The effectiveness of the Enhanced Recovery After Surgery (ERAS) protocols in gastric cancer surgery remains controversial.
MethodsMulticentre prospective cohort study of adult patients undergoing surgery for gastric cancer. Adherence with 22 individual components of ERAS pathways were assessed in all patients, regardless of whether they were treated in a self-designed ERAS centre. Each centre had a three-month recruitment period between October 2019 and September 2020. The primary outcome was moderate-to-severe postoperative complications within 30 days after surgery. Secondary outcomes were overall postoperative complications, adherence to the ERAS pathway, 30 day-mortality and hospital length of stay (LOS).
ResultsA total of 743 patients in 72 Spanish hospitals were included, 211 of them (28.4 %) from self-declared ERAS centres. A total of 245 patients (33 %) experienced postoperative complications, graded as moderate-to-severe complications in 172 patients (23.1 %). There were no differences in the incidence of moderate-to-severe complications (22.3% vs. 23.5%; OR, 0.92 (95% CI, 0.59 to 1.41); P = 0.068), or overall postoperative complications between the self-declared ERAS and non-ERAS groups (33.6% vs. 32.7%; OR, 1.05 (95 % CI, 0.70 to 1.56); P = 0.825). The overall rate of adherence to the ERAS pathway was 52% [IQR 45 to 60]. There were no differences in postoperative outcomes between higher (Q1, > 60 %) and lower (Q4, ≤ 45 %) ERAS adherence quartiles.
ConclusionsNeither the partial application of perioperative ERAS measures nor treatment in self-designated ERAS centres improved postoperative outcomes in patients undergoing gastric surgery for cancer.
Trial RegistrationClinicalTrials.gov Identifier NCT03865810
La efectividad de los protocolos de recuperación intensificada o ERAS en la cirugía del cáncer gástrico sigue siendo controvertida.
MétodosEstudio de cohortes prospectivo multicéntrico de pacientes intervenidos de cáncer gástrico. Se evaluó la adherencia a 22 elementos ERAS en todos los pacientes, independientemente de la existencia de un protocolo ERAS. Cada centro tuvo un período de reclutamiento de tres meses, con un seguimiento de 30 días. La medida de resultado primario fue el numero de complicaciones posoperatorias moderadas a graves. Las medidas de resultado secundarias fueron el número total de complicaciones, la adherencia a los elementos ERAS, la mortalidad y la estancia.
ResultadosSe incluyeron 743 pacientes en 72 hospitales, 211 (28,4 %) en centros ERAS. 245 pacientes (33 %) experimentaron complicaciones posoperatorias, moderadas o graves en 172 (23,1 %). No hubo diferencias en la incidencia de complicaciones moderadas a graves (22,3 % vs. 23,5 %; OR, 0,92 (IC 95 %, 0,59 a 1,41); P = 0,068), o complicaciones posoperatorias totales entre los centros ERAS y no ERAS (33,6 % vs. 32,7 %; OR, 1,05 (IC 95 %, 0,70 a 1,56); P = 0,825). La adherencia a los elementos ERAS fue del 52% [IQR 45 a 60]. No hubo diferencias entre los cuartiles de cumplimiento ERAS más alto (Q1, > 60 %) y más bajo (Q4, ≤ 45 %).
ConclusionesNi la aplicación parcial de medidas ERAS ni el tratamiento en centros ERAS mejoraron los resultados en pacientes sometidos a cirugía gástrica por cáncer.
Gastric cancer is the fifth most frequent malignant tumour worldwide, and accounts for 732,000 deaths per year, ranking it as the fourth leading cause of cancer-related death.1 Gastrectomy for cancer is a technically challenging procedure, with postoperative complications occurring in 20 to 46 % of patients2,3
Enhanced Recovery After Surgery (ERAS) is a multidisciplinary perioperative approach that provides standardized evidence-based recommendations for the care of patients undergoing specific types of surgery.4 ERAS began in patients undergoing colorectal surgery,5 and resulted in a decrease in postoperative complications and length of hospital stay (LOS) when adequate adherence to the protocols was achieved.6,7 The ERAS model is well established for colorectal surgery as the optimal perioperative care. ERAS implementation in gastric surgery8 has shown discrete decreases in LOS.9 Moreover, despite the clinical reported benefits of ERAS, its implementation in clinical practice has been quite slow for a variety of reasons, such as lack of convincing data, low level of knowledge about ERAS, or expertise and institutional limitations,10,11 and lack of large studies assessing the association between ERAS adherence and postoperative outcomes.
The aim of this study was to assess perioperative care in patients undergoing elective gastric surgery for cancer in Spain, and to analyse the association between the individual ERAS elements and postoperative complications.
MethodsStudy design and participantsThe POWER 4study was a multicentre, prospective, three-month cohort study. The study was approved by the Ethics Committee of the Instituto Aragonés de Ciencias de la Salud, Zaragoza, Spain (C.P.-C.I. PI19/106, March 27, 2019) and was prospectively registered (NCT03865810). The study protocol was published,12 and approved by the ethics committees or institutional review boards of each centre. All patients signed a written informed consent before inclusion. This study followed the STROBE reporting guideline for cohort studies,13 and the Reporting on ERAS Compliance, Outcomes, and Elements Research Checklist.14 The hospital and investigator involvement were provided through the Spanish Perioperative Audit and Research Network (RedGERM). All Spanish centres were invited to participate regardless of having or not an established ERAS pathway.
ProceduresAll consecutive adult patients scheduled for elective gastric surgery for cancer were assessed for inclusion during a single period of three months of recruitment at each participating hospital between October 2019 and October 2020. Subtotal and total gastrectomies were included, both laparoscopic and open. Exclusion criteria were emergency surgery, endoscopic procedures, non-oncological gastric surgery and patient refusal. Each patient was followed up for 30 days after surgery. Patient information was acquired from hospital and primary care medical records.
Data was collected using the Castor EDC platform15 in a case record form designed specifically for POWER 4. Centres declared themselves as centres with or without an established multidisciplinary ERAS pathway for gastric surgery, regardless of the items that conformed the pathway and their current adherence.
The definition of the individual ERAS components was based on the ERAS Society® Guidelines for gastric surgery.8 For a simpler and more accurate data collection, some of the 25 items of these guidelines8 were grouped and POWER4 included 22 elements of perioperative care12(Table S1 supplementary data).
Data included patient characteristics, surgical procedure, surgical approach, duration of surgery, preoperative laboratory results, ERAS elements, and 30-day outcomes (postoperative complications, LOS, readmission, reoperation, and 30-day mortality). Thirty-day postoperative complications were predefined and graded as mild, moderate, or severe as described by the European Perioperative Clinical Outcome definitions (EPCO)16 (Table S2, supplementary data).
Data was censored at 30 days following surgery for patients who remained at the hospital. To ensure the validity of the data, they were validated and audited by another site investigator who was not involved in the initial data collection. Our aim was to recruit as many hospitals and patients in Spain as possible.
OutcomesThe primary outcome measure was 30-day moderate-to-severe postoperative complications. Secondary outcomes were occurrence of overall postoperative complications, adherence to the ERAS pathway, 30 day-mortality and LOS.
Adherence to the ERAS pathway was defined as the percentage of ERAS items that were applied to each patient over the total number of interventions recommended by the ERAS Society.
Statistical analysisThe results were analysed according to whether the patient underwent surgery in a self-designed ERAS centre or in a non-ERAS centre. Discrete variables were described as absolute frequencies and percentages and their differences were analysed using Fisher's or Pearson's exact tests. Continuous variables were presented with medians with their corresponding interquartile ranges (IQR) and statistical differences were calculated using the Wilcoxon rank sum test. Adherence to the ERAS pathway was calculated for each individual patient according to the number of the ERAS items achieved over the 22 analysed items (ERAS adherence). Subsequently, the analysis was repeated, subdividing the entire sample into four quartiles according to the rate of adherence to the ERAS items (Q1: highest adherence, Q4: lowest adherence), regardless of whether or not the patients was treated at a self-designed ERAS centre. Highest and lowest adherence quartiles were compared, and linear adjustments of adherence were performed for each ERAS element. We then analysed the rate of moderate-to-severe complications for each ERAS element using Fisher's exact test, and we performed a multivariate analysis to study the association between the rate of each of the ERAS elements and the clinical and demographic variables. We also used the same model in a multilevel multivariable logistic regression model to explore independent factors associated with moderate-to-severe postoperative complications assessing the variability of each centre.
In order to avoid errors in the multiple comparisons, the respective q value was calculated for each P value in order to maintain a false discovery rate of less than 5 %. Comparisons in which the P value and q value were less than 0.05 were considered statistically significant.
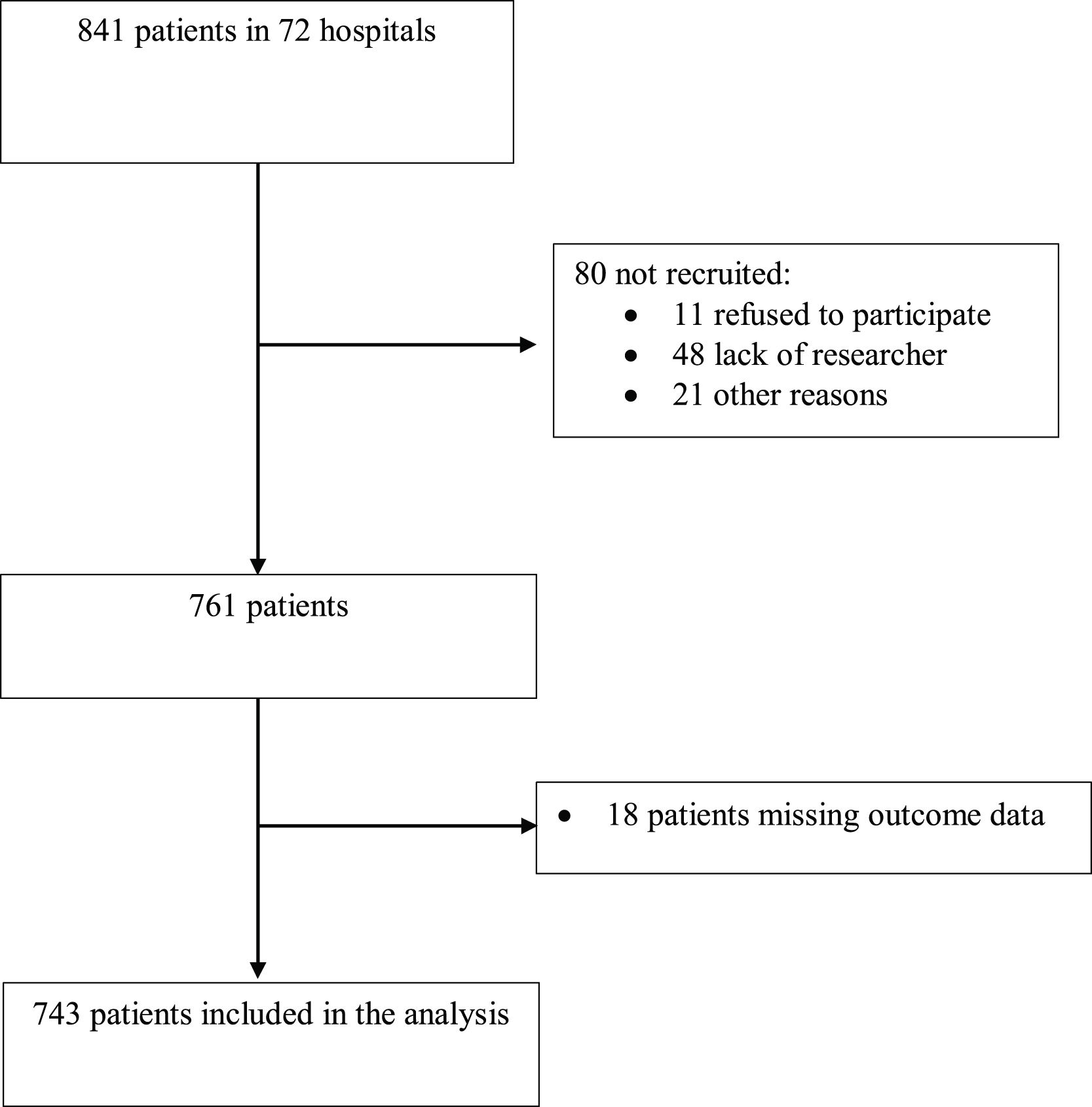
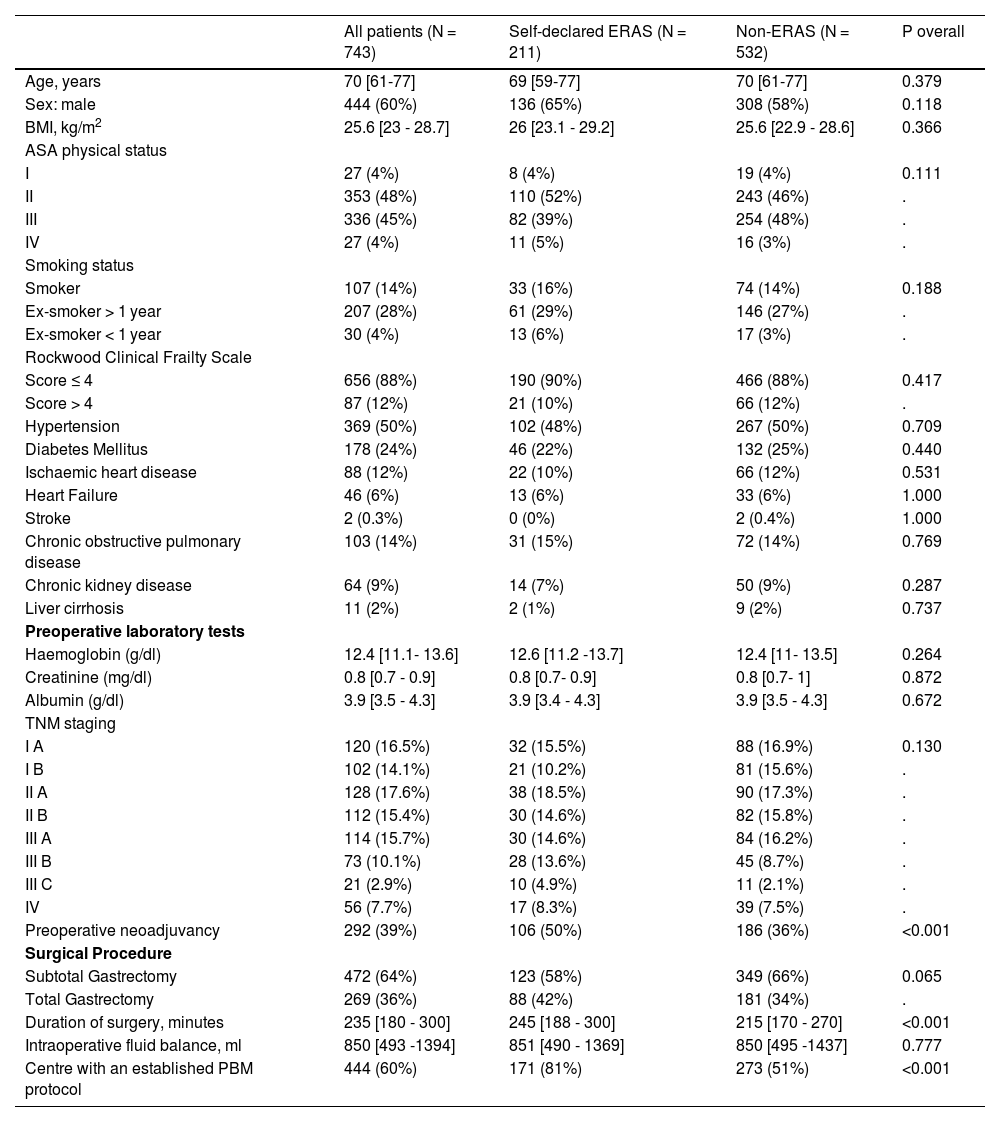
ResultsParticipantsA total of 743 patients from 72 centres were included in the analysis (Fig. 1), of which 211 (28.4%) were included in self-declared ERAS centres; 444 (60 %) were men, and median age was 70 years [IQR 61 to 77]. Other characteristics are shown in Table 1. More patients in the ERAS group received preoperative neoadjuvant therapy and were treated in a centre with a Patient Blood Management (PBM) program. Duration of surgery was longer in the ERAS group. (Table 1).
Patient characteristics.
| All patients (N = 743) | Self-declared ERAS (N = 211) | Non-ERAS (N = 532) | P overall | |
|---|---|---|---|---|
| Age, years | 70 [61-77] | 69 [59-77] | 70 [61-77] | 0.379 |
| Sex: male | 444 (60%) | 136 (65%) | 308 (58%) | 0.118 |
| BMI, kg/m2 | 25.6 [23 - 28.7] | 26 [23.1 - 29.2] | 25.6 [22.9 - 28.6] | 0.366 |
| ASA physical status | ||||
| I | 27 (4%) | 8 (4%) | 19 (4%) | 0.111 |
| II | 353 (48%) | 110 (52%) | 243 (46%) | . |
| III | 336 (45%) | 82 (39%) | 254 (48%) | . |
| IV | 27 (4%) | 11 (5%) | 16 (3%) | . |
| Smoking status | ||||
| Smoker | 107 (14%) | 33 (16%) | 74 (14%) | 0.188 |
| Ex-smoker > 1 year | 207 (28%) | 61 (29%) | 146 (27%) | . |
| Ex-smoker < 1 year | 30 (4%) | 13 (6%) | 17 (3%) | . |
| Rockwood Clinical Frailty Scale | ||||
| Score ≤ 4 | 656 (88%) | 190 (90%) | 466 (88%) | 0.417 |
| Score > 4 | 87 (12%) | 21 (10%) | 66 (12%) | . |
| Hypertension | 369 (50%) | 102 (48%) | 267 (50%) | 0.709 |
| Diabetes Mellitus | 178 (24%) | 46 (22%) | 132 (25%) | 0.440 |
| Ischaemic heart disease | 88 (12%) | 22 (10%) | 66 (12%) | 0.531 |
| Heart Failure | 46 (6%) | 13 (6%) | 33 (6%) | 1.000 |
| Stroke | 2 (0.3%) | 0 (0%) | 2 (0.4%) | 1.000 |
| Chronic obstructive pulmonary disease | 103 (14%) | 31 (15%) | 72 (14%) | 0.769 |
| Chronic kidney disease | 64 (9%) | 14 (7%) | 50 (9%) | 0.287 |
| Liver cirrhosis | 11 (2%) | 2 (1%) | 9 (2%) | 0.737 |
| Preoperative laboratory tests | ||||
| Haemoglobin (g/dl) | 12.4 [11.1- 13.6] | 12.6 [11.2 -13.7] | 12.4 [11- 13.5] | 0.264 |
| Creatinine (mg/dl) | 0.8 [0.7 - 0.9] | 0.8 [0.7- 0.9] | 0.8 [0.7- 1] | 0.872 |
| Albumin (g/dl) | 3.9 [3.5 - 4.3] | 3.9 [3.4 - 4.3] | 3.9 [3.5 - 4.3] | 0.672 |
| TNM staging | ||||
| I A | 120 (16.5%) | 32 (15.5%) | 88 (16.9%) | 0.130 |
| I B | 102 (14.1%) | 21 (10.2%) | 81 (15.6%) | . |
| II A | 128 (17.6%) | 38 (18.5%) | 90 (17.3%) | . |
| II B | 112 (15.4%) | 30 (14.6%) | 82 (15.8%) | . |
| III A | 114 (15.7%) | 30 (14.6%) | 84 (16.2%) | . |
| III B | 73 (10.1%) | 28 (13.6%) | 45 (8.7%) | . |
| III C | 21 (2.9%) | 10 (4.9%) | 11 (2.1%) | . |
| IV | 56 (7.7%) | 17 (8.3%) | 39 (7.5%) | . |
| Preoperative neoadjuvancy | 292 (39%) | 106 (50%) | 186 (36%) | <0.001 |
| Surgical Procedure | ||||
| Subtotal Gastrectomy | 472 (64%) | 123 (58%) | 349 (66%) | 0.065 |
| Total Gastrectomy | 269 (36%) | 88 (42%) | 181 (34%) | . |
| Duration of surgery, minutes | 235 [180 - 300] | 245 [188 - 300] | 215 [170 - 270] | <0.001 |
| Intraoperative fluid balance, ml | 850 [493 -1394] | 851 [490 - 1369] | 850 [495 -1437] | 0.777 |
| Centre with an established PBM protocol | 444 (60%) | 171 (81%) | 273 (51%) | <0.001 |
ASA: American Society of Anesthesiology; ERAS: Enhanced recovery after surgery; PBM: Patient Blood Management.
Percentage reflects over non-missing sample size. Discrete variables n (%). Continuous variables Median[Q1-Q3].
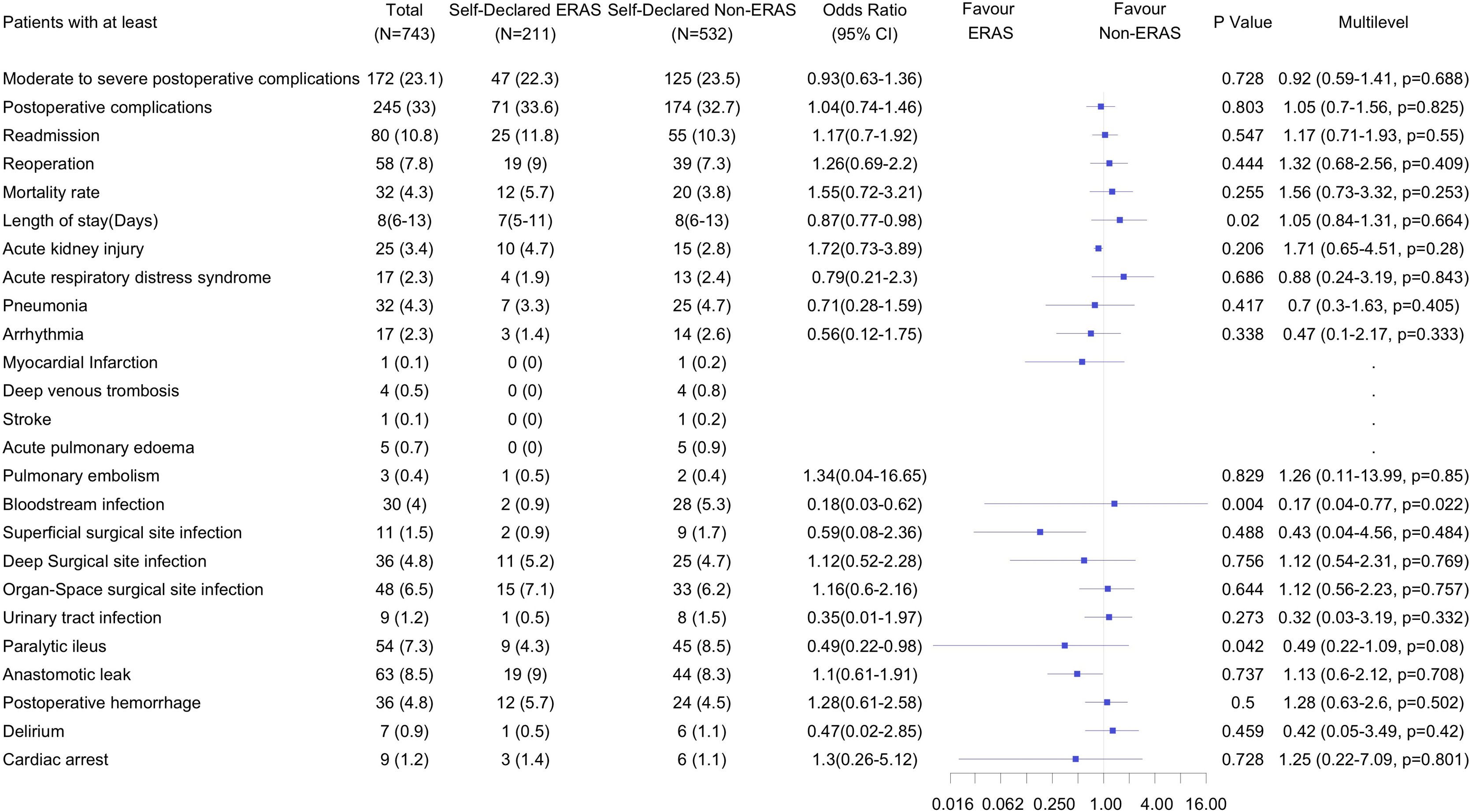
A total of 172 patients (23.1%) suffered moderate-to-severe complications, and 245 patients (33 %) experienced overall postoperative complications. There were no differences in the incidence of moderate-to-severe complications (47 (22.3%) vs. 125 (23.5%); OR, 0.92 (95% CI, 0.59 to 1.41); P = 0.068) or overall postoperative complications (71 (33.6%) vs. 174 (32.7%); OR, 1.05, 95 % CI, 0.70 to 1.56; P = 0.825) between the self-declared ERAS and non-ERAS groups. Patients in the ERAS group had less bloodstream infection compared to the non-ERAS group. There were no differences in the rest of the predefined complications and there were no differences in readmissions, reoperations, mortality and LOS. (Fig. 2).
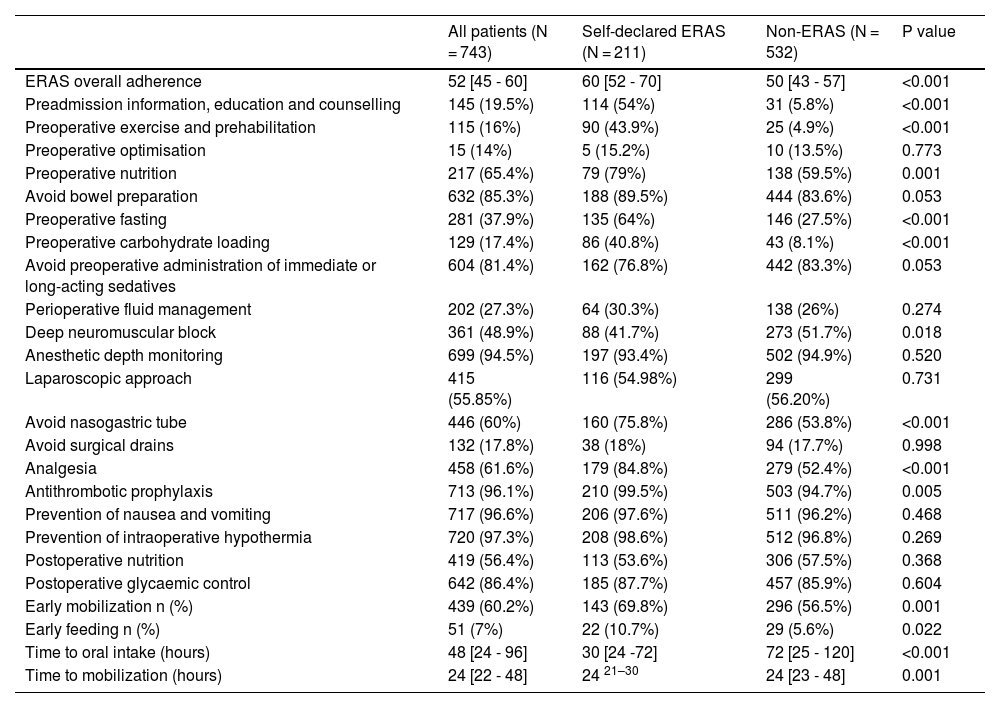
Adherence dataThe overall adherence rate to the ERAS individual items was 52% [IQR 45 to 60]. Adherence was significantly higher in self-declared ERAS centres compared to non-ERAS centres 60% [IQR 52 to 70] vs. 50% [IQR 43 to 57] (P < 0.001). Adherence to most of the individual ERAS elements was higher in the self-declared ERAS cohort. Table 2 shows the adherence for each of the individual ERAS elements.
Adherence to ERAS elements in self-declared ERAS and non-ERAS centres.
| All patients (N = 743) | Self-declared ERAS (N = 211) | Non-ERAS (N = 532) | P value | |
|---|---|---|---|---|
| ERAS overall adherence | 52 [45 - 60] | 60 [52 - 70] | 50 [43 - 57] | <0.001 |
| Preadmission information, education and counselling | 145 (19.5%) | 114 (54%) | 31 (5.8%) | <0.001 |
| Preoperative exercise and prehabilitation | 115 (16%) | 90 (43.9%) | 25 (4.9%) | <0.001 |
| Preoperative optimisation | 15 (14%) | 5 (15.2%) | 10 (13.5%) | 0.773 |
| Preoperative nutrition | 217 (65.4%) | 79 (79%) | 138 (59.5%) | 0.001 |
| Avoid bowel preparation | 632 (85.3%) | 188 (89.5%) | 444 (83.6%) | 0.053 |
| Preoperative fasting | 281 (37.9%) | 135 (64%) | 146 (27.5%) | <0.001 |
| Preoperative carbohydrate loading | 129 (17.4%) | 86 (40.8%) | 43 (8.1%) | <0.001 |
| Avoid preoperative administration of immediate or long-acting sedatives | 604 (81.4%) | 162 (76.8%) | 442 (83.3%) | 0.053 |
| Perioperative fluid management | 202 (27.3%) | 64 (30.3%) | 138 (26%) | 0.274 |
| Deep neuromuscular block | 361 (48.9%) | 88 (41.7%) | 273 (51.7%) | 0.018 |
| Anesthetic depth monitoring | 699 (94.5%) | 197 (93.4%) | 502 (94.9%) | 0.520 |
| Laparoscopic approach | 415 (55.85%) | 116 (54.98%) | 299 (56.20%) | 0.731 |
| Avoid nasogastric tube | 446 (60%) | 160 (75.8%) | 286 (53.8%) | <0.001 |
| Avoid surgical drains | 132 (17.8%) | 38 (18%) | 94 (17.7%) | 0.998 |
| Analgesia | 458 (61.6%) | 179 (84.8%) | 279 (52.4%) | <0.001 |
| Antithrombotic prophylaxis | 713 (96.1%) | 210 (99.5%) | 503 (94.7%) | 0.005 |
| Prevention of nausea and vomiting | 717 (96.6%) | 206 (97.6%) | 511 (96.2%) | 0.468 |
| Prevention of intraoperative hypothermia | 720 (97.3%) | 208 (98.6%) | 512 (96.8%) | 0.269 |
| Postoperative nutrition | 419 (56.4%) | 113 (53.6%) | 306 (57.5%) | 0.368 |
| Postoperative glycaemic control | 642 (86.4%) | 185 (87.7%) | 457 (85.9%) | 0.604 |
| Early mobilization n (%) | 439 (60.2%) | 143 (69.8%) | 296 (56.5%) | 0.001 |
| Early feeding n (%) | 51 (7%) | 22 (10.7%) | 29 (5.6%) | 0.022 |
| Time to oral intake (hours) | 48 [24 - 96] | 30 [24 -72] | 72 [25 - 120] | <0.001 |
| Time to mobilization (hours) | 24 [22 - 48] | 24 21–30 | 24 [23 - 48] | 0.001 |
Percentage reflects over non-missing sample size. Discrete variables n (%).
Continuous variables Median [Q1-Q3].
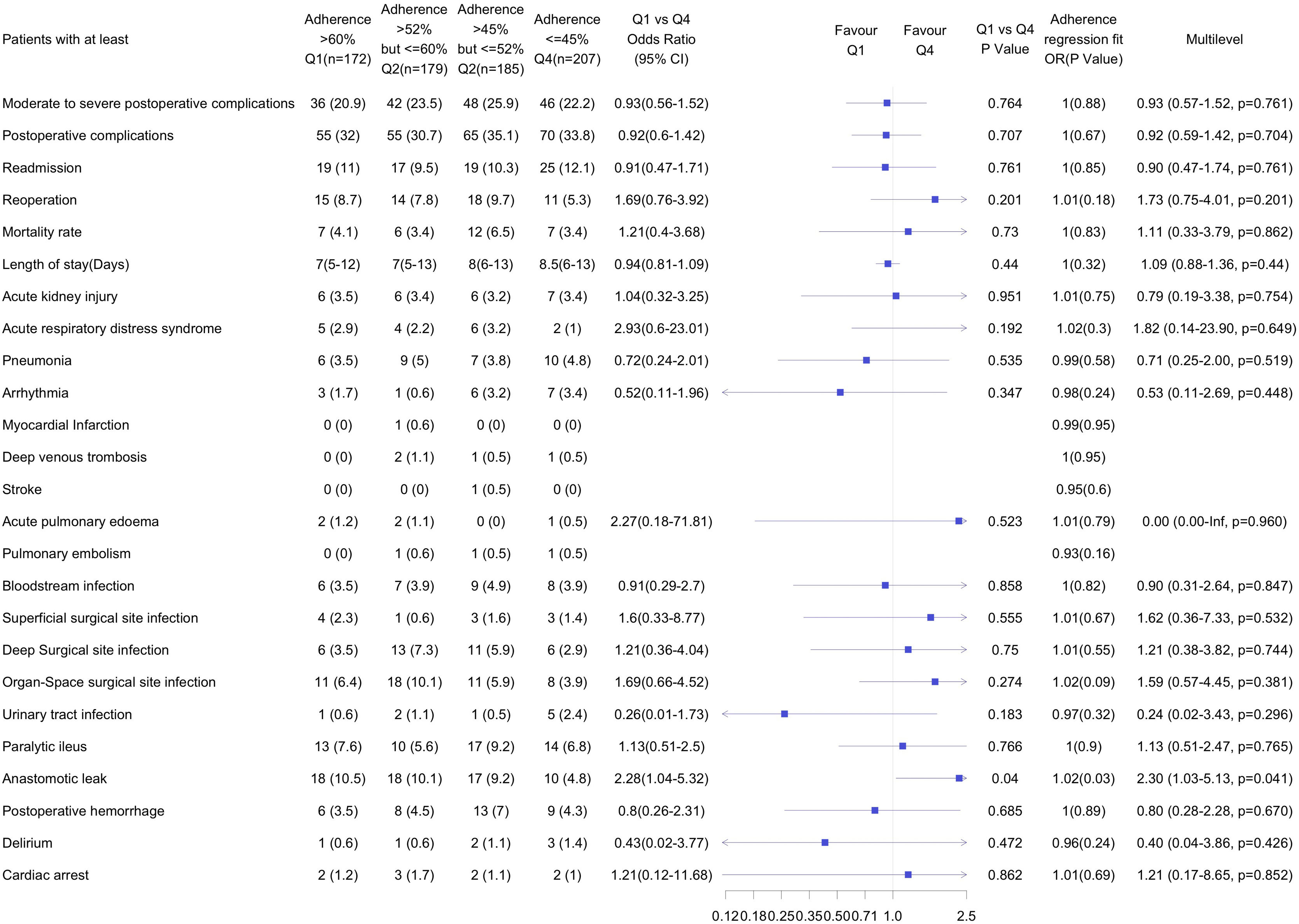
Adherence to the ERAS elements in the highest adherence quartile (Q1) was > 60 %, while in the lowest adherence quartile (Q4) ≤ 45 %. There were no differences in the number of patients with moderate to severe complications, overall complications, readmissions, mortality or LOS. Patients in the Q1 had more anastomotic leak compared with those in the low adherence group (Q4). There were no other differences in any of the predefined complications between the highest and lowest adherence groups (Fig. 3). Linear adherence adjustment also showed that additional adherence to the ERAS pathway in the Q1 did not improve any of the outcomes compared to Q4.
Individual ERAS elements, patients characteristics and moderate-to-severe postoperative complicationsMultivariable and multilevel analyses of individual ERAS elements showed a statistically significant reduction of moderate-to-severe complications in those patients in which early mobilization was achieved while patients not receiving preoperative mechanical bowel preparation and postoperative nutritional care had more moderate-severe postoperative complications. No other associations were found for the other ERAS elements (Table S3, supplementary data.).
Patients who received neoadjuvant therapy had a lower risk of moderate-severe postoperative complications. Frail patients, patients with cirrhosis, patients undergoing total gastrectomy, and patients with a longer lasting surgery had a higher risk of moderate-to-severe complications (Table S3, supplementary data).
Individual ERAS elements and patients characteristics and LOSLOS was significantly lower in patients in whom nasogastric tube was not used, and in those who received early feeding. However, patients who underwent total gastrectomy and those who received postoperative nutritional support had a longer LOS. None of the other ERAS elements, neither being treated at a self-declared ERAS centre, were associated with shorter LOS (Table S4, supplementary data).
DiscussionThe main finding of this study was that neither treatment at a self-declared ERAS centre nor high compliance (Q1) with ERAS measures were associated with improved outcomes after elective gastric cancer surgery.
Given the success of ERAS in colorectal surgery, this multimodal perioperative care approach has emerged as an optimal perioperative strategy to improve clinical outcomes in gastric cancer surgery and in many other procedures. However, numerous controversies exist regarding the practice of ERAS in patients undergoing gastrectomy. A recent meta-analysis of randomized controlled trials which assessed the role of ERAS in radical gastrectomy showed that ERAS results in accelerated convalescence, improved nutritional status, and improved quality of life for gastric cancer patients.17 Similarly to our findings, Blumenthaler et al did not find that the application of an ERAS pathway in gastric surgery resulted in fewer postoperative complications.18 It has been reported that the efficacy of protocols that are incompletely or incipiently applied is markedly reduced.18,19 Although this has been mainly reported for colorectal surgery 6,20 it was also recently shown that greater adherence to ERAS recommendations for gastric surgery resulted in improved postoperative outcomes.21 However, we did not find that greater adherence to protocols was associated with better outcomes, although the adherence in our highest adherence quartile was lower than previously reported as required to improve postoperative outcomes6, so it is possible that our low overall adherence in Spain was one of the determinants of the ineffectiveness of the ERAS protocol in this cohort.
Early mobilization was the only ERAS element that was associated with fewer moderate-severe complications. Several studies have shown that the application of this part of the ERAS program can significantly accelerate the recovery of postoperative intestinal function22,23 and improve postoperative outcomes.7,24,25 On the other hand, those who received postoperative nutritional support had more postoperative complications and higher LOS probably due to a previous poorer nutritional status or the presence of postoperative complications.. The length of stay was also shorter in patients who did not have a nasogastric tube and who received an early feeding. The number and relative combination of ERAS elements implemented, and the relative importance of individual items, especially those considered as "core elements'' of ERAS is currently a debated topic.26,27 It is accepted that early mobilization is a core element in most ERAS guidelines4, its fulfilment may be due to both a dedicated effort by the postoperative multidisciplinary team and a sign of favourable clinical outcome,28 and conversely, the inability to mobilize early in the immediate postoperative period may be considered as a warning sign of poor clinical outcome.29 Early deviation of postoperative ERAS elements appears to be the most significant in terms of association with ERAS failure and delayed discharge.29 Early restoration of the oral feeding promotes early recovery of normal bowel function, so it seems logical that patients who achieve early tolerance will have a shorter LOS,30 while those with impediments to achieving this, such as the presence of a nasogastric tube or the need for nutritional support, will have a longer LOS. Once again, this postoperative element could be considered as a success of the pathway, or as an early warning sign in those cases in which compliance is not achieved.31
A recent study provided unique insight into changes in gastric cancer presentation, management and outcomes over a 30-year period and highlighted the importance of perioperative chemotherapy in long-term survival, as well as the reduction in the number of total gastrectomy as a milestone in the management of patients undergoing gastric cancer surgery to improve postoperative outcomes.32 We found similar results, patients with neoadjuvant therapy presented fewer complications, but longer surgeries and total gastrectomy were associated with more postoperative complications. On the other hand, although the partial application of ERAS did not lead to better outcomes, it was not associated with more readmissions or more reoperations, so its practice seems safe, and efforts should be undertaken to achieve greater adherence to the protocol, since it is ultimately the standard of care in many cases.
Most of the studies on gastric surgery were performed in the Asian population, and this study is the largest study to date on gastric surgery in the ERAS setting in a Western population; however, we must acknowledge some limitations of the study. First, in an optimally designed study, the groups would be blindly assigned to ERAS or non-ERAS for the same period of time to avoid allocation bias. However, we consider that a randomized clinical trial comparing an ERAS protocol versus a clinical practice in which none of the ERAS elements were used would be unethical. On the other hand, information bias may be inherent in the design of this study, and could have influenced an increase in adherence to the ERAS elements, so that the actual ERAS adherence could even be lower. The elements of ERAS themselves are interrelated. For example, conservative intraoperative fluid administration is arguably more applicable in patients who did not receive preoperative bowel preparation, and administration of carbohydrate drinks mandates adequate adherence to preoperative fasting time. In addition, the use of some of the ERAS elements are related to the severity of the patient, e.g., goal-directed fluid therapy, usually recommended for the high-risk patient.33,34 We tried to avoid selection bias by including a majority of Spanish centres that were performing gastrectomy, regardless of whether they self-designated as ERAS or not. The effect of these problems was partly offset by the large sample size and the number of institutions reporting data, but we also have to recognize that gastrectomy is not as frequent a procedure as, for example, colorectal surgery, and many centres recruited a low number of patients.
The application of ERAS protocols for gastric surgery in our cohort was very low. Neither partial compliance with ERAS protocols nor treatment with self-designated ERAS centres improved postoperative outcomes in patients undergoing gastric surgery for cancer.
FundingSupport was only provided by institutional sources. The POWER 4 study was supported by the Spanish Perioperative Audit and Research Network (RedGERM) and the Grupo Español de Rehabilitación Multimodal (GERM).
RedGERM conducted and designed the study; provided collection and management of the data; but had no role in the analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
COI/DisclosuresDr. Ripollés-Melchor reports personal fees from Edwards Lifesciences, Vifor Pharma, MSD and Fresenius Kabi outside the submitted work. Dr. Alfredo Abad-Gurumeta reports personal fees from Edwards Lifesciences, MSD, 3 M, Braun, Ferrer, Rovi and ALTAN outside the submitted work. Dr. Aldecoa reports personal fees from Fresenius Kabi and Octapharma outside the submitted work. Dr García-Erce declares no conflict of interest with this study. However, he has previously given talks, chaired tables at congresses and conferences and organized courses with scholarships or funding from Amgen, Jansen, Sandoz, Vifor Pharma and Zambon. Carlos Jericó has given talks and received consultancy fees from Viphor Pharma España SL, Bial and Zambon. Ane Abad-Motos, Marcos Bruna-Esteban, María García-Nebreda, Isabel Otero-Martínez, Omar Abdel-lah Fernández, María P. Tormos-Pérez, Gloria Paseiro-Crespo, Raquel García-Álvarez, María A Mayo-Ossorio, Orreaga Zugasti-Echarte, Paula Nespereira-García, Lucia Gil-Gómez, Margarita Logroño-Ejea, Raquel Risco, Felipe C Parreño-Manchado, Silvia Gil-Trujillo, Carmen Benito, María I De-Miguel-Cabrera, Bakarne Ugarte-Sierra, Cristina Barragán-Serrano, Henar Muñoz-Hernández, Sabela del- Río-Fernández, María L. Herrero-Bogajo, Alma M. Espinosa-Moreno, Vanessa Concepción-Martín, Andrés Zorrilla-Vaca, Laura Vaquero-Pérez, Irene Mojarro, Manuel Llácer-Pérez, Leticia Gómez-Viana, María T. Fernández-Martín, Carlos Ferrando-Ortolà, José M. Ramírez-Rodríguez report no potential conflicts of interest.
Appendix 1: POWER 4 Investigators GroupAlthaia. Xarxa Assistencial i Universitària de Manresa
Mercè Güell Farré1,2, Roser Farré Font, Rafael Diaz del Gobbo, Raquel Sánchez Jimenez (Surgery Department) Isabel Pérez Reche, Belen Gil Calvo, Jordi Llorca García, Laura Carrasco Sánchez, Cristina Prat llimargas (Anesthesia Department).
Complejo Asistencial de Zamora
Ana María García-Sánchez1, Gema Martínez-Ragüés1, María Gómez-Fernández (Anesthesia Department); Álvaro del-Castillo-Criado2, Ruth Martínez-Díaz, Sara Alegría-Rebollo (Surgery Department)
Complejo Asistencial Universitario de León
Javier Ferrero-de-Paz1,2, Cristina García-Pérez, Sergio Marcos-Contreras, Diana Fernández-García, Maeva Torío-Marcos (Anesthesia Department)
Complejo Asistencial Universitario de Salamanca
Omar Abdel-lah Fernández1,2, Felipe Carlos Parreño-Manchado1 (Surgery Department); María Ángeles Martín (Anesthesia Department)
Complejo Hospitalario de Mérida
José María Tena-Guerrero1,2, Estefanía Palma-González (Anesthesia Department); Gustavo Flores-Flores1 (Surgery Department)
Complejo Hospitalario de Navarra
Orreaga Zugasti-Echarte1,2, Elena Pérez-Bergara, Susana Hernández-García, Marta Martín-Vizcaíno, Francisco Javier Yoldi-Murillo, Maider Valencia-Alzueta (Anesthesia Department); Carlos Chaveli-Díaz, Coro Miranda-Murua, Concepción Yárnoz-Irazábal (Surgery Department); Sonsoles Botella-Martínez (Nutrition Department)
Complejo Hospitalario de Toledo
Rafael López-Pardo1,2 (Surgery Department); Marta Torres-Montalvo (Anesthesia Department)
Complejo Hospitalario Don Benito Villanueva de la Serena
Isabel Pinilla-Pico, Enrique del-Cojo-Peces1,2, María del Pilar Rodríguez-Chaparro (Anesthesia Department)
Complejo Hospitalario Universitario A Coruña
Manuel Ángel Gómez-Ríos1,2, Sara del-Río-Regueira, Eva Mosquera-Rodríguez (Anesthesia Department)
Complejo Hospitalario Universitario de Canarias
Jessica Hernández-Beslmeisl1,2, María Alejandra Perozo-Medina1, Beneharo Darias-Delbey, María del Carmen Martín- Lorenzo, Vanessa González-Fariña, Montserrat Rodríguez-Domínguez, Jaime Fernández-de-la-Vega-Medina, Pilar Berrotarán-Ayub (Anesthesia Department)
Complejo Hospitalario Universitario Insular Materno Infantil de Gran Canaria
José Valín-Martínez1,2, Laura Concepción-Santana, Raúl Cruz-Zorio, Dolores Betancort-Gutiérrez Rodríguez (Anesthesia Department)
Complexo Hospitalario Universitario de Ourense
Leticia Gómez-Viana1,2, Olalla Figueiredo, Ariadna Rodríguez, Manuel González (Anesthesia Department); David Iglesias, José Manuel Domínguez-Carrera (Surgery Department); Jorge do Olmo (Adminisitrative support)
Complexo Hospitalario Universitario de Santiago
Sabela Del-Río-Fernández1,2, Laura Dos-Santos-Carregal, María Jesús Rodríguez-Forja, Olga Campaña-Figueira, Julián Álvarez-Escudero (Anesthesia Department); Lucía Lesquereux-Martínez, Purificación Parada-González, Ricardo Montenegro-Romero, Manuel Bustamante-Montalvo (Surgery Department); Ana Rosa García-Placín, Carmen Carpintero-Rama, Sergio Brea-Bahamonde (Nursing Department)
Fundación Jímenez Díaz
José Ramón Torres-Alfonso1, Cristina Barragán-Serrano1,2, María Posada-González, Gabriel Salcedo-Cabañas, Peter Vorwald-Wolfgang (Surgery Department)
Hospital Álvaro Cunqueiro de Vigo (Complejo Hospitalario Universitario de Vigo)
Isabel Otero-Martínez1,2, Patricia Jove-Alborés, Marta López-Otero, Hermelinda Pardellas-Rivera, Ignacio Maruri-Chimeno, Sonia González-Fernández, Raquel Sánchez-Santos (Surgery Department); Luis Luna-Mendoza (Anesthesia Department)
Hospital Arnau de Vilanova, Valencia
Enrique Lloria-Pons1,2, Francisco Gramuntell-Marco (Anesthesia Department) ; Raúl Cánovas-de-Lucas, Cristina Sancho-Moya (Surgery Department)
Hospital Clínic de Barcelona
Raquel Risco1,2, Anna Fernández-Esmerats, Josep Martí Sanahuja-Blasco, Manuel López-Baamonde, Marta Ubré, Graciela Martínez-Pallí (Anesthesia Department); Dulce Momblán (Surgery Department); Nuria Rivas-Gallardo (Nurse)
Hospital Clínico San Carlos
Rubén Sánchez-Martín1,2, Pedro Moral, Rosalía Navarro, Luis Santé (Anesthesia Department); Esther Almenta (Surgery Department)
Hospital Clínico Universitario de Valladolid
Henar Muñoz-Hernández1,2, Rita Pilar Rodríguez-Jiménez, Nuria Ruiz, María Teresa Peláez, Juan José Rojo, Carlota Gordaliza (Anesthesia Department); Carlos Jezieniecki (Surgery Department)
Hospital Comarcal de Inca
Carlos Jimenez-Viñas1,2, Carlo Brugiotti, Ulices Onchalos-Lopez (Surgery Department)
Hospital Costa del Sol, Marbella (Málaga)
Manuel Llácer-Pérez1,2 (Anesthesia Department)
Hospital de la Santa Creu i Sant Pau
Astrid Batalla1,2, Gonzalo Azparren, Micaela Bastitta, Luisa Cueva, Mar Felipe, Marta Giné, Ana M. Gómez, Inmaculada India, Santiago Piñol (Anesthesia Department)
Hospital de Sagunto
Maria José Gimeno-Campos1,2, Aurora Moreno-Gázquez1, Julio Llorens-Herrerías, Gema Del-Castillo-Rodrigo (Anesthesia Department)
Hospital General La Mancha Centro
María Luz Herrero-Bogajo (Surgery Department)
Hospital General Universitario de Castellon
María Isabel De Miguel-Cabrera1,2, José Miguel España-Pamplona, María Gellida-Vilarroig, Laura Jordá-Sanz (Anesthesia Department)
Hospital General Universitario de Ciudad Real
Silvia Gil-Trujillo1,2, Laura Calatayud-Gómez, Juan David Valencia-Echeverri, Víctor Baladrón-González (Anesthesia Department); Aurora Gil-Rendo1,2, Susana Sanchez-García, Esther García-Santos (Surgery Department)
Hospital General Universitario Gregorio Marañón de Madrid
Carmen Benito1,2, Cristina Lisbona, Pilar Benito (Anesthesia Department)
Hospital General Universitario Santa Lucia
Olga Cecilia Correa-Chacón1,2, Alejandro Alcázar-Urrea, Ana Belén Fernández-López, Ramiro Betancourt-Bastidas (Anesthesia Department); Inmaculada Navarro-García, Elena Romera-Barba (Surgery Department)
Hospital Joan XXIII de Tarragona
Nuria Mira-Jovells1,2, Liz Karime Estevez-Perez, Judit Saludes-Serra (Anesthesia Department); Carles Olona-Casas2, Jordi Vadillo-Bargalló. Rosa Jorba-Martín (Surgery Department)
Hospital Juan Ramon Jimenez, Huelva
Irene Mojarro1,2, Juan Victor Lorente, Pablo Longo-Guridi, Ana Maria Quintero-Moreno, David Soriano-López, Maria de la Peña Gómez-Dominguez, Cecilia Prieto-Candau, Marisol Hernández-Castillo, Irene Jarana, Francisco García-Andreu, Jose González-González, María Dolores Díaz-Lara (Anesthesia Department)
Hospital Lluís Alcanyís
Elena Añón-Iranzo, Javier Aguiló-Lucia (Surgery Department)
Hospital Medina del Campo
Maria Teresa Fernandez-Martin1,2, Yessica Guerra (Anesthesia Department)
Hospital Nuestra Señora del Prado. Talavera de la Reina
Pablo Gimeno-Fernández1,2, Carla Iglesias-Morales, Ana María Ríos Villalba (Anesthesia Department)
Hospital Povisa, Vigo
Paula Nespereira-García1,2, María José Castro-Neira, María Ochoa-Díez (Anesthesia Department)
Hospital Sierrallana, Torrelavega
Silvia García-Orallo (Anesthesia Department); Maialen Mozo-Segurado, Jose Manuel Gutiérrez-Cabezas1,2, Ruben Gonzalo-González (Surgery Department)
Hospital Universitario 12 de Octubre
Raquel García-Álvarez1,2, Álvaro Ramiro-Ruíz1,2, María González-Cofrade, Estefanía Carvajal-Revuelta, Ana Bellido-López, Guillermo Redondo-Cristóbal (Anesthesia Department);
Hospital Universitario Central de Asturias
Sonia Amoza-Pais1,2, Tamara Díaz-Vico, María Moreno-Gijón, Sandra Sanz-Navarro, Lourdes M Sanz-Álvarez, Estrella Olga Turienzo-Santos, Jose Luis Rodicio-Miravalles, Amaya Rizzo-Ramos (Surgery Department); Ana Cuéllar-Martínez, Rosa Ana Álvarez-Fernández, Carmen Fernández-Seijo, Amelia Álvarez-Barrial, Carmen Gónzalez-Ortea, Silvia Fernández-Rodriguez, Nerea García-González, Lorena Varela-Rodriguez, Elena Berzal-Canga, Ángela García-Díaz-Negrete, Helena García-Sánchez, Javier Saenz-Abos, Beatriz Ayuso-Iñigo, María Vega-Colón (Anesthesia Department)
Hospital Universitario Clínico San Cecilio, Granada
Ángela Catalina Palacios-Córdoba1, María Teresa Quel-Collado2, Mercedes Olvera-García, Agustina Martinez-Sánchez, Carmen Serrano-Alvarez (Anesthesia Department)
Hospital Universitario Cruces
Eunate Ganuza-Martínez, María Jesús Maroño-Boedo2, Alberto Sánchez-Campos, Blanca Anuncia Escontrela-Rodríguez, Alberto Martínez-Ruiz1 (Anesthesia Department); Gaizka Errazti-Olartecoechea, Iratxe Rodeño-Esteban, Tamara Moreno-Allende, Oihane Gutierrez-Grijalba, Yanina Kataryniuk-Di-Costanzo, Irene Alvarez-Abad, Patricia Sendino-Cañizares, Patricia Mifsut-Porcel, Aingeru Sarriugarte-Lasarte1, Mikel Guerra-Lerma (Surgery Department)
Hospital Universitario de Álava
Margarita Logroño-Ejea1,2, María Del Carmen Iturricastillo-Pérez, María Gastaca-Abasolo
María José Muñoz-Sanz, Ibai Iriarte-Zaranton, Erika Olea-De-La-Fuente, Ana Mendiguren-Murua (Anesthesia Department); Lorena Reka-Mediavilla, Alberto Gastón-Moreno, Gabriel Jesús Martínez-De-Aragón-Remirez-De-Esparza, Valentín Sierra-Esteban (Surgery Department)
Hospital Universitario de Badajoz
Gema Montero-Mejías, Claudia Pardo-Martínez, Teresa Valadés-Periañez, Ana Marín-Moreno, Juan Ricardo Caro-González, María José Rodríguez- Pérez1,2 (Anesthesia Department)
Hospital Universitario de Basurto
José Carlos Herrero-Herrero1,2, Pablo Renedo-Corcóstegui, Alejandro Martín-Oliva, Ana Isabel Fernández-Herrero, Nerea Andrieu-Estefanía (Anesthesia Department)
Hospital Universitario de Cabueñes
Javier Albaladejo-Magdalena, María Álvarez-Rodriguez (Anesthesia Department); Valentina Sosa-Rodríguez, Raquel María Fresnedo-Pérez (Surgery Department)
Hospital Universitario de Fuenlabrada
Cristina Gil-Lapetra1,2, Beatriz Bolzoni-Marciel, Maria Isabel Herrera-López, Fernando Setién-Moreno, Ana Zulema Castro-Costoya, José Olarra-Nuel (Anesthesia Department); Marta De-Vega-Irañeta (Surgery Department)
Hospital Universitario de Galdakao
Bakarne Ugarte-Sierra1,2, Irune Vicente-Rodríguez, Francisco Javier Fernández-Pablos, Rafael Alberto Abad-Alonso, Roberto Maniega-Alba, Francisco Javier Ibáñez-Aguirre (Surgery Department); Susana Postigo-Morales, Karmelo Intxaurraga-Fernández, Sorkunde Telletxea-Benguria (Anesthesia Department); Ángela Outón-Guerrero (Nurse)
Hospital Universitario de Gran Canaria Dr. Negrín
María Asunción Acosta-Mérida1,2 (Surgery Department)
Hospital Universitario de Guadalajara
Roberto de la Plaza-Llamas1,2, José Manuel García-Gil, Ignacio Antonio Gemio-del-Rey (Surgery Department); Mercedes Cabellos-Olivares (Anesthesia Department)
Hospital Universitario de Móstoles
Raquel Fernández-García1,2, Lidia Castro Freitas, Beatriz Nacarino Alcorta, María Martín Ayuso (Anesthesia Department); María Moral González, David García Teruel (Surgery Department)
Hospital Universitario de Vic
Jordi Serrat-Puyol1,2 (Anesthesia Department), Joan Molinas-Bruguera, Sara Fernández (Surgery Department)
Hospital Universitario Doctor Peset
Nuria Peris-Tomás1,2, Jose Ángel Díez-Ares, Dolores Periáñez-Gómez, Paula Gonzálvez-Guardiola, Ramón Trullenque-Juan, Ezequiel Martínez-Mas (Surgery Department); Estefanía Martínez, Julia Martín, Raquel Higueras, Sara Cuenca (Anesthesia Department)
Hospital Universitario Fundación de Alcorcón
Beatriz Martín-Vaquerizo1, Alma M. Espinosa-Moreno1,2, Miriam Sánchez-Merchante, Rebeca Alcañiz-Sobrino, Guillermo Egea-Hita, Santiago García-del-Valle-y-Manzano (Anesthesia Department)
Hospital Universitari i Politècnic La Fe
Virginia Moreno-Blanco1 (Research Nurse); Marcos Bruna-Esteban2, Fernando Mingol-Navarro, Javier Vaqué-Urbaneja (Surgery Department); Óscar Díaz-Cambronero (Anesthesia Department); Beatriz Luján-Sandemetrio, Bisila Copariate-Piqueras, Sonia Giménez-Salcedo (OR Nurse)
Hospital Universitario Infanta Leonor
Javier Ripolles-Melchor1, Ane Abad-Motos1,2, Alfredo Abad-Gurumeta, Norma Aracil-Escoda, Elena Saez-Ruiz, Rut Salvachua-Fernandez (Anesthesia Department); Gloria Paseiro-Crespo, María García-Nebreda (Surgery Department) ; Begoña Toribio (OR Nurse)
Hospital Universitario Infanta Sofía
Esther Ferrero-Celemin1,2, Luis García-Sancho-Téllez, José Daniel Sánchez-López, Sara Núñez-O'Sullivan, Mariana García-Virosta, Carmen Rodriguez-Haro, María Hernández-O'Reilly (Surgery Department)
Hospital Universitario La Paz
Alejandro Suarez-de-la-Rica1,2, Mercedes Lopez-Martinez, Bryant Croes, Jaime Mujica, Emilio Maseda (Anesthesia Department);
Hospital Universitario La Princesa
Enrique Alday-Muñoz1,2, Esperanza Mata-Mena (Anesthesia Department); Iñigo García-Sanz,
Cristina Marín (Surgery Department)
Hospital Universitario La Zarzuela
Antonio Pedraza Muñoz, Jorge Puertas Dominguez (Anesthesia Department)
Hospital Universitario Lucus Augusti
Rocío González-López1,2, Gisela Navarro-Quirós1,2, M. Isabel Pérez-Moreiras, María Conde-Rodriguez, Manuel Muinelo-Lorenzo (Surgery Department)
Hospital Universitario Marqués de Valdecilla
Miren Jasone Díez-Zapirain1,2, Guillermo Tejón-Pérez1, María Asunción Álvarez-Cebrián, Eva Barrero-Ruiz, Eva Capa-Fuertes, Mariana Carrillo-Rivas, Isabel Hernandez-Sanchez, Valvanuz Monteagudo-Cimiano, Ángela Pascual-Casado, Jose Luis Rábago-Morillón (Anesthesia Department)
Hospital Universitario Miguel Servet de Zaragoza
Javier Martinez-Ubieto1, Ana Pascual-Bellosta1, Sonia Ortega-Lucea, Berta Perez-Otal2, Lucia Tardos-Ascaso2 (Anesthesia Department); Luis Antonio Ligorred-Padilla (Surgery Department)
Hospital Universitari Mútua Terrassa
Jaume Tur-Martínez1,2, Joaquin Rodríguez-Santiago, Noelia Pérez-Romero, Noelia Puértolas-Rico, Laura Hernández-Giménez (Surgery Department)
Hospital Universitario Nuestra Señora de Candelaria
Vanessa Concepción-Martín1,2 (Surgery Department)
Hospital Universitario Puerta de Hierro, Majadahonda
Viktoria Molnar1,2, Macarena Barbero, Belén San-Antonio (Anesthesia Department); Alberto Pueyo (Surgery Department)
Hospital Universitario Puerta del Mar
M de los Angeles Mayo-Ossorio1,2, Ander Bengoechea-Trujillo, José Manuel Pacheco-Garcia (Surgery Department)
Hospital Universitario Ramón y Cajal
Yolanda Diez-Remesal1,2, Alba Gonzalo-Millán, Alberto Berruezo-Camacho, Azucena Alaez-Cortés, Begoña Ortolá-Rocher, Berta Iglesias-Gallego, Eduardo Martín-Montero, Gerardo Arias-Cuesta, Inés de la Hoz-Polo, Isabel Recuero-Torrijos, Lucia Pereira-Torres, Maria Cañamas-Catalá, Maria C. Martin-González, Paloma Zambrana-García, Sergio Llorente -Damas
Hospital Universitario Rio Hortega
Cesar Aldecoa1,2, Alba Perez-Gonzalez, Clara Bolaño-Perez, Delia Velasco-Andres, Esther Aguado-Saster, Irene Arranz, Itziar Mendez-Torrubiano, Laura Vaquero-Perez, Maria Jesus Sanz-de-Leon, Pablo Rodicio, Silvia Martin-Alfonso, David Martin-Tappi
Mario Madrid-Tribano, Rocio Rioja-Garrido, Borja Morales-Jaquete (Dept. of Anesthesia)
Hospital Universitario Severo Ochoa
Marta Vicente-Orgaz1,2, Adriana Carolina Orozco-Vinasco, Gema Fraga-Casais; Any Minerva Miyagi-Yonamine, Raquel Ramos-de-Castro, Rossel Alina Mejía-Arnaud, Rubén Saz-Castro (Anesthesia Department); Patricia Díaz-Peña (Surgery Department)
Hospital Universitario Vall d’Hebrón
Angels Camps-Cervantes1, M Pilar Tormos-Perez, Patricia Galan-Menendez1,2, Elena Esclapez-Sampere, Laura Villarino-Villa, Yuri Loaiza-Aldeán, Ivette Chocron-Dapratt, Olga Martinez-Silva, Silvia Matarin-Olmo (Surgery Department)
Hospital Universitario Virgen Macarena
Héctor Berges-Gutiérrez1,2, Macarena Pérez-Serrano, Juan Javier Diaz-Castillo, Álvaro Sancho-Muñoz-de-Verger, Jesús Alejandro Villanueva-Mena-Bernal (Anesthesia Department)
Hospital Sant Joan Despi Moises Broggi
Maria Lucia Gil-Gómez1,2, Jesús Fernanz-Anton2, Sandra Marmaña-Mezquita (Anesthesia Department); Gonzalo Galofré-Pujol (Surgery Department); Carlos Jericó (Internal Medicine Department.)
Hospital Virgen de los Lirios de Alcoy
José Jacob Motos-Micó1,2, Francisco José Orts-Micó1, Carlos Serra-Díaz, Francisco Arlandis-Félix, Nieves Pérez-Climent (Surgery Department); Lorena Blanes-Pastor, Jose Luis Jover-Pinillos (Anesthesia Department)
MD Anderson Cancer Center Madrid
David Salvaterra1,2, Manuel Linero, Javier Galipienzo (Anesthesia Department);
Óscar Alonso, Gloria Ortega, Santiago Gonzalez, Irene Lopez-Rojo (Surgery Department);
Javier García-Fernández as President Elect of the Spanish Society of Anesthesiology and Critical Care (SEDAR); the Spanish Surgeons Association (AEC), and the European Society of Regional Anaesthesia and Pain Management-Spain (ESRA Spain) provided non compensated pre recruitment announcement of the study on their websites and social networks. Alejandro Bona-Enguita (GERM Secretary) was the POWER 4 website webmaster. Alberto Cebollada-Solanas provided statistical analysis. No financial compensation was provided.