with acute abdominal pain is the main diagnostic sign of gastrointestinal (GI) perforation,1 which usually requires surgical treatment. GI tract perforation is a disruption in the integrity of the gastrointestinal wall that may be caused by various etiologies. Classically, simple standing chest radiography including the diaphragm is the first imaging test that is done in order to identify the presence of extraluminal gas, although it is sometimes difficult to establish the diagnosis because the symptoms are non-specific and pneumoperitoneum is only observed on 30%–59% of simple radiographs.2,3 Several studies have demonstrated that computed tomography (CT) is the best technique for detecting free intraperitoneal air and for the diagnosis of GI perforation.4 The pre-operative localization of the intestinal perforation site can help the surgeon in the therapeutic approach. For the surgical treatment of GI tract perforations, less-aggressive laparoscopic procedures are currently preferred over open laparotomy5,6 but in lower GI tract perforations, laparotomy is usually required.7 Thus, it is useful for the surgeon to know the location of the perforation before initiating the surgical procedure.
Multidetector CT (MDCT) provides multiplanar reconstruction (MPR) with optimal spatial resolution and high quality, which increases the sensitivity of CT for detecting the site of the perforation.8,9 In recent years, several papers have been published with MDCT that have analyzed the value of different radiological signs in identifying the perforation site.9–11
The objective of our study is to analyze the capacity of MDCT to identify the site of GI perforations and to determine which radiological signs, either direct or indirect,10 are the most predictive. We will also analyze the interobserver agreement for both diagnosis and identification of the localization.
Material and MethodsPatientsThis is a retrospective study carried out in the emergency radiology area at Hospital Universitario La Paz for a period of 28 months (April 2007 to August 2009). We analyzed all the MDCT exams in our database of patients who came to the emergency room with acute abdominal symptoms and were later diagnosed with pneumoperitoneum or GI perforation on CT. In all patients, the presence of gastrointestinal perforation was confirmed during surgery.
Excluded from the study were those patients who had undergone surgery within the previous 15 days and those cases in which the exact site of the perforation was not confirmed during surgery.
ProcedureThe studies were done with an MDCT (Toshiba Asteion) using the following parameters: FOV 400, cut thickness 0.5mm, Pitch 3.00, 120kV, and 180mA. In most cases, the studies were done after the administration of IV contrast, except in those cases in which the use of iodine was contraindicated. The patients were administered 120mL at 2.5mL/s with an injection pump and a 60-s delay.
Oral/rectal contrasts were not administered systematically because in some cases this would have delayed the completion of the test. In our study, 90% of the patients received only IV contrast, 7% received IV and oral/rectal contrast, and in 2% no IV contrast was used.
Image AnalysisThe images were reviewed at a workstation (Vitrea®). Two expert radiologists, with no previous knowledge of the clinical histories or the surgical or histological results, evaluated the axial images and the multiplanar reconstruction.
The two radiologists independently analyzed the images and determined the presence of the following radiological signs and the affected segment:
- 1)
Extravasation of oral contrast.
- 2)
Intestinal wall focal defects.
- 3)
Free air in the supramesocolic compartment.
- 4)
Free air in the inframesocolic compartment.
- 5)
Free air in supra- and inframesocolic compartments.
- 6)
Gas bubbles adjacent to the intestinal wall.
- 7)
Localized extraluminal fluid.
- 8)
Segmental wall thickening (>3mm).
- 9)
Perivisceral fat stranding.
- 10)
Abscesses.
- 11)
Sagittal and coronal MPR to view the focal wall defect.
Signs 1, 2, and 11 were considered direct signs, meaning that they are signs that indicate where there is a discontinuity in the GI wall. The remainder were indirect signs of the location of the GI perforation: some indicate the distribution of the extraluminal gas (signs 3, 4, 5, and 6) and others indicate inflammatory changes (signs 8, 9, and 10) that help estimate the affected GI segment.
For the localization of the site of the perforation, the following segments of the digestive tract were considered:
- 1)
Stomach/duodenum.
- 2)
Jejunum and ileum.
- 3)
Appendix.
- 4)
Ascending, transverse, and descending colon.
- 5)
Sigma/rectum.
- 6)
Undetermined (site not identified).
The Kappa correlation coefficient was determined between the two radiologists for detecting the perforation site and for the identification, presence or absence of each sign analyzed. For the remainder of the statistical analysis, the data from radiologist 1 were chosen and the Kappa correlation coefficient was calculated between the predicted location of the perforation site detected by MDCT and the actual site revealed during surgery, which is considered the reference procedure.
The results were considered to be in agreement when the perforation site identified by MDCT was the same as what was identified during surgery (true positive) or when the perforation site was not recognized in either of the two procedures (true negative). Results were considered to be in disagreement when the MDCT did not identify the perforation site (false negative) or defined the origin of the perforation to be different from that found during surgery (false positive).
The frequency of appearance was calculated for each sign and for each radiologist. The data from radiologist 1 were used to calculate the sensitivity (S), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) for each sign in the prediction of the perforation site. A Kappa correlation coefficient greater than 0.4 was considered acceptable; while greater than 0.6 was good and greater than 0.8 was excellent.
ResultsThe study included 98 patients, 46 of whom were men and 52 women, with a mean age of 59 (range 15–97).
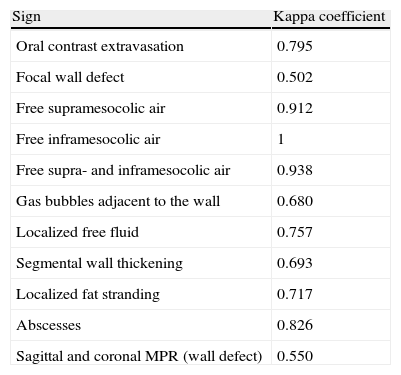
The Kappa correlation coefficient between radiologists for predicting the localization of the GI perforation was 0.919. Table 1 shows the Kappa index between the two radiologists for each radiological sign for gastrointestinal perforation.
Kappa Correlation Coefficient for Each Sign of Gastrointestinal Perforation Between the 2 Radiologists.
| Sign | Kappa coefficient |
| Oral contrast extravasation | 0.795 |
| Focal wall defect | 0.502 |
| Free supramesocolic air | 0.912 |
| Free inframesocolic air | 1 |
| Free supra- and inframesocolic air | 0.938 |
| Gas bubbles adjacent to the wall | 0.680 |
| Localized free fluid | 0.757 |
| Segmental wall thickening | 0.693 |
| Localized fat stranding | 0.717 |
| Abscesses | 0.826 |
| Sagittal and coronal MPR (wall defect) | 0.550 |
MPR, multiplanar reconstructions.
The perforation sites found by radiologist 1 on the MDCT in the 98 patients were: 14 (14.3%) stomach or duodenum; 15 (15.3%) small intestine; 14 (14.3%) appendix; 11 (11.2%) ascending, transverse or descending colon; 40 (40.8%) sigma/rectum; and in four patients (4.1%) the site was not determined. For radiologist 2, the perforation sites identified on MDCT for the 98 patients were: 13 (12.7%) stomach or duodenum; 15 (14.7%) small intestine; 14 (13.7%) appendix; 10 (9.8%) ascending, transverse or descending colon; 40 (39.2%) sigma/rectum; and in six patients (5.8%) the site was not determined.
The locations of the perforations found during surgery in the 98 patients were: 20 (20.4%) stomach or duodenum; 16 (16%) small intestine; 15 (15.3%) appendix; 14 (14.3%) ascending, transverse or descending colon; and 33 (33.7%) sigma/rectum.
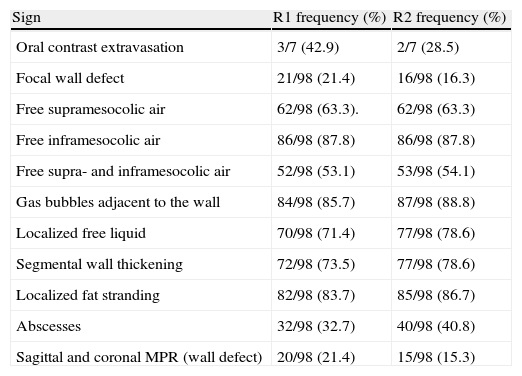
The prediction of the perforation site in the gastrointestinal tract by means of MDCT coincided with the surgical findings (true positive) in 80 (80.4%, Kappa 0.804) out of 98 patients. In 18 patients, the prediction did not concur with the findings. In 14 (14.3%) cases, MDCT identified an incorrect perforation site (false positive) and in four (4.1%) out of 98 patients, MDCT did not identify the location of the GI perforation (false negative). The frequency for each sign is shown in Table 2. The S, Sp, PPV, and NPV for each sign for predicting the perforation site are shown in Table 3.
Frequency of Each Radiological Sign.
| Sign | R1 frequency (%) | R2 frequency (%) |
| Oral contrast extravasation | 3/7 (42.9) | 2/7 (28.5) |
| Focal wall defect | 21/98 (21.4) | 16/98 (16.3) |
| Free supramesocolic air | 62/98 (63.3). | 62/98 (63.3) |
| Free inframesocolic air | 86/98 (87.8) | 86/98 (87.8) |
| Free supra- and inframesocolic air | 52/98 (53.1) | 53/98 (54.1) |
| Gas bubbles adjacent to the wall | 84/98 (85.7) | 87/98 (88.8) |
| Localized free liquid | 70/98 (71.4) | 77/98 (78.6) |
| Segmental wall thickening | 72/98 (73.5) | 77/98 (78.6) |
| Localized fat stranding | 82/98 (83.7) | 85/98 (86.7) |
| Abscesses | 32/98 (32.7) | 40/98 (40.8) |
| Sagittal and coronal MPR (wall defect) | 20/98 (21.4) | 15/98 (15.3) |
MPR, multiplanar reconstructions; R1, radiologist 1; R2, radiologist 2.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of Each Radiological Sign.
| Sign | S % | Sp % | PPV % | NPV % |
| Oral contrast extravasation | 2.5 (2/80) | 94 (17/18) | 66 (2/3) | 17 (17/95) |
| Focal wall defect | 20 (16/80) | 72 (13/18) | 76 (16/21) | 16 (13/77) |
| Free supramesocolic air | 60 (48/80) | 22 (4/18) | 77 (48/62) | 11 (4/36) |
| Free inframesocolic air | 90 (72/80) | 22 (4/18) | 83 (72/86) | 33 (4/12) |
| Free supra- and inframesocolic air | 52 (42/80) | 44 (8/18) | 80 (42/52) | 17 (8/46) |
| Gas bubbles adjacent to the wall | 91 (73/80) | 38 (7/18) | 86 (73/84) | 50 (7/14) |
| Localized free fluid | 75 (60/80) | 44 (8/18) | 85 (60/70) | 28 (8/28) |
| Segmental wall thickening | 83 (67/80) | 61 (11/18) | 90 (67/74) | 45 (11/24) |
| Localized fat stranding | 88 (71/80) | 38 (7/18) | 86 (71/82) | 43 (7/16) |
| Abscesses | 35 (28/80) | 77 (14/18) | 87 (28/32) | 21 (14/66) |
| Sagittal and coronal MPR (wall defect) | 20 (16/80) | 66 (12/18) | 80 (16/20) | 15 (12/76) |
MPR, multiplanar reconstructions; NPV, negative predictive value; PPV, positive predictive value; S, sensitivity; Sp, specificity.
Several authors have demonstrated the utility of CT, and especially MDCT, in localizing GI perforations (S=86%).8,11 In our study, we have retrospectively analyzed the capacity to detect the perforation site, which was 80%. In explorations with conventional CT, the S for detecting the GI perforation site was low (36%).12 The recent introduction of MDCT has provided faster imaging study acquisition, thinner slices, and MPR reconstructions.13 These factors have led to not only better and easier identification of minimal quantities of extraluminal air (Fig. 1) but also the possibility to detail the diagnosis and determine where the gas had leaked, the etiology, and the perforation site (Fig. 2). A correct preoperative diagnosis of the perforated GI region can be very useful for surgeons.
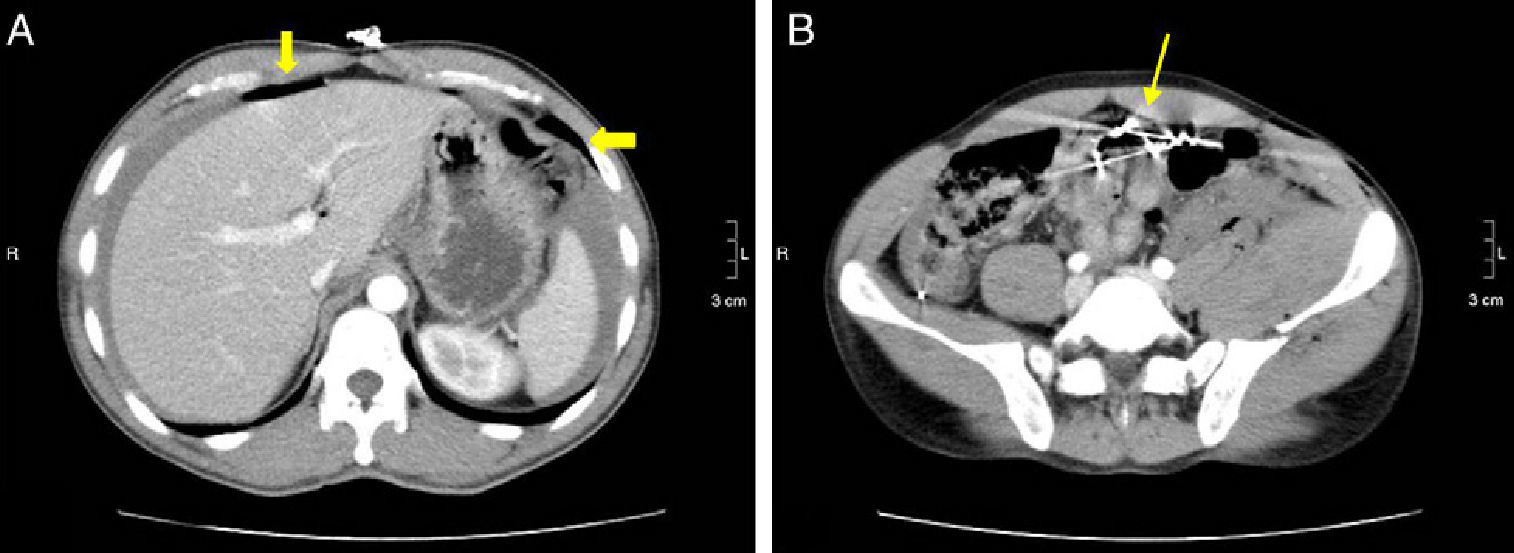
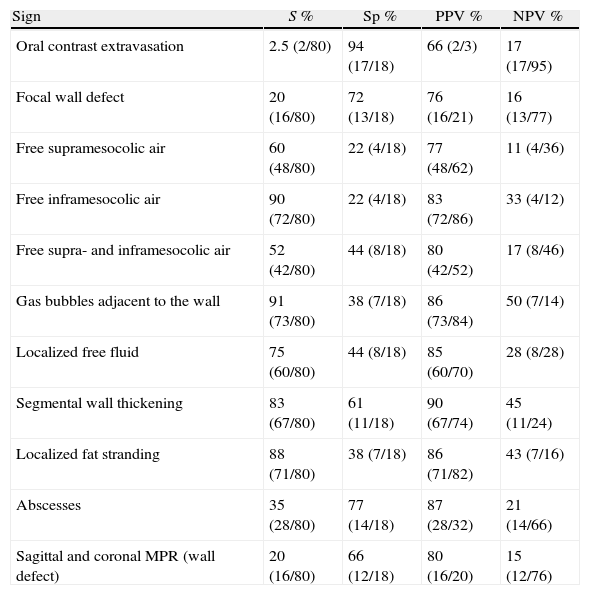
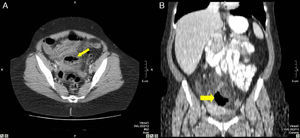
37-Year-old male with perforation of the small intestine by birdshot pellets: (A) MDCT with IV contrast showing an axial slice of the upper abdomen with pneumoperitoneum (bold arrow); (B) axial slice of the same patient with multiple intraabdominal birdshot pellets (arrow). The perforation of the small intestine was identified during surgery.
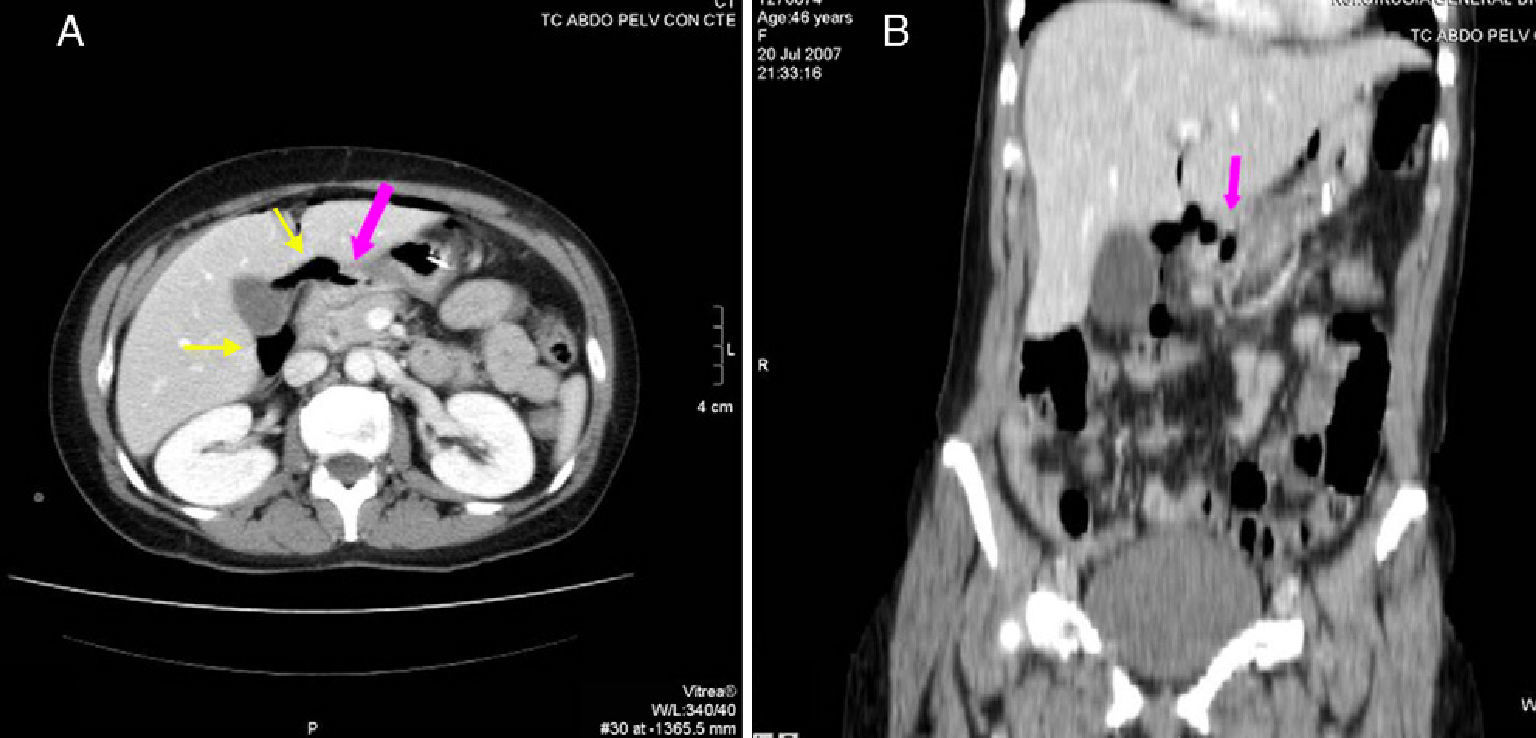
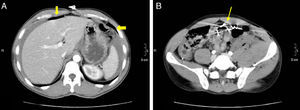
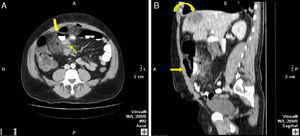
46-Year-old woman with pneumoperitoneum due to a perforated ulcer: (A) on MDCT with IV contrast, an important perihepatic and periportal pneumoperitoneum is identified (thin arrows), suggesting upper gastrointestinal perforation; (B) coronal reconstruction was able to confirm the defect in the anterior wall of the gastric antrum (bold arrow), which was also observed in axial slice A. Surgery confirmed the presence of a perforation in the gastric antrum.
The Kappa correlation coefficient between radiologists for predicting the localization of the perforation in our study is 0.919, which is excellent. Once again, this supports the fact that CT is an objective test for the diagnosis of GI perforation.4 In a recent study, Kim et al.14 have also obtained excellent interobserver agreement.
The three most frequent signs observed in our study are: free extraluminal air in the inframesocolic compartment, stranding of adjacent fat, and gas bubbles adjacent to the wall (Fig. 3).
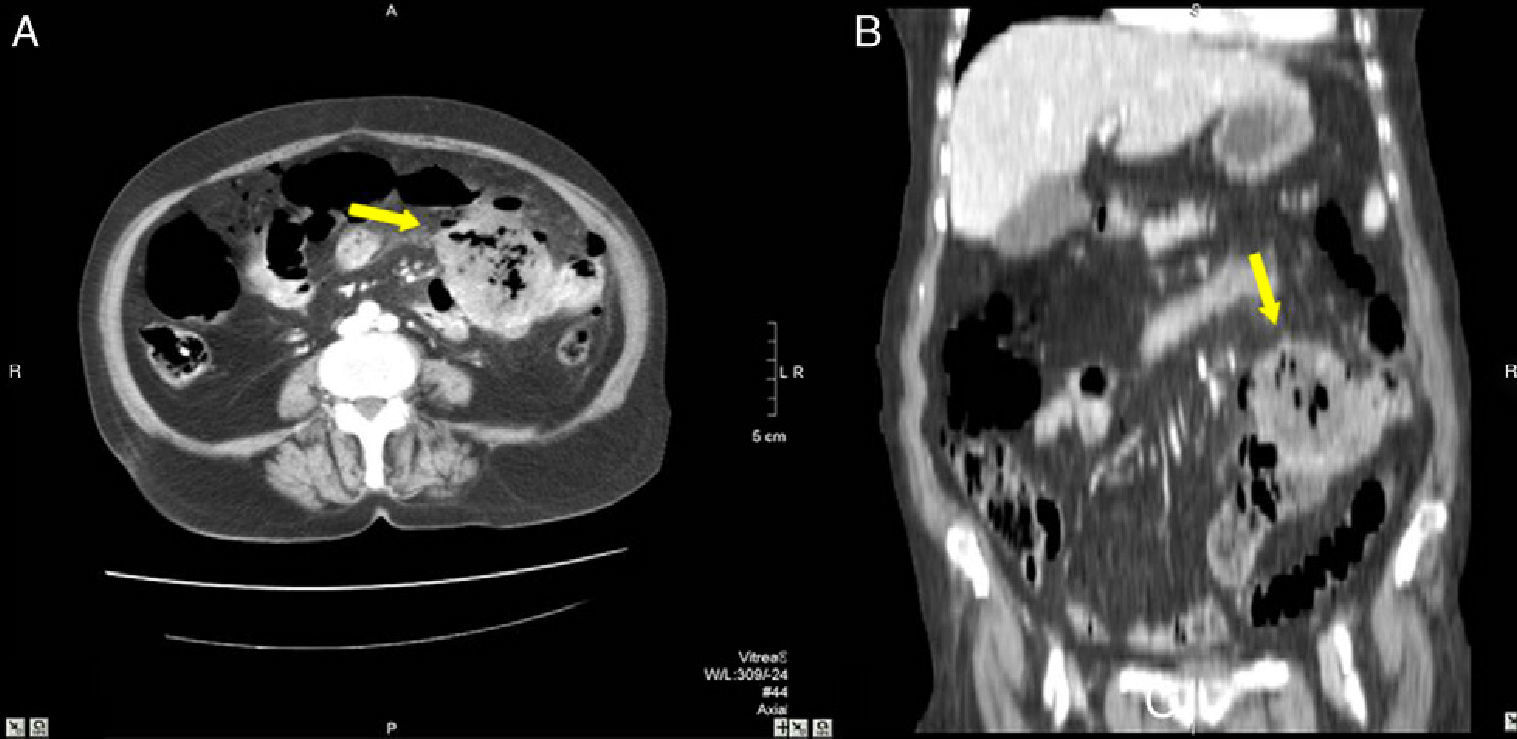
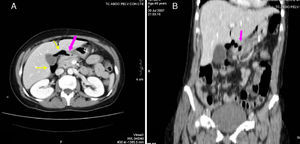
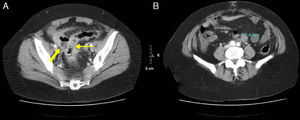
82-Year-old woman with pneumoperitoneum: (A) MDCT axial slice with IV contrast showing multiple extraluminal gas bubbles and a 7cm collection (arrow) with irregular wall, adjacent to the jejunum; (B) coronal reconstruction demonstrating an abscess (arrow) and jejunum. The pathology report confirmed perforated GIST of the small intestine.
The three most sensitive signs in our study for detecting the perforation site were the presence of gas bubbles adjacent to the wall (S=91%), free extraluminal air in the inframesocolic compartment (S=90%), and stranding of adjacent fat (S=88%). All three are indirect signs (Fig. 4). Hainaux et al.11 determined that the most sensitive signs were: fat stranding (92%), concentration of extraluminal bubbles (89%), and free fluid (67%). Oguro et al.10 analyzed the S of the direct and indirect signs for detecting upper or lower GI perforation, concluding that the direct signs are more sensitive (95.5%) than the indirect signs (50%) for detecting upper gastrointestinal perforations. Meanwhile, for lower gastrointestinal perforations, indirect signs (78.9%) were more sensitive than direct signs (63.2%).
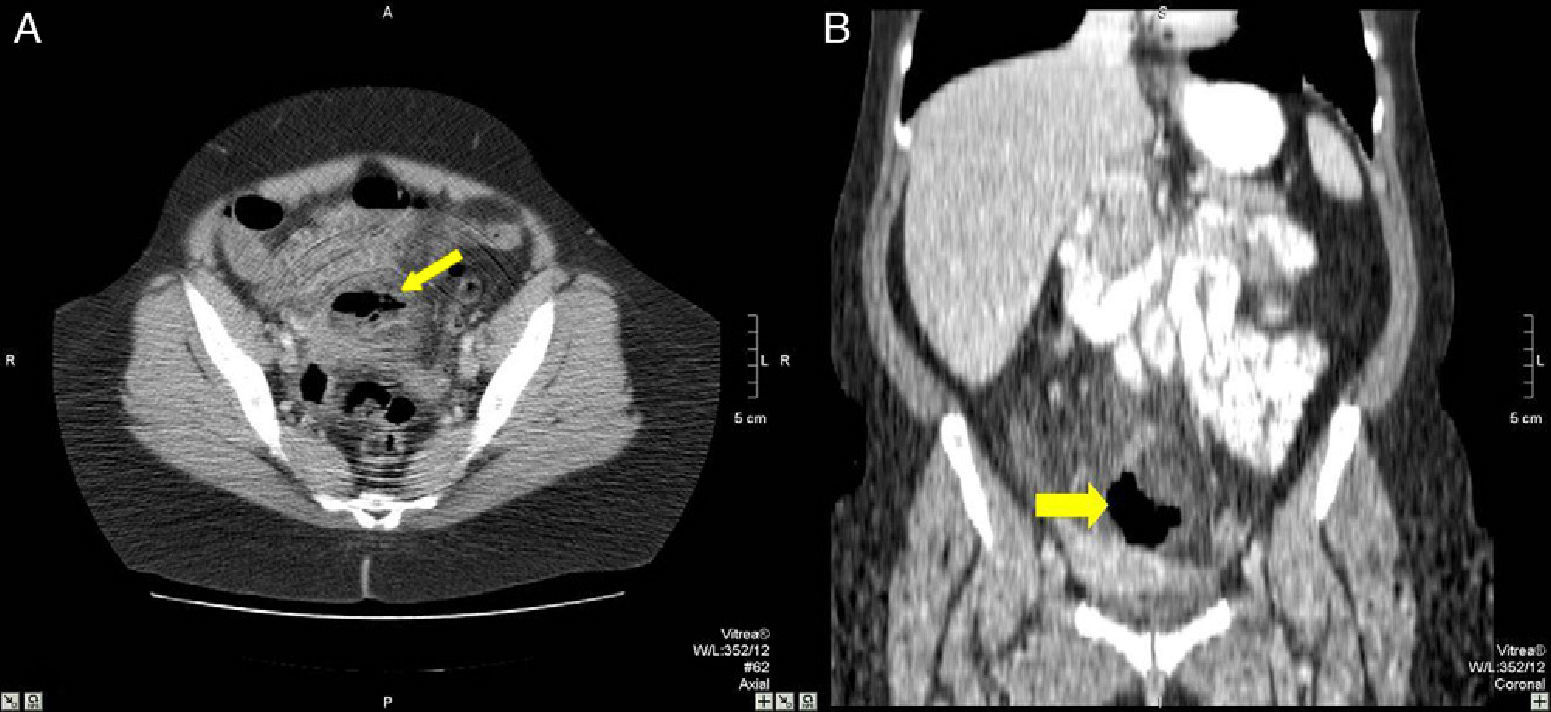
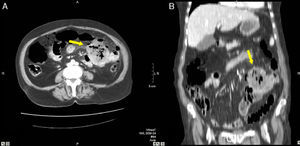
48-Year-old woman with perforated acute appendicitis: (A) MDCT demonstrating an abscess (arrow) and free liquid in the pelvis, without identification of the appendix; (B) coronal reconstruction of the same patient with a collection of gas (arrow) in the pelvis. Anatomic pathology confirmed perforated appendicitis.
The most specific signs in our study were extravasation of the oral contrast (94%), observation of a wall defect (72%), and the presence of abscesses (77%). Extravasation of oral and rectal contrast is infrequent in several studies and is considered a sign of perforation. In closed abdominal trauma with perforation of hollow viscera, it has shown an S of 19%–42%,15 and in penetrating abdominal trauma with GI perforation it has been observed with a frequency of 15%.16 Due to the limited number of studies (seven out of 98 patients) with oral contrast, the presence of the direct sign of contrast extravasation was found in three cases with low S but high Sp at 94%. In our study, the MDCT were revised retrospectively and oral/rectal contrast was not administered systematically because it depended on the criteria of the radiologist and the state of the patient (Fig. 5). Oral contrast should be administered approximately 60min before the test, which delays the timing of the study, and most patients with acute abdominal symptoms cannot wait that long.
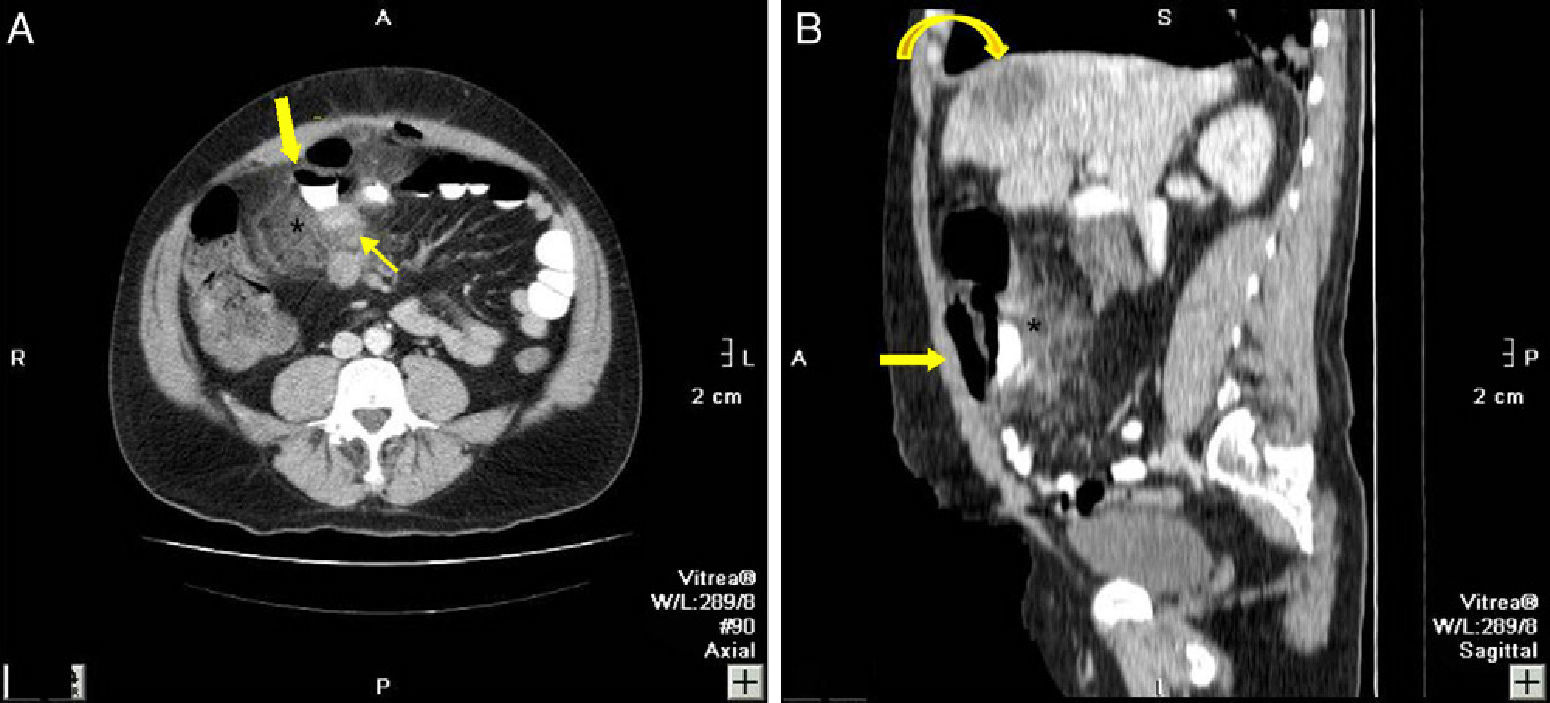
44-Year-old male with pneumoperitoneum: (A) MDCT axial slice with oral and IV contrast showing extraluminal gas and extravasation of oral contrast (bold arrow), intense adjacent fat stranding (*), and thickening of the small bowel loops (thin arrow); (B) sagittal reconstruction showing the extravasation of oral contrast (arrow) and fat stranding (*); an SOL in the liver is also observed (curved arrow). Surgery confirmed perforation of the small intestine, while pathology confirmed the presence of a large-cell carcinoma in the distal ileum with infiltration in the mesentery and hepatic metastasis.
The frequency of the direct visualization of the perforation site presents much variability, as seen in our bibliographic review, which depends on the type of CT used, collimation, and experience of the radiologist. We have found percentages ranging from 0% to 43%–53%, and reaching up to 72% in upper GI perforations. Greater percentages of visualization of this sign were obtained with MDCT (8- and 16-detector), using fine axial reconstructions (1.25mm) and coronal and sagittal MPR reconstructions compared with the 5mm cuts.17 In our study, we also wanted to analyze this sign in the reconstructions that the MDCT provides automatically in coronal and sagittal views. The sign analyzed is “visualization of the wall defect on MPR”, present in 21% of the patients on axial images. With the MPR reconstructions, the detection rate did not increase.
In a series with MDCT (64 detectors) in 41 patients, Cho et al.18 correctly diagnosed the perforation site by recognizing the focal wall defect in 80% (2-mm thick axial images and 1mm MPR); this direct sign was more sensitive and precise in upper GI perforations (S 95.5%).
In our study, the signs with a PPV for identifying the GI perforation site are: segmental wall thickening (90%), the presence of abscesses (87%), and gas bubbles adjacent to the wall (86%) (Fig. 6). Hainaux et al.11 determined that the signs with greater PPV are: the concentration of extraluminal air bubbles adjacent to an intestinal loop, segmental intestinal wall thickening, and a focal defect in the wall of the intestine.
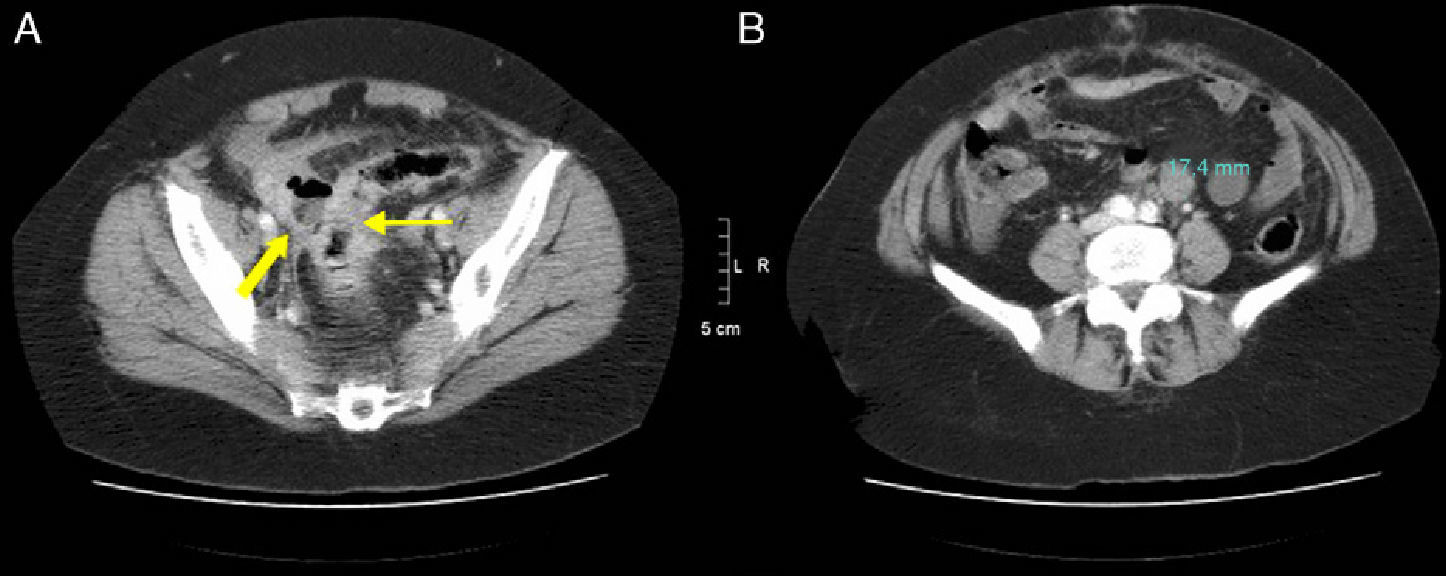
54-Year-old woman with acute abdominal symptoms and pneumoperitoneum seen on MDCT: (A) MDCT axial slice with IV contrast of the pelvis revealing asymmetrical thickening of the sigma wall (thin arrow), free fluid, and an adjacent abscess with air-fluid level (bold arrow); (B) axial slice at a more cranial level where a pathologic retroperitoneal lymphadenopathy is observed (17mm). The pathology study confirmed a perforated adenocarcinoma of the sigma with positive retroperitoneal lymph nodes.
One of the main limitations of our study is that it is a retrospective study, and each test was used according to the criteria of the radiologist. Another limitation is that the CT that we have available in our emergency department is only a 2-detector scanner, which provides MPR reconstructions but not with the higher quality offered by the latest MDCT models.
MDCT is able to locate gastrointestinal perforation sites with a high sensitivity and excellent interobserver correlation. The radiological signs that identify GI perforation sites with the highest sensitivity were the presence of gas bubbles adjacent to the wall, extraluminal free air in the inframesocolic compartment, and adjacent fat stranding. The most specific were the extravasation of oral contrast, the observation of a wall defect, and the presence of abscesses.
Conflict of InterestsThe authors declare having no conflict of interests.
The authors would like to express their gratitude toward the Departments of Surgery and Statistics at the Hospital Universitario La Paz.
Please cite this article as: Cadenas Rodríguez L, Martí de Gracia M, Saturio Galán N, Pérez Dueñas V, Salvatierra Arrieta L, Garzón Moll G. Utilidad de la tomografía computarizada multidetector para identificar la localización de las perforaciones gastrointestinales. Cir Esp. 2013;91:316–323.
This paper was presented as an electronic poster at the 30 Congreso Nacional de la SERAM in La Coruña, Spain on 27 May, 2010.