Bariatric surgery is a relatively safe surgical procedure with a high success rate. However, recent reports indicate a higher prevalence of alcohol or substance abuse disorder in this patient group. The purpose of this study was to review the related evidence to serve as a reference for multidisciplinary teams who treat these patients.
MethodsWe searched the PubMed and CENTRAL databases. The odds ratios were extracted from the different articles, comparing the prevalence of the abuse of alcohol or other substances in the postoperative period versus preoperative levels. We also compared the prevalence of alcohol use disorder after different types of bariatric surgery.
ResultsA total of 49 121 bariatric patients (80.8% female) were evaluated for alcohol use disorder. In general, bariatric surgery was found to be associated with an increase in the prevalence of alcohol abuse (4.58 ± 5.3 vs. 1.58 ± 10.7% in the preoperative period). We also found that the population of patients who underwent RYGB procedures had a higher prevalence of alcohol use disorder than patients who underwent another type of surgery (OR: 1.83; 95% CI: 1.51–2.21). The prevalence of substance abuse disorder (other than alcohol) after this procedure is less studied, although there appears to be an increased risk of abuse of certain substances.
ConclusionsBariatric surgery is the best treatment for obesity and its complications. The evidence reviewed suggests that it correlates with a modest but consistent increase in the prevalence of abuse of alcohol and other substances. Medical teams who treat bariatric patients must be informed about this eventuality for its timely prevention, diagnosis and treatment.
La cirugía bariátrica es un procedimiento quirúrgico relativamente seguro y con alta tasa de éxito. Sin embargo, reportes recientes indican una mayor prevalencia de abuso de alcohol u otras sustancias en este grupo de pacientes. El propósito del presente estudio fue revisar la evidencia que existe al respecto para que sea tomada en cuenta por el equipo multidisciplinario que atiende a este grupo de pacientes.
MétodosSe realizaron búsquedas en las bases de datos de PubMed y CENTRAL, y se extrajeron las razones de momio de los distintos artículos, comparando la prevalencia por abuso de alcohol o de otras sustancias en el periodo posquirúrgico vs. los niveles prequirúrgicos. También se comparó la prevalencia de abuso de alcohol tras distintos tipos de cirugía bariátrica.
ResultadosUn total de 49.121 pacientes bariátricos (80,8% mujeres) fueron evaluados para abuso de alcohol. De manera general, se encontró que la cirugía bariátrica estaba asociada con un aumento en la prevalencia por abuso de alcohol (4,58 ± 5,3 vs. 1,58 ± 10,7% en el periodo prequirúrgico). También encontramos que la población de pacientes que se sometieron a cirugía de tipo RYGB tenía mayor prevalencia de abuso de alcohol que aquellos que se sometieron a otro tipo de cirugía (OR: 1,83; IC 95%: 1,51–2,21). La prevalencia de abuso de sustancias distintas al alcohol tras este procedimiento está menos estudiada, aunque parece existir un aumento en el riesgo por abuso a ciertas sustancias.
ConclusionesLa cirugía bariátrica es el mejor tratamiento para la obesidad y sus complicaciones. La evidencia revisada sugiere que se relaciona con un aumento modesto, pero consistente en la prevalencia por abuso de alcohol y otras sustancias. El equipo médico a cargo del paciente bariátrico deberá estar informado acerca de esta eventualidad para su oportuna prevención, diagnóstico y tratamiento.
Obesity is considered one of the largest public health problems worldwide. In 2016, according to body mass index ranges established by the WHO, the combined prevalence of excess weight and obesity in the adult population around the world was 38% (representing more than 1.9 billion adults). The prevalence of obesity alone at that time was 13% (650 million), with higher rates found in females (15%) than in males (11%). In response to this substantial increase, which tripled the number of obese people in the world in just over 40 years, a wide range of treatments1 have been implemented in 3 levels: first psychosocial, then pharmacological and, finally, surgical. Bariatric surgery has been shown to be the most effective long-term treatment for patients with severe obesity, both in terms of weight loss as well as the resolution of comorbidities.2,3
Currently, the most widely used bariatric surgical procedures are laparoscopic sleeve gastrectomy (LSG), Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (LAGB), vertical banded gastroplasty (VBG) and one-anastomosis gastric bypass (mini gastric bypass). Each has specific indications and results.4 The International Federation for the Surgery of Obesity and Metabolic Disorders has conducted an international survey on the number of bariatric procedures carried out since 2003, which also included geographic and population distributions as well as the evolution of this type of procedure.5–8 Meta-analysis studies show excellent positive results, including excess weight loss over 60% that is maintained for a period of at least 5 years,9–11 control of hypertension12 and glycemic control.13 In general, weight loss surgery increases life expectancy (which correlates with the percentage of weight lost) while it also improves quality of life during these added years.9,10,14,15
However, a peculiar complication has been reported in certain patients after undergoing bariatric surgery: a postoperative increase in addictive behaviors, particularly alcohol abuse disorder (AUD).16 Additionally, some authors have observed a higher risk of developing AUD in patients who had RYGB compared to other procedures,17,18 although others have not observed a clear difference.19,20 The purpose of this study is to evaluate the evidence that currently exists about the rates of addictive disorders after different types of bariatric surgery. To this end, we reviewed original research articles published between January 2010 and October 2020, which is about 4 years after the period covered by the last systematic review on alcohol abuse disorder before and after bariatric surgery.21 What makes our study different is that we have compared the prevalence of AUD after RYGB with other types of bariatric surgeries, and we include an analysis of the effect of bariatric surgery on the prevalence of substance abuse other than alcohol.
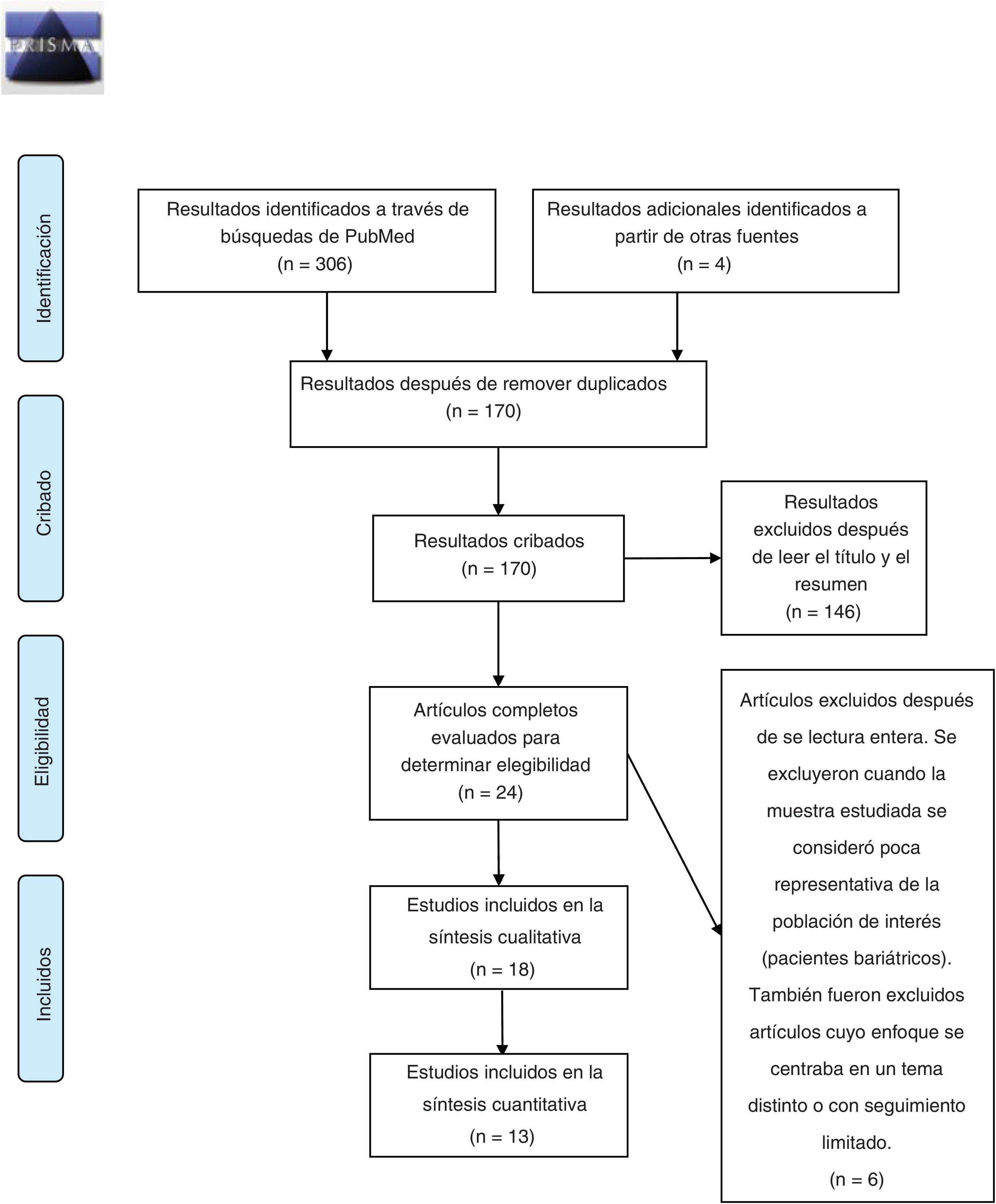
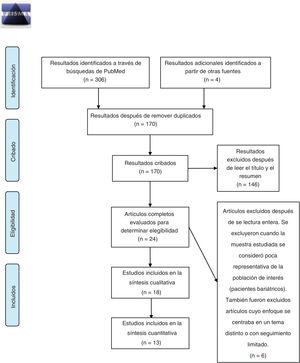
MethodsThis review was carried out in accordance with the PRISMA-P protocol for reporting systematic reviews.22 The primary source selection process is summarized in Fig. 1. Bibliographic searches were carried out in the PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) databases, using the keywords: “bariatric surgery obesity alcohol abuse”, “bariatric surgery obesity alcohol drug addiction” and “bariatric surgery obesity substance abuse”, selecting studies carried out from January 1, 2010, to October 31, 2020. The search and subsequent selection of articles (by reading the title and abstract) was done by 2 of the authors (JPM and GCS), who worked independently. Differences between reviewers were resolved by discussion and subsequently subjected to the independent criteria of a third author (GRR). The inclusion criteria were: articles whose main topic of research dealt with the prevalence of addiction disorders to alcohol and/or other drug use in adults (over-18) in the years following bariatric surgery; written in Spanish or English; and original research articles. Duplicate articles were removed using the EndNote program. The information from the included articles was extracted by 3 authors (JPM, RCZ and GCS) and is summarized in Table 1. Finally, 2 authors (JPM and RCZ) independently carried out the risk of bias assessment using the Newcastle-Ottawa scale for cohort studies23; discrepancies between evaluations were discussed by the authors and resolved.
Characteristics of the studies included in this review.
| Design | Purpose of the study | Nature and size of the sample | Measurement tool and disorder evaluated | Substance and result | Reference |
|---|---|---|---|---|---|
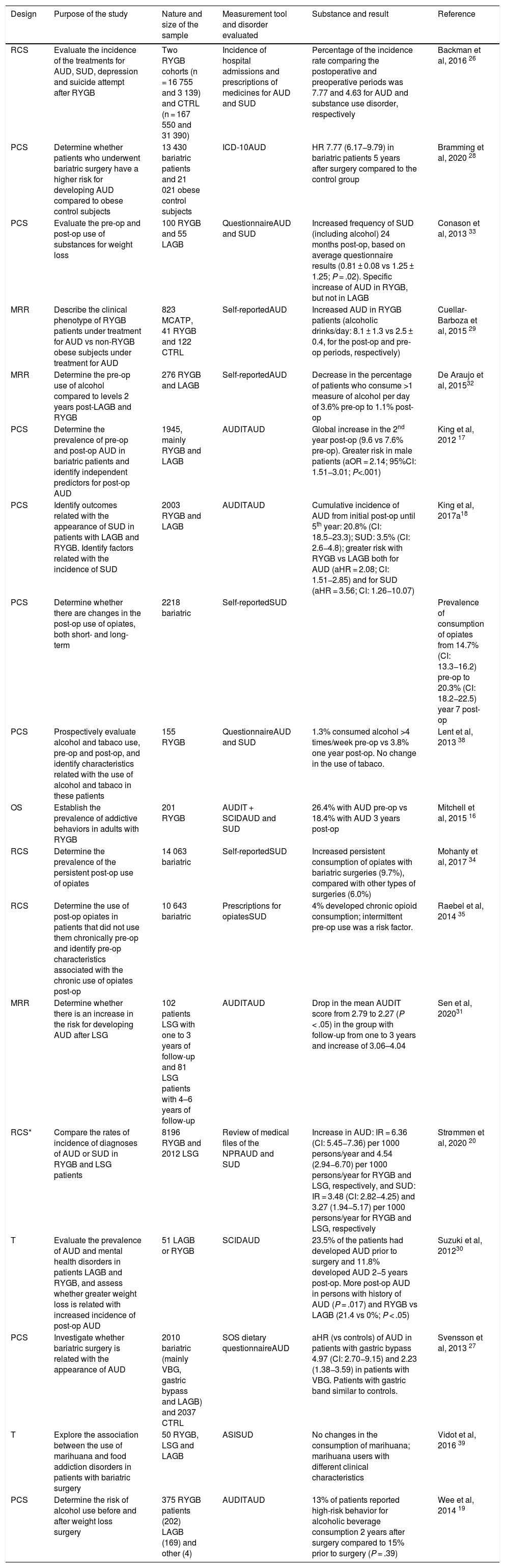
| RCS | Evaluate the incidence of the treatments for AUD, SUD, depression and suicide attempt after RYGB | Two RYGB cohorts (n = 16 755 and 3 139) and CTRL (n = 167 550 and 31 390) | Incidence of hospital admissions and prescriptions of medicines for AUD and SUD | Percentage of the incidence rate comparing the postoperative and preoperative periods was 7.77 and 4.63 for AUD and substance use disorder, respectively | Backman et al, 2016 26 |
| PCS | Determine whether patients who underwent bariatric surgery have a higher risk for developing AUD compared to obese control subjects | 13 430 bariatric patients and 21 021 obese control subjects | ICD-10AUD | HR 7.77 (6.17−9.79) in bariatric patients 5 years after surgery compared to the control group | Bramming et al, 2020 28 |
| PCS | Evaluate the pre-op and post-op use of substances for weight loss | 100 RYGB and 55 LAGB | QuestionnaireAUD and SUD | Increased frequency of SUD (including alcohol) 24 months post-op, based on average questionnaire results (0.81 ± 0.08 vs 1.25 ± 1.25; P = .02). Specific increase of AUD in RYGB, but not in LAGB | Conason et al, 2013 33 |
| MRR | Describe the clinical phenotype of RYGB patients under treatment for AUD vs non-RYGB obese subjects under treatment for AUD | 823 MCATP, 41 RYGB and 122 CTRL | Self-reportedAUD | Increased AUD in RYGB patients (alcoholic drinks/day: 8.1 ± 1.3 vs 2.5 ± 0.4, for the post-op and pre-op periods, respectively) | Cuellar-Barboza et al, 2015 29 |
| MRR | Determine the pre-op use of alcohol compared to levels 2 years post-LAGB and RYGB | 276 RYGB and LAGB | Self-reportedAUD | Decrease in the percentage of patients who consume >1 measure of alcohol per day of 3.6% pre-op to 1.1% post-op | De Araujo et al, 201532 |
| PCS | Determine the prevalence of pre-op and post-op AUD in bariatric patients and identify independent predictors for post-op AUD | 1945, mainly RYGB and LAGB | AUDITAUD | Global increase in the 2nd year post-op (9.6 vs 7.6% pre-op). Greater risk in male patients (aOR = 2.14; 95%CI: 1.51−3.01; P<.001) | King et al, 2012 17 |
| PCS | Identify outcomes related with the appearance of SUD in patients with LAGB and RYGB. Identify factors related with the incidence of SUD | 2003 RYGB and LAGB | AUDITAUD | Cumulative incidence of AUD from initial post-op until 5th year: 20.8% (CI: 18.5−23.3); SUD: 3.5% (CI: 2.6−4.8); greater risk with RYGB vs LAGB both for AUD (aHR = 2.08; CI: 1.51−2.85) and for SUD (aHR = 3.56; CI: 1.26−10.07) | King et al, 2017a18 |
| PCS | Determine whether there are changes in the post-op use of opiates, both short- and long-term | 2218 bariatric | Self-reportedSUD | Prevalence of consumption of opiates from 14.7% (CI: 13.3−16.2) pre-op to 20.3% (CI: 18.2−22.5) year 7 post-op | |
| PCS | Prospectively evaluate alcohol and tabaco use, pre-op and post-op, and identify characteristics related with the use of alcohol and tabaco in these patients | 155 RYGB | QuestionnaireAUD and SUD | 1.3% consumed alcohol >4 times/week pre-op vs 3.8% one year post-op. No change in the use of tabaco. | Lent et al, 2013 38 |
| OS | Establish the prevalence of addictive behaviors in adults with RYGB | 201 RYGB | AUDIT + SCIDAUD and SUD | 26.4% with AUD pre-op vs 18.4% with AUD 3 years post-op | Mitchell et al, 2015 16 |
| RCS | Determine the prevalence of the persistent post-op use of opiates | 14 063 bariatric | Self-reportedSUD | Increased persistent consumption of opiates with bariatric surgeries (9.7%), compared with other types of surgeries (6.0%) | Mohanty et al, 2017 34 |
| RCS | Determine the use of post-op opiates in patients that did not use them chronically pre-op and identify pre-op characteristics associated with the chronic use of opiates post-op | 10 643 bariatric | Prescriptions for opiatesSUD | 4% developed chronic opioid consumption; intermittent pre-op use was a risk factor. | Raebel et al, 2014 35 |
| MRR | Determine whether there is an increase in the risk for developing AUD after LSG | 102 patients LSG with one to 3 years of follow-up and 81 LSG patients with 4–6 years of follow-up | AUDITAUD | Drop in the mean AUDIT score from 2.79 to 2.27 (P < .05) in the group with follow-up from one to 3 years and increase of 3.06–4.04 | Sen et al, 202031 |
| RCS* | Compare the rates of incidence of diagnoses of AUD or SUD in RYGB and LSG patients | 8196 RYGB and 2012 LSG | Review of medical files of the NPRAUD and SUD | Increase in AUD: IR = 6.36 (CI: 5.45−7.36) per 1000 persons/year and 4.54 (2.94−6.70) per 1000 persons/year for RYGB and LSG, respectively, and SUD: IR = 3.48 (CI: 2.82−4.25) and 3.27 (1.94−5.17) per 1000 persons/year for RYGB and LSG, respectively | Strømmen et al, 2020 20 |
| T | Evaluate the prevalence of AUD and mental health disorders in patients LAGB and RYGB, and assess whether greater weight loss is related with increased incidence of post-op AUD | 51 LAGB or RYGB | SCIDAUD | 23.5% of the patients had developed AUD prior to surgery and 11.8% developed AUD 2−5 years post-op. More post-op AUD in persons with history of AUD (P = .017) and RYGB vs LAGB (21.4 vs 0%; P < .05) | Suzuki et al, 201230 |
| PCS | Investigate whether bariatric surgery is related with the appearance of AUD | 2010 bariatric (mainly VBG, gastric bypass and LAGB) and 2037 CTRL | SOS dietary questionnaireAUD | aHR (vs controls) of AUD in patients with gastric bypass 4.97 (CI: 2.70−9.15) and 2.23 (1.38−3.59) in patients with VBG. Patients with gastric band similar to controls. | Svensson et al, 2013 27 |
| T | Explore the association between the use of marihuana and food addiction disorders in patients with bariatric surgery | 50 RYGB, LSG and LAGB | ASISUD | No changes in the consumption of marihuana; marihuana users with different clinical characteristics | Vidot et al, 2016 39 |
| PCS | Determine the risk of alcohol use before and after weight loss surgery | 375 RYGB patients (202) LAGB (169) and other (4) | AUDITAUD | 13% of patients reported high-risk behavior for alcoholic beverage consumption 2 years after surgery compared to 15% prior to surgery (P = .39) | Wee et al, 2014 19 |
MRR: medical record review; aHR: adjusted hazard ratio; aOR: adjusted odds ratio; ASI: Addiction Severity Index; AUD: alcohol use disorder; AUDIT: Alcohol Use Disorders Identification Test; PCS: prospective cohort study; RCS: retrospective cohort study; CTRL: control patients; CI: 95% confidence interval; ICD-10: International Classification of Disease, 10th Revision; M: males; LAGB: laparoscopic adjustable gastric band; LSG: laparoscopic sleeve gastrectomy; F: females; MCATP: Mayo Clinic Addiction Treatment Program; NPR: Norwegian Patient Registry; OS: observational study; Post-op: postoperative; Pre-op: preoperative; RYGB: Roux-en-Y gastric bypass; SCID: Structured Clinical Interview for DSM-IV Axis I Disorders; SOS: Swedish Obese Subjects; SUD: substance use disorder; T: transversal study; IR: incidence rate; VBG: vertical banded gastrectomy.
Fig. 2 presents a summary of the primary sources consulted for the preparation of this study. In total, 18 original articles were analyzed. We selected studies that provided specific and complete information about the prevalence of AUD before and after surgery, the prevalence of substance use disorder other than alcohol (SUD) before and after surgery, or the prevalence of illicit substance use before and after surgery, which are presented in a quantitative synthesis in the text (n = 13). The results of the remainder (n = 5) are briefly described in the main text and in Table 1. The quantitative synthesis of data was carried out based on previously published criteria,24 and all calculations were done with the MedCalc program.25 For each of the comparisons and the evaluation of their respective outcomes, the data were extracted to obtain odds ratios (OR) for the pre- vs postoperative period for AUD and/or SUD. Our choice of this measure of association was based on the fact that many of the studies included evaluated prevalence by comparing the results of questionnaires that had been applied preoperatively vs postoperatively. For the studies that reported incidence rates of AUD and/or SUD and, therefore, focused on new cases over time, prevalence was considered at the longest reported postoperative time, with a minimum follow-up of 2 years and a maximum of 6 years in order to reduce clinical diversity. We also compared the prevalence of postoperative AUD for different types of surgery (RYGB vs others). For this latter comparison, we have grouped all procedures other than RYGB into a single category because of their relative scarcity and because previous studies have suggested a greater risk of AUD after RYGB compared to other procedures.17 Likewise, laparoscopic and open RYGB are represented in a single RYGB category.
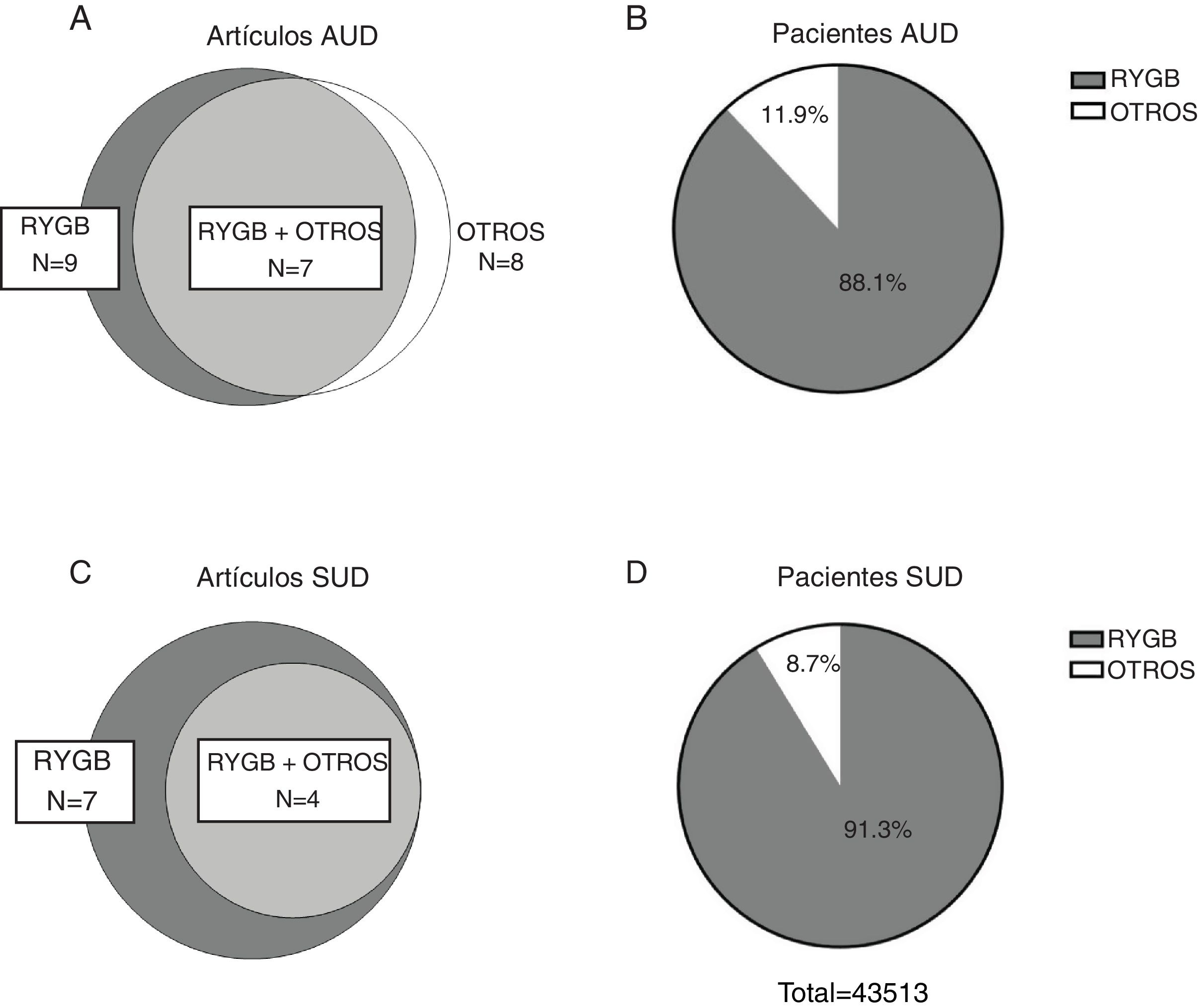
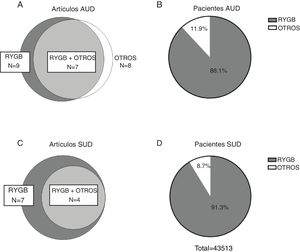
ResultsDescription of the results reviewedThe initial search carried out in the PubMed and Cochrane databases yielded 170 results after eliminating duplicates, 146 of which were excluded after reading the title and abstract, and another 6 were excluded after reading the full article. Therefore, the systematic review of the effect of bariatric surgery on the rate of addiction disorders was conducted by analyzing 16 original peer-reviewed research articles and 2 additional studies found from primary sources, for a total of 18 original articles (Fig. 1). Enough data could be extracted from 13 of these to carry out a quantitative synthesis, which is presented in the text. The 18 articles analyzed are comprised of 4 prospective cohort studies (PCS), 3 medical record reviews (MRR), one observational study (OS), 8 retrospective cohort studies (RCS) (one of which is population-based) and 2 cross-sectional studies (Table 1). Regarding the type of surgery performed in most of the articles, 11 of them studied populations whose surgeries were varied (including RYGB, LAGB, LSG and VBG), while 5 articles analyzed only patients with RYGB surgery. Regarding the size of the samples that were analyzed in the articles, they varied from 50 patients to about 20 000 (Table 1). In cases of both AUD and SUD, the vast majority of patients underwent RYGB (Fig. 2).
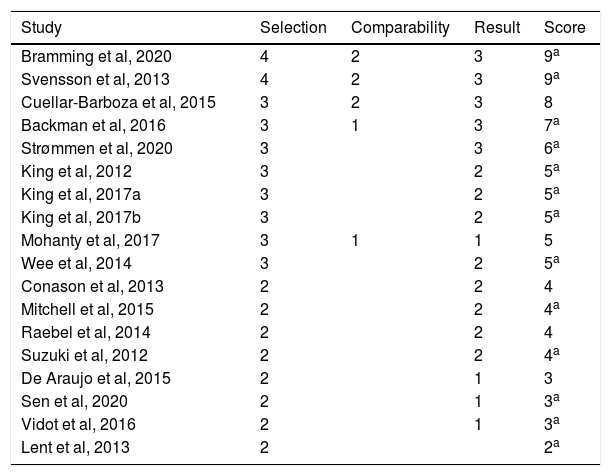
The evaluation tools that were used included: the incidence of hospital admissions and its relationship with the prescription of medications for AUD and SUD, questionnaires such as the Swedish Obese Subjects (SOS) dietary survey, the Alcohol Use Disorders Identification Test (AUDIT) and the Structured Clinical Interview for DSM-IV (SCID). In addition, patient self-reported data and reviews of medical records were used. Although there is great methodological diversity among the studies reviewed, in all of them the main objective was to evaluate the correlation between bariatric surgery and the incidence of AUD and/or substance use in the postoperative period. The risk of bias assessment was carried out with the Newcastle-Ottawa scale for cohort studies (Table 2). Most of the studies analyzed had selected cohorts (for example: patients who required medical services at certain hospitals, or who were part of a certain treatment program), in such a way that their representativeness is very low. Even with this limitation, the sample of some studies was quite large. On the other hand, only 3 studies included a sample of control patients (Backman et al, Cuellar-Barboza et al. and Svensson et al.), meaning that they included, for comparative purposes, a group of obese patients who were non-exposed (no bariatric surgery).26–28 Another study29 included obese patients with AUD/SUD and no history of bariatric surgery as a control group. In all the cases analyzed, the authors corroborated the exposure (in this case bariatric surgery) and evaluated the presence of AUD and/or SUD before the operation. The comparability was a parameter in which most of the studies analyzed had low scores, since only 3 of them used control groups, as mentioned above. Finally, when we analyzed the studies with a minimum follow-up of 2 years, 7 studies measured the results based on the review of medical records, while the others did so using self-reported data. In addition, 13 of the 18 articles analyzed had followed the patients for at least 2 years, while 15 studies had a follow-up of at least 80% of the initial participants. Finally, most of the studies had a mean scale score between 3 and 5; 5 articles were considered of excellent quality (6 points or more), and only one article obtained a rating lower than 3 (Table 2).
Risk of bias assessment of the studies reviewed using the Newcastle–Ottawa scale for cohort studies. The studies are in decreasing order according to the score obtained.
| Study | Selection | Comparability | Result | Score |
|---|---|---|---|---|
| Bramming et al, 2020 | 4 | 2 | 3 | 9a |
| Svensson et al, 2013 | 4 | 2 | 3 | 9a |
| Cuellar-Barboza et al, 2015 | 3 | 2 | 3 | 8 |
| Backman et al, 2016 | 3 | 1 | 3 | 7a |
| Strømmen et al, 2020 | 3 | 3 | 6a | |
| King et al, 2012 | 3 | 2 | 5a | |
| King et al, 2017a | 3 | 2 | 5a | |
| King et al, 2017b | 3 | 2 | 5a | |
| Mohanty et al, 2017 | 3 | 1 | 1 | 5 |
| Wee et al, 2014 | 3 | 2 | 5a | |
| Conason et al, 2013 | 2 | 2 | 4 | |
| Mitchell et al, 2015 | 2 | 2 | 4a | |
| Raebel et al, 2014 | 2 | 2 | 4 | |
| Suzuki et al, 2012 | 2 | 2 | 4a | |
| De Araujo et al, 2015 | 2 | 1 | 3 | |
| Sen et al, 2020 | 2 | 1 | 3a | |
| Vidot et al, 2016 | 2 | 1 | 3a | |
| Lent et al, 2013 | 2 | 2a |
Eleven studies included in the present review reported data for the prevalence of AUD in both the preoperative and postoperative periods, totaling 49 121 patients (80.8% women) with postoperative data for 43 380. The reviewed studies show great variability in terms of follow-up time (between 2 and 22 years). Therefore, for comparative purposes, we extracted data at a maximum of 5–6 years from studies with very long follow-up times, and we have not included times shorter than 2 years, resulting in a range of 2–6 years (median: 3.6 years). The results obtained presented a very high variability (OR between 0.24; 95% CI: 0.09–0.6830 and 19.98; 95% CI: 1.15–345.8831), probably due to the great methodological diversity (various measurement tools to assess AUD) and, to a lesser extent, considerable clinical diversity (different surgical procedures, different follow-up times, different populations, etc.). An increase in the prevalence of AUD after surgery was reported in 9 out of the 11 studies reviewed. In addition, the weighted mean (±SD) prevalence of AUD observed in the reviewed studies in the postoperative period was 4.58% ± 5.3%, compared to 1.58% ± 10.7% in the preoperative period and 1.11% ± 0.25% in control subjects. Therefore, although bariatric surgery seems to be related to an increased risk of developing AUD, this occurs in a relatively small percentage of patients.
One of the most prominent studies that reported an increased risk of AUD with bariatric surgery analyzed 2 cohorts of 16 755 and 3 139 Swedish patients who had RYGB surgery (with 167 550 and 31 390 referral subjects, respectively), and compared the incidence of depression, substance abuse and alcohol abuse in the preoperative vs postoperative periods. Notably, the percentage of patients receiving treatment for AUD 4 years after surgery was 2.6%, compared to 0.4% one year before the procedure and 0.3% in the control group.26 When the authors analyzed the sexes separately, they reported that women in the RYGB group were slightly more likely to receive treatment for AUD before surgery compared to controls (IR: 7.25 vs 5.20 per 10 000 persons/year), with a more pronounced increase in this treatment in the group of men after surgery (incidence rate ratio [IRR]: 9.11; 95% CI: 6.91–11.99) for men vs 7.22 (95% CI: 5.86–8.89) for women.26 These data coincide with a previous study in which the male sex was also identified as a risk factor (aOR: 2.14; 95% CI: 1.51−3.01; P < .001).17 Another study that could not be included in our quantitative synthesis reviewed the medical records of patients treated by the Mayo Clinic Addiction Treatment Program. This is the only study included in the present review that used the inverse strategy, by selecting a population of patients being treated for addictive disorder and evaluating the proportion who had received bariatric surgery and describing their characteristics and evolution. The authors found that 41 out of the 823 patients had a history of RYGB surgery. In these patients, a gradual increase in alcohol consumption was reported with every passing year after surgery procedure, reaching 8.1 ± 1.3 alcoholic drinks per day vs 2.5 ± 0.4 in the preoperative period (P = .009). In this group of patients, no relationship was found between sex, age or body mass index and alcohol consumption habits.29 Among the studies we have reviewed, only one decreased alcohol consumption after bariatric surgery. In this retrospective documentary study in the Portuguese population, 276 obese patients who underwent RYGB or LAGB were evaluated and reported a lower preoperative prevalence of alcohol consumption (24.2%) compared to a representative sample of the Portuguese population at large (34.0%). Furthermore, these patients showed a clear decrease in their alcohol consumption, which went from 24.2% to 9.1% 2 years after surgery.32 It is noteworthy, however, that this study did not directly analyze the prevalence of AUD in these populations, but rather consumption habits.
Risk of alcohol abuse disorder in patients with Roux-en-Y gastric bypass vs other types of surgerySome studies have suggested that not all types of bariatric surgery are associated with the same risk of AUD.17 Therefore, we subsequently proceeded to the analysis of the propensity to develop postoperative AUD based on surgery type. Due to the relative paucity of data on procedures other than gastric bypass, these were grouped into a single category, and a significant increase in the risk of AUD was reported in 3 of the 8 studies reviewed. The respective OR were between 0.05 (95% CI: 0.00−0.88)30 and 19.98 (95% CI: 1.15–345.88).31 When we exclusively analyzed patients with RYGB, we observed increased risk 8 out of the 10 studies, and each of these 8 had follow-up times of ≥3 years. As for the 2 studies that found no effect, one had a follow-up time of 2 years19 and the other had a follow-up of 2–5 years, although it only included 28 patients.30 The other studies had an OR of 1.40 (95% CI: 1.06–1.85), which compared the prevalence of preoperative AUD diagnosis with its prevalence 2 years after surgery using the AUDIT,17 and 7.33 (95% CI: 5.86–9.18) in a very recent study of 11 686 patients with RYGB and a median follow-up of 6.9 years.28 Finally, the weighted mean difference of the OR obtained for RYGB compared to other types of surgery was 1.83 (95% CI: 1.51–2.21).
Among the studies that compared the risk of AUD in patients with RYGB vs other types of procedures, one published in 2017 was based on a prospective cohort study of adult patients from the US who underwent bariatric surgery (Longitudinal Assessment of Bariatric Surgery-2). This study reported that RYGB was associated with a significantly higher risk compared to LAGB (average postoperative time 43.4 ± 6.8 months).18 These observations confirmed previous results published by the same group that showed an increase in the prevalence of AUD in the second postoperative year (9.6% compared to 7.6% pre-op), identifying RYGB surgery as a risk factor (aOR: 2.07; 95% CI: 1.40–3.08; P < .001), as well as male sex (OR: 2.14; 95% CI: 1.51−3.01; P < .001).17 Another study in obese patients undergoing bariatric surgery found an increase in alcohol consumption and abuse after gastric bypass (adjusted hazard ratio [aHR]: 4.97; 95% CI: 2.70−9.15) and VBG (aHR: 2.23; 95% CI: 1.38−3.59), but not in patients with gastric band (aHR: 1.57; 95% CI: 0.73–3.35).27 Similarly, a cross-sectional study in 28 RYGB patients and 23 LAGB patients showed that 21.4% of RYGB patients had AUD vs 0% in the LAGB group.30 This apparent discrepancy could be explained, at least in part, by the fact that the vast majority of the patients evaluated in this study were women (90.2%), most of whom were treated with LAGB. Finally, a recent study in a cohort of Norwegian RYGB and LSG patients from the registry found an incidence rate of AUD of 6.06 per 1000 persons/year in a postoperative period covering 33 352 persons/year and in which there were no significant differences in the risk rate between the 2 procedures.20
Use of other drugs and its relationship with addictive disorders after bariatric surgeryThe prevalence of other types of addictions other than alcoholism after bariatric surgery has also been studied; however, there are far fewer reports in this regard. A total of 10 studies comparing the prevalence of dependence disorders for substances other than alcohol before and after having undergone bariatric surgery met our selection criteria, 7 of which had a postoperative follow-up of at least 2 years (median: 3.3 years) and reported sufficient data to extract OR. These included 17 914 patients (74.8% women) with preoperative data, 12 839 of whom had postoperative data. The substances analyzed were mainly opiates, benzodiazepines, tobacco and cannabis. The postoperative period varied between 2 and 7 years. The OR ranged from 0.73 (95% CI: 0.40–1.32) in a study that only evaluated the prevalence of tobacco use16 to 2.48 (95% CI: 1.86–3.29) in another with an extensive cohort that evaluated the use of different substances.20 As for the other three studies, one published in 2013 evaluated 155 patients, mainly women, based on a questionnaire developed by the authors themselves. They reported that the frequency of use of illicit substances did not increase significantly in the two years after bariatric surgery. However, when they combined the incidences of AUD and substance abuse, a greater increase was reached compared to preoperative levels than when the incidence of AUD was considered alone.33 Another study reported that in a group of patients who had never used opioid-type analgesics before bariatric surgery, 10% reported persistent use one year after surgery, which is 60% more than the general surgical patient population.34 Therefore, although less frequent, there appears to be a trend towards a specific increase in dependence on opioid-type analgesics related with bariatric surgery.
Although they seem conclusive, these observations must be considered with caution. A large retrospective cohort study published in 2014 showed that, although 4% of patients without a history of chronic opiate use developed dependence after surgery, the risk of developing this condition was strongly related to the intermittent use of opiates or other abused substances before surgery.35 Notably, none of the articles reviewed here reported a significant change in the prevalence of smoking after bariatric surgery,36–38 so it is impossible to confirm that postoperative tobacco use is affected. On the other hand, when marijuana abuse was studied in a mostly female population (76%) who underwent RYGB (62%), 18% of the sample reported consuming marijuana, 38% of which reported recent use and 21.4% an increase in postoperative use. However, when evaluated two years after surgery, more than 30% of patients with a history of preoperative marijuana use reported not having used it in the last year.39 Therefore, this study does not allow us to conclude that bariatric surgery induces an increased risk of developing a cannabis dependence; longer-term evaluations and additional studies will be required to reach a conclusion.
Regarding the specific type of bariatric surgery, its relevance as a risk factor in the development of dependence disorders for substances other than alcohol is not clear. In the recent cohort study of bariatric patients from the Norwegian population mentioned above, an increased incidence of diagnoses of non-alcohol substance abuse disorder was also observed in both the post-RYGB period (incidence rate 3.48 per 1000 persons/year) and the LSG (incidence rate 3.27 per 1000 persons/year).20 Another study included in our analysis35 with a one-year follow-up period established a lower risk of developing opioid dependence with gastric band compared to bypass (OR: 0.42; 95%CI: 0.25−0.70 for band vs bypass). However, a recently published study that followed patient cohorts over a 7-year period found no difference between the two procedures (RYGB vs LAGB) in terms of the relative risk of developing opioid dependence,40 which is in clear contrast with observations made in another study by the same group about the risk of developing AUD (see previous section).
Summary and limitationsThe evidence reviewed in this paper includes several additional studies that are in line with the findings of a previously published meta-analysis21 that found a pooled OR of 1.852 and a significant increase in AUD after bariatric surgery over a postoperative period of 3 or more years. In addition, we have conducted a meta-analysis, whose results suggest that there is a significantly higher risk of developing AUD in the years after RYGB-type surgery than other types of surgery. Furthermore, we have observed that, although there is evidence to support increased abuse of substances other than alcohol after bariatric surgery, the results are not definitive. In different studies reviewed herein,20,34,40 the incidence of substance abuse (especially sedatives such as opioids) increased following a similar kinetic to alcohol abuse, with increased risk starting in the 3rd year. Interestingly, and contrary to what was observed with AUD, a recent study reported that there was no increased risk in terms of opioid abuse in RYGB vs other procedures with a 7-year postoperative follow-up40 or in substance abuse (mainly sedatives) between RYGB vs LSG patients with a 6-year postoperative follow-up.20 These data are interesting in light of the change in alcohol absorption kinetics, which we will briefly discuss later. Moreover, it is striking that none of the articles reviewed in this text found any increase in the incidence of stimulant use – no relationship was observed for tobacco, and the data obtained for other substances (eg, cocaine or amphetamines) are very limited. It is therefore likely that the risk assessment for developing a substance use disorder after a bariatric procedure will have to be assessed separately for each substance or group of substances.
An important limitation of this manuscript is that, as was the case with a previously published systematic review on a similar topic,21 the measure of association that we decided to extract from the various studies included in our quantitative synthesis was the OR. Although most of the initial studies included in this review express the data in OR or in prevalence at a given time after surgery, many of the more recent studies with longer follow-up times report the data in terms of hazard ratios. Therefore, potentially relevant information is not presented regarding the dynamics of risk in the postoperative period. Furthermore, our study only considered papers published in indexed journals for the quantitative synthesis, and publication bias was not taken into account, which may have exaggerated the reported effect.
Also, analogous to previous studies,21 another limitation of the conclusions described in this review is that most of the studies reviewed lack a control group (ie, patients with morbid obesity not treated with bariatric surgery), and it is possible that these patients had developed AUD or dependence on some other substance regardless of having undergone any such surgery. We should mention, however, that a very recent article that does include this control observed that the hazard ratio for developing AUD 5 years after bariatric surgery compared to preoperative levels (HR: 7.77; 95%CI: 6.17−9.79) was almost identical to what was obtained by comparing the bariatric surgery group 5 years after surgery with the control group of obese patients without surgery (HR: 7.29; 95%CI: 5.60–9.48),28 which argues in favor of a specific effect of bariatric surgery on the risk of AUD. Moreover, the presence of AUD and/or SUD is a relative exclusion criterion for bariatric surgery, depending on the surgeon. Meanwhile, if patients who wish to be selected for bariatric surgery are also aware of this fact, they may voluntarily misrepresent their alcohol consumption when asked. In both situations, the result could be an artificial decrease in preoperative prevalence, which increases the risk of type 1 error of a significant postoperative increase without it being real. On the other hand, the fact that several studies have observed a ‘J curve’ effect in the rate of substance abuse behaviors (which tend to decrease in the first year and later rise to exceed baseline levels33,40) argues against said possibility.
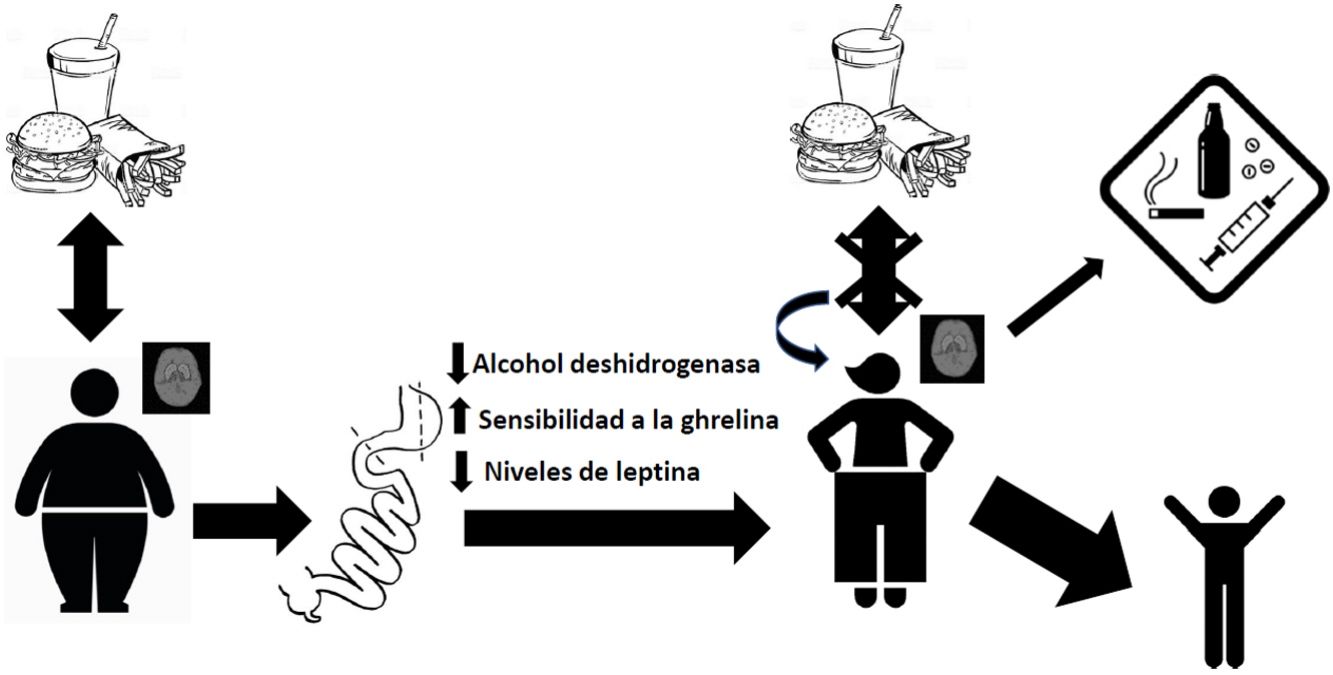
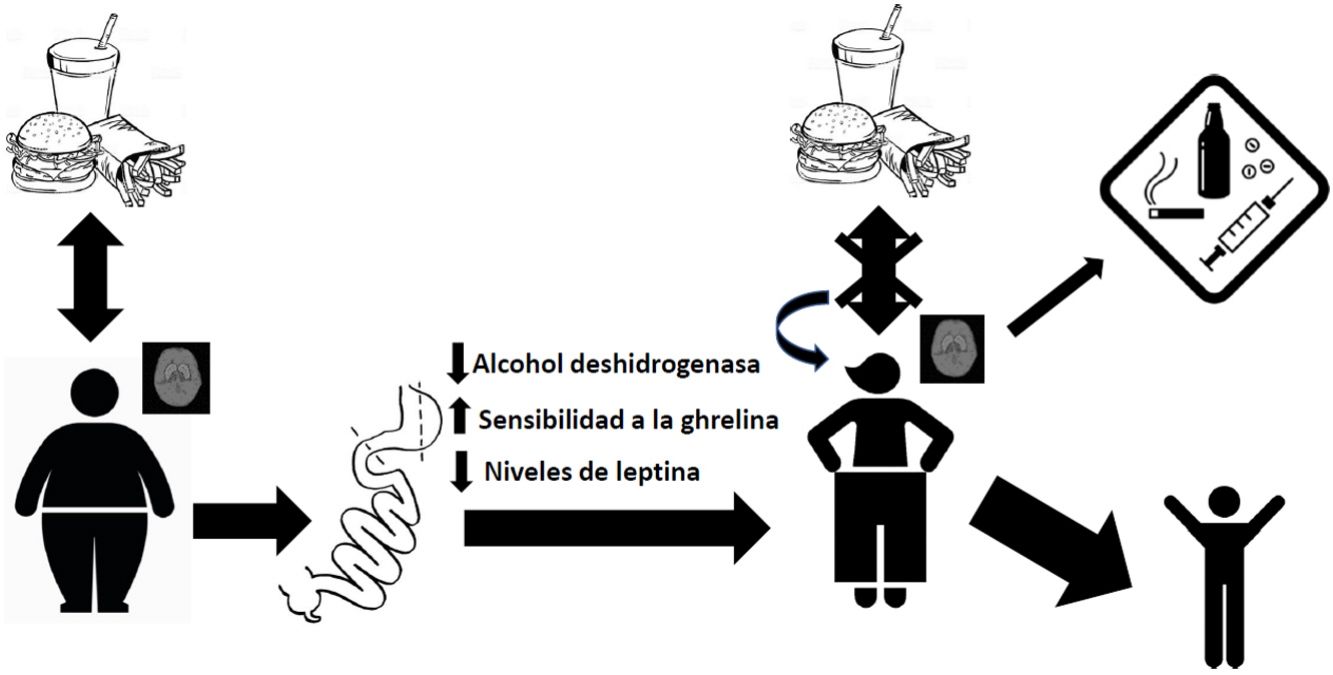
DiscussionAbsorption kinetics and addictive potential of alcohol after bariatric surgeryThe mainstay of weight control surgery consists of modifying the normal anatomy of the gastrointestinal tract and, consequently, modifying the physiology of nutrient absorption. With gastric bypass, an increase has been observed in the rate of absorption of alcohol into the bloodstream due to reduced first-pass effect through a decrease in the alcohol dehydrogenase enzyme in the stomach.41,42 This, together with the reduction in stomach volume and the acceleration of gastric emptying time after surgery, leads to more rapid and extensive absorption in the jejunum.43 This has been demonstrated in patients with RYGB, with observed blood alcohol levels higher than 0.08% after ingesting a single drink, compared to their controls at 0.05%.44,45 This exposure to peaks with a higher concentration of alcohol can generate a greater addictive potential in these patients.46 It was also found that the elimination rate was reduced, and therefore the exposure time to alcohol was longer. Thus, the gastric bypass group needed, on average, 108 min to reach an alcoholic breath level of 0, while the control group reached this level after an average of 72 min.44 Interestingly, these values increase with the passage of postoperative time, as reported by Woodard et al. in 201145 in a study where 19 patients with RYGB had breath tests after drinking 5 ounces of red wine. The results showed increasing blood alcohol levels at 3 months (0.059%) and 6 months (0.088%) after surgery, while the preoperative level had been 0.024%. Regarding the time to return to the value 0, at 3 months post-op it was 61 min and at 6 months post-op 88 min, compared to 49 min before surgery.45
Endocrine disturbances after bariatric surgery and its relationship with the abuse of alcohol and other substancesBariatric surgery generates disturbances in the endocrine regulation of satiety. By modifying the distribution of the gastrointestinal tract anatomically, changes in the concentration of hormones occur, which causes different physiological adaptations at both the peripheral and central levels. For example, we know that there is a greater sensitivity to ghrelin in patients treated with bariatric surgery, and it has been suggested that this increased sensitivity to this hormone may contribute to increased ethanol consumption.47 In this regard, studies carried out in murine models show that ghrelin antagonists block the gratifying effects of alcohol,48,49 which is consistent with a role of ghrelin in mediating the rewarding nature of this drug. Another hormone that modulates the reward centers of the brain is leptin, which plays a particularly important role in activating brain reward circuitry in response to cues related to both highly appetizing food and abused drugs. Higher endogenous levels of leptin have been associated with exaggerated emotional responses to images of food in adolescents with obesity.50 On the other hand, a negative correlation has been reported between its plasma levels and the intense desire to consume alcohol, as well as the neuronal activity related to cues that remember obtaining ethyl substances.51
General conclusionsThe evidence examined in this review supports the idea that there is an increased risk of alcohol abuse in patients who are treated with bariatric procedures, mainly RYGB. This review emphasizes the need for timely detection of patients at a higher risk of presenting postoperative AUD16 before undergoing these types of procedures, thereby reducing the appearance of alcohol and other substance abuse after surgery. If a higher risk is found, the surgeon should take this into account in their choice of surgical procedure as the data reviewed here show a relatively higher risk for RYGB versus other procedures. The need for neuropsychological follow-up adapted to this type of patient could also be identified. Alternatively, a referral for a different treatment could be considered after consulting with the patient. However, bariatric surgery is by far the most effective treatment for obesity and its related conditions, with an excellent long-term prognosis; meanwhile, the appearance of AUD only occurs in a relatively small percentage of cases. On the other hand, better knowledge of the neurobehavioral characteristics of patients who develop addictive behaviors after bariatric surgery will definitely improve the selection process and postoperative management. For instance, brain imaging studies before the surgical procedure associated with a longitudinal follow-up (as in as recent study52) will provide valuable information to complement patient medical files, family history and other data.
FundingJPM and GRR have received grants from the university research support program Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) numbers IA206019 and IN224019, Dirección General de Asuntos del Personal Académico, UNAM.
Conflict of interestsThe authors declare that this article has been completed with no commercial or financial relations that could be construed as a possible conflict of interests.
Please cite this article as: Cerón-Solano G, Zepeda RC, Romero Lozano JG, Roldán-Roldán G, Morin JP. Cirugía bariátrica y trastorno por abuso de alcohol y otras sustancias: una revisión sistemática. Cir Esp. 2021;99:635–647.