Obesity and rapid weight loss after bariatric surgery (BS) are risk factors for the development of cholelitiasis. The aim of this study is to know the incidence of the de novo symptomatic cholelitiasis (DNSC) after BS and to analyze the risk factors for its development.
MethodsSingle-centre retrospective observational study of patients undergoing BS between January 2010 and December 2017. The incidence of DNSC has been studied and sex, age, comorbilities, surgical tecnique, initial BMI and percentage of excess BMI lost (%EBMIL) at 6th, 12th and 24th postoperative months have been analyzed.
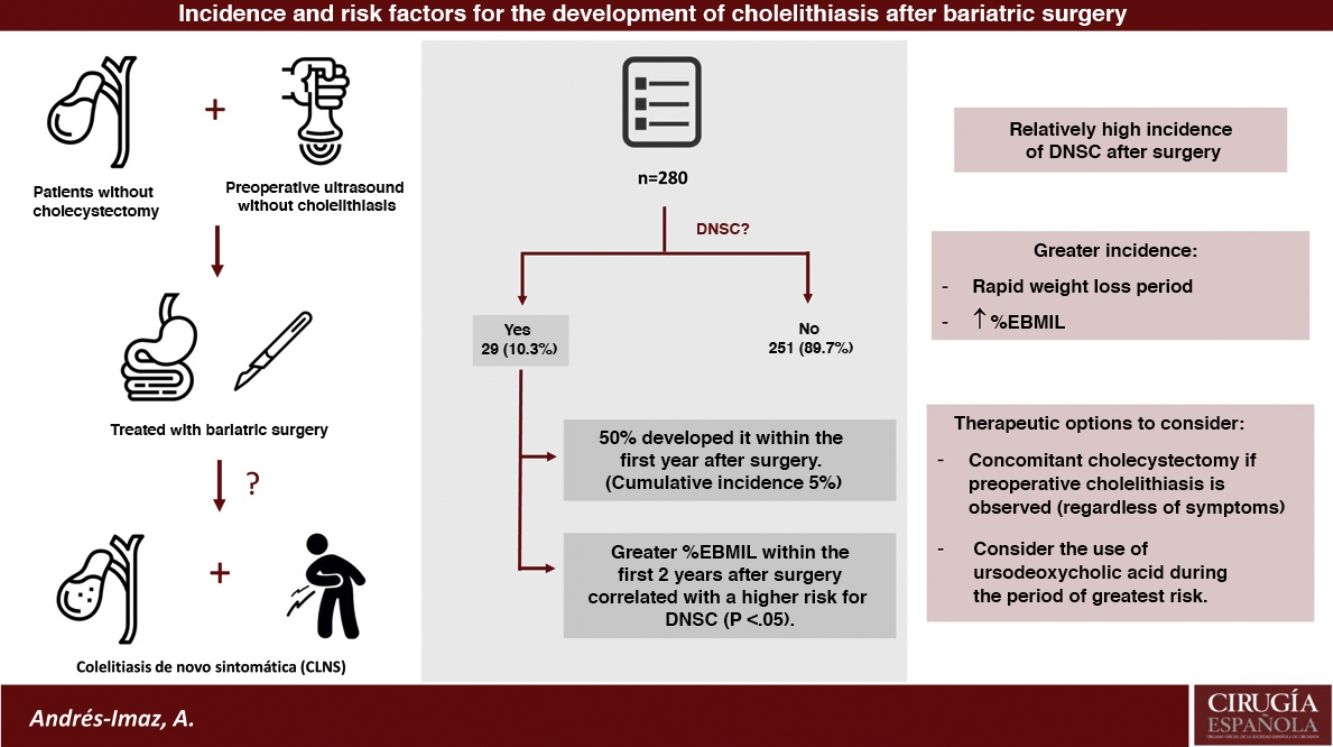
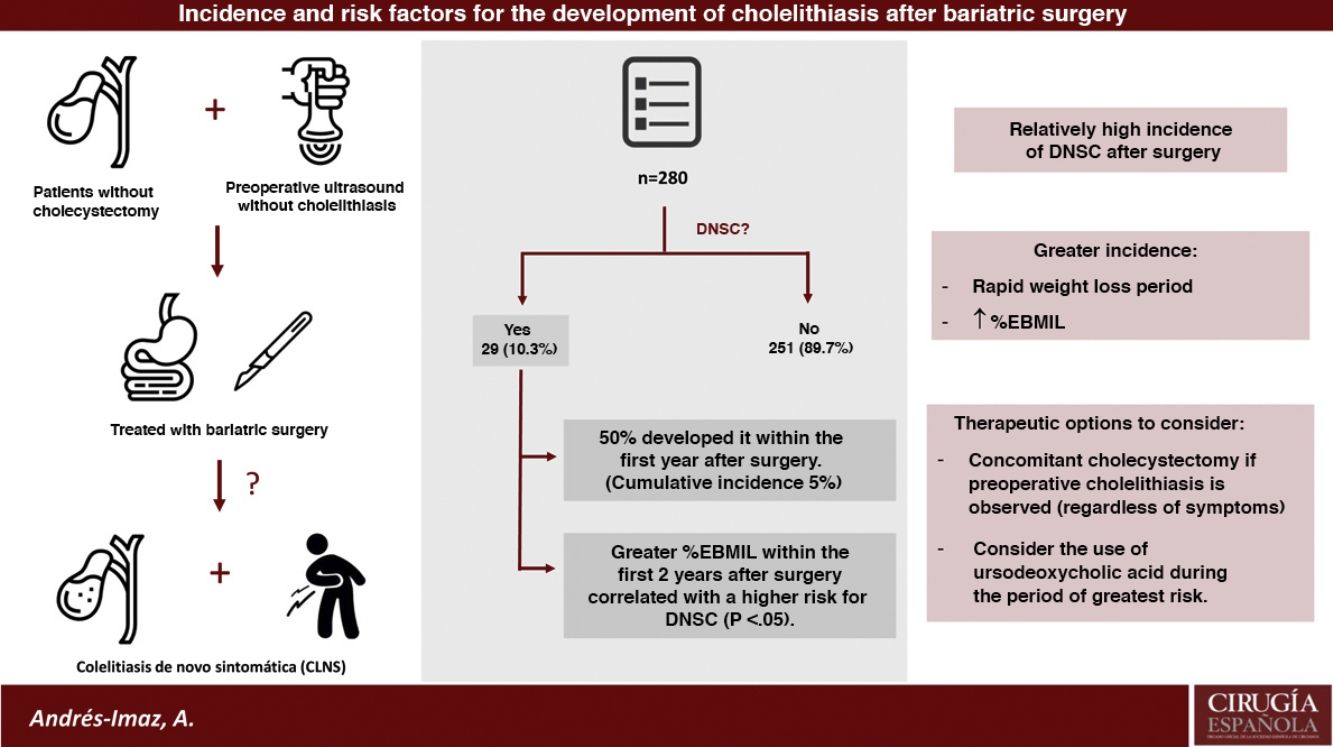
ResultsAmong the 415 patients who underwent BS, 280 have been studied since they were not previously cholecystectomized and had a preoperative negative abdominal ultrasound. Twenty-nine developed DNSC (10,35%), with a remarkably higher increase in cumulative incidence during the first postoperative year (CI 5%, IC 95% 2,4–7,6). A higher percentage of excess BMI lost at the 6, 12 and 24 postoperative months was statistically significantly correlated with an increased risk of DNSC.
ConclusionsIncidence of DNSC and cholecystectomy after BS are relatively high, mainly during rapid weight loss period and even more the higher the percentage of excess BMI lost is. Concomitant cholecystectomy during BS in case of preoperative cholelithiasis regardless of symptoms and the use of ursodeoxycholic acid during the period of greater risk for DNSC development are two therapeutic options to consider.
La obesidad y la rápida pérdida de peso tras cirugía bariátrica (CB) son factores de riesgo para la formación de colelitiasis. El objetivo de este trabajo es conocer la incidencia de colelitiasis de novo sintomática (CLNS) tras CB y analizar los factores de riesgo para su desarrollo.
MétodosEstudio observacional retrospectivo unicéntrico de los pacientes sometidos a CB entre Enero de 2010 y Diciembre de 2017. Se ha estudiado la incidencia de CLNS y se han analizado el género, la edad, las comorbilidades, la técnica quirúrgica, el IMC inicial y el % exceso de IMC perdido (%EIMCP) al 6°, 12° y 24° mes postoperatorio.
ResultadosDe los 415 pacientes intervenidos de CB, 280 han sido estudiados ya que no estaban colecistectomizados previamente y tenían una ecografía preoperatoria negativa para colelitiasis. Veintinueve desarrollaron CLNS (10,35%), con un aumento en la incidencia acumulada notablemente más alto durante el primer año postoperatorio (IA 5%, IC 95% 2,4–7,6). Un mayor %EIMCP al 6°, 12°, 24° mes postoperatorio se correlacionó de forma estadísticamente significativa con un mayor riesgo de CLNS.
ConclusionesLa incidencia de CLNS y colecistectomía tras CB es relativamente alta, principalmente durante el periodo de pérdida de peso rápida y más cuanto mayor sea el %EIMCP. La colecistectomía concomitante en caso de colelitiasis preoperatoria independientemente de la sintomatología y el uso de ácido ursodesoxicólico durante el periodo de mayor riesgo para el desarrollo de CLNS son dos opciones terapéuticas a tener en cuenta.
Obesity is a known risk factor for the formation of cholelithiasis. The excess excretion of cholesterol into the bile and the decreased contractile response of the gallbladder smooth muscle to cholecystokinin produce bile stasis, which favors the precipitation of crystals and the consequent formation of calculi. It is estimated that the morbidly obese population (body mass index [BMI] >40 kg/m2) has an annual incidence of cholelithiasis of 2%1,2.
In contrast, rapid weight loss, either after bariatric surgery (BS) or by non-surgical means, also predisposes patients to the formation of de novo cholelithiasis (DNC). The mechanism by which DNC occurs is still under discussion. On the one hand, lipolysis produces greater excretion and mobilization of cholesterol, generating lithogenic bile; on the other hand, duodenal exclusion after certain surgical procedures leads to less cholecystokinin secretion and, therefore, less gallbladder motility2.
Several studies reveal that 30%–53% of the population undergoing BS develop DNC and that 7%–14% will present symptoms secondary to biliary disease (de novo symptomatic cholelithiasis, or DNSC), mainly in the form of colic and cholecystitis3–5. In fact, cholecystectomy is the most frequently performed procedure after BS6. Studies have also indicated7–10 that one of the risk factors for the formation of gallstones is the percentage of excess body mass index lost (%EBMIL) during the first year, which then decreases when a stable weight is reached. Thus, perhaps malabsorptive surgical techniques carry a greater risk than purely restrictive ones.
Therefore, performing concomitant cholecystectomy with BS has been a historically controversial issue. Generally, there are no doubts when patients present with symptomatic cholelithiasis at the time of surgery, but the indication is debated if the cholelithiasis is asymptomatic or when there is no cholelithiasis. In the absence of randomized clinical trials, the most consolidated and accepted approach today is selective cholecystectomy, meaning that associated cholecystectomy is only performed in cases of symptomatic lithiasis, since routine cholecystectomy may not be technically simple and is not free of complications7–10.
The objective of this study is to determine the incidence of DNSC after BS and to analyze the risk factors for its development.
MethodsWe conducted a single-center retrospective observational study based on data from the prospective database of the Esophageal-Gastric and Obesity Surgery Division. The study was approved by the Ethics Committee of our hospital (Protocol Code: AND-COL-2020-01).
Before BS, all patients were assessed by a multidisciplinary committee of endocrinologists, psychologists, anesthesiologists, nurses, and surgeons. The preoperative study was completed with laboratory tests, upper gastrointestinal endoscopy with screening for Helicobacter pylori infection, abdominal ultrasound, respiratory function tests, and psychological evaluation. Patients who presented cholelithiasis on preoperative ultrasound underwent cholecystectomy during BS, regardless of whether they presented symptoms or not. In the nutrition and psychology consultations, behavior and eating habit assessments were carried out, which were taken into consideration when making the indication for surgery. All patients followed a very low-calorie liquid diet the week before surgery. Two types of bariatric techniques were performed: sleeve gastrectomy (SG) was indicated for patients with a BMI of 35–40 with comorbidities associated with obesity, non-nibblers, and as a first phase in super-obese patients with BMI ≥ 55, while patients with a BMI of 40–55, classified as nibblers, underwent Roux-en-Y gastric bypass (RYGB).
Patients who had undergone BS at our center between January 2010 and December 2017 were included in the study. We analyzed the frequency of DNSC after BS and the risk factors for its development. DNSC was defined as all abdominal symptoms secondary to biliary lithiasic disease (colic, cholecystitis, pancreatitis, etc) with imaging studies confirming cholelithiasis, all of whom had a negative ultrasound prior to BS. The date of diagnosis was defined as the day the imaging test was performed. A minimum follow-up of 24 months was carried out in the outpatient consultations until February 2020 (inclusive). Patients with another prior BS technique or with a follow-up of less than 24 months were excluded. Sex, age, comorbidities, surgical technique, initial BMI, and %EBMIL were analyzed 6, 12, and 24 months after surgery.
Data were collected using Excel for Windows®, and the analyses were performed using Excel for Windows® and Statistical Package for the Social Sciences® 21.0 (SPSS IBM®, Chicago, IL, USA). The variables were described using the most appropriate statistic for the nature and scale of measurement for each one: mean and standard deviation for quantitative variables, and absolute and relative frequencies in percentages for qualitative variables.
A univariate analysis was performed to assess the importance of the study variables in relation to the outcome of interest. The Student’s t test was used to study the continuous variables and the chi-squared test for the nominal variables. Since the appearance of symptoms (DNSC) is dependent on the follow-up time, its incidence was studied using Kaplan–Meier curves, analyzing statistical significance using the Log-rank test. For all tests of statistical significance, a P value <.05 was considered statistically significant.
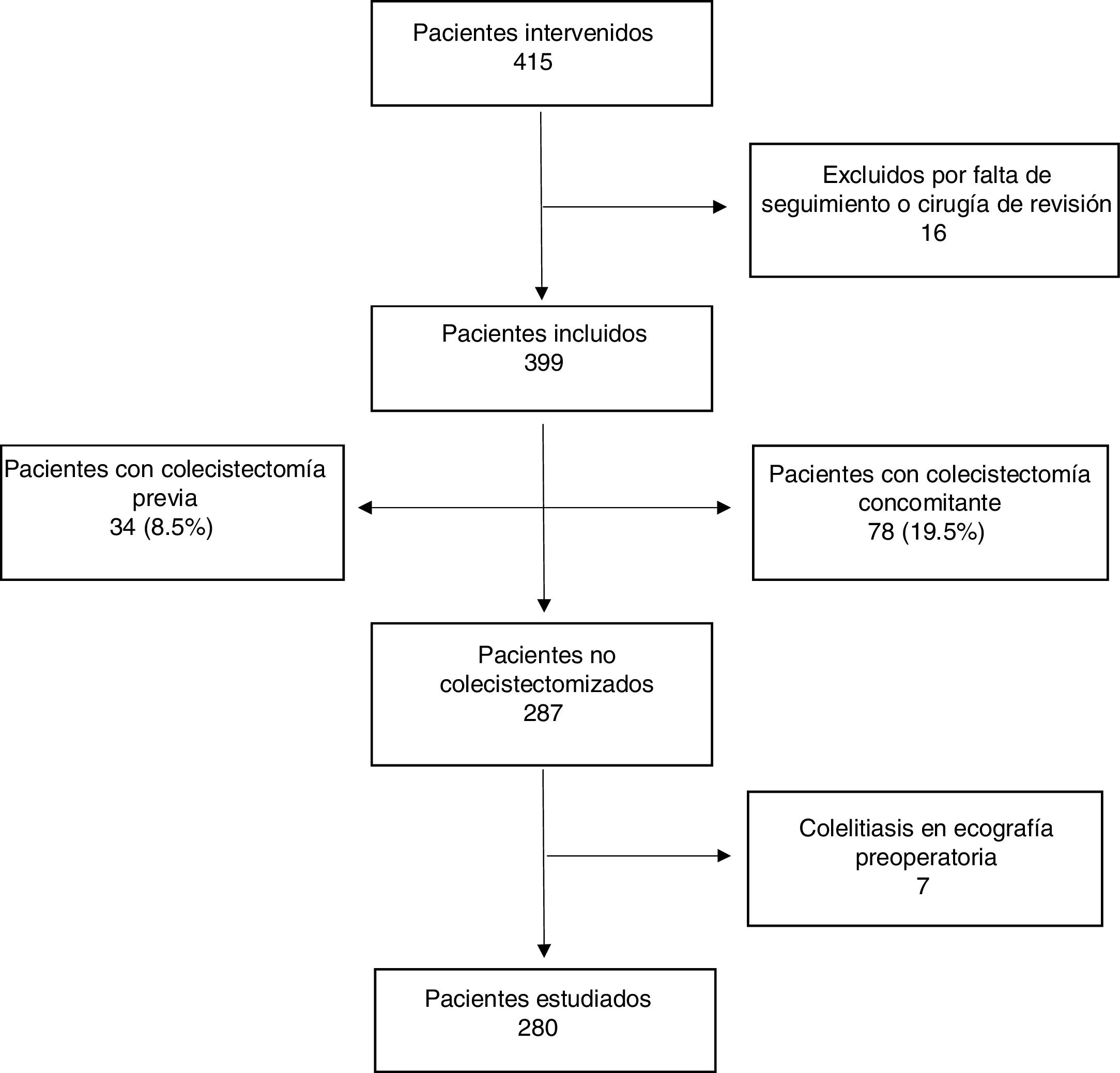
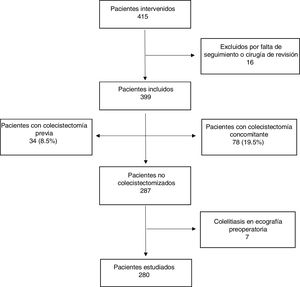
ResultsDuring the study period, a total of 415 patients underwent BS (Fig. 1). Sixteen were excluded due to previous BS or a postoperative follow-up of less than 24 months.
Out of the 399 patients included, 34 had been previously cholecystectomized (8.5%), 78 underwent concomitant cholecystectomy (19.5%), and 7 (1.7%) had preoperative asymptomatic cholelithiasis but were not cholecystectomized due to poor exposure of Calot’s triangle because of the position of the trocars.
Mean age was 44.92 years, and 71.9% were women. Two hundred and fifty-five patients underwent RYGB (63.91%) and 144 SG (36.09%). The most frequent comorbidity was arterial hypertension (HTN) in 43.86%, followed by diabetes mellitus (DM) in 28.57%. The initial BMI was 46.03 and the %EBMIL after 6, 12 and 24 months were 57.49%, 70.48% and 70.85%, respectively.
The number of patients not previously cholecystectomized and with negative preoperative ultrasound was 280. From this total, 29 developed DNSC (10.35%) (group A) and the rest remained asymptomatic (group B). The mean follow-up time for both groups as a whole (n = 280) was 77.6 months (SD 24.97; range 26.32–121.46).
Regarding group A, the majority began with recurrent biliary colic (15; 48.3%), but there were also cases of acute cholecystitis (7), acute pancreatitis (4), cholecystopancreatitis (one), cholangitis (one), choledocholithiasis (one) and epigastralgia (one). Most cases started during the first 24 months after surgery (21; 75%). Twenty-seven required cholecystectomy (93.1%), which was performed electively in the majority (78%) and urgently in the rest (22%), the latter due to the presence of acute cholecystitis. The approach was laparoscopic in 89% of the cases. The open approach was necessary on 3 occasions due to technical difficulties in the context of acute cholecystitis and significant inflammatory changes. No patients had major complications in the postoperative period. Two patients with DNSC were not cholecystectomized: one of them due to a single episode of postoperative colic, and the other presented other more relevant diseases.
Regarding the 251 patients who remained asymptomatic (group B), 88 had an imaging test performed after BS for another reason (kidney stones, intestinal obstruction, etc), 14 of which had asymptomatic DNC in these tests (15.6%).
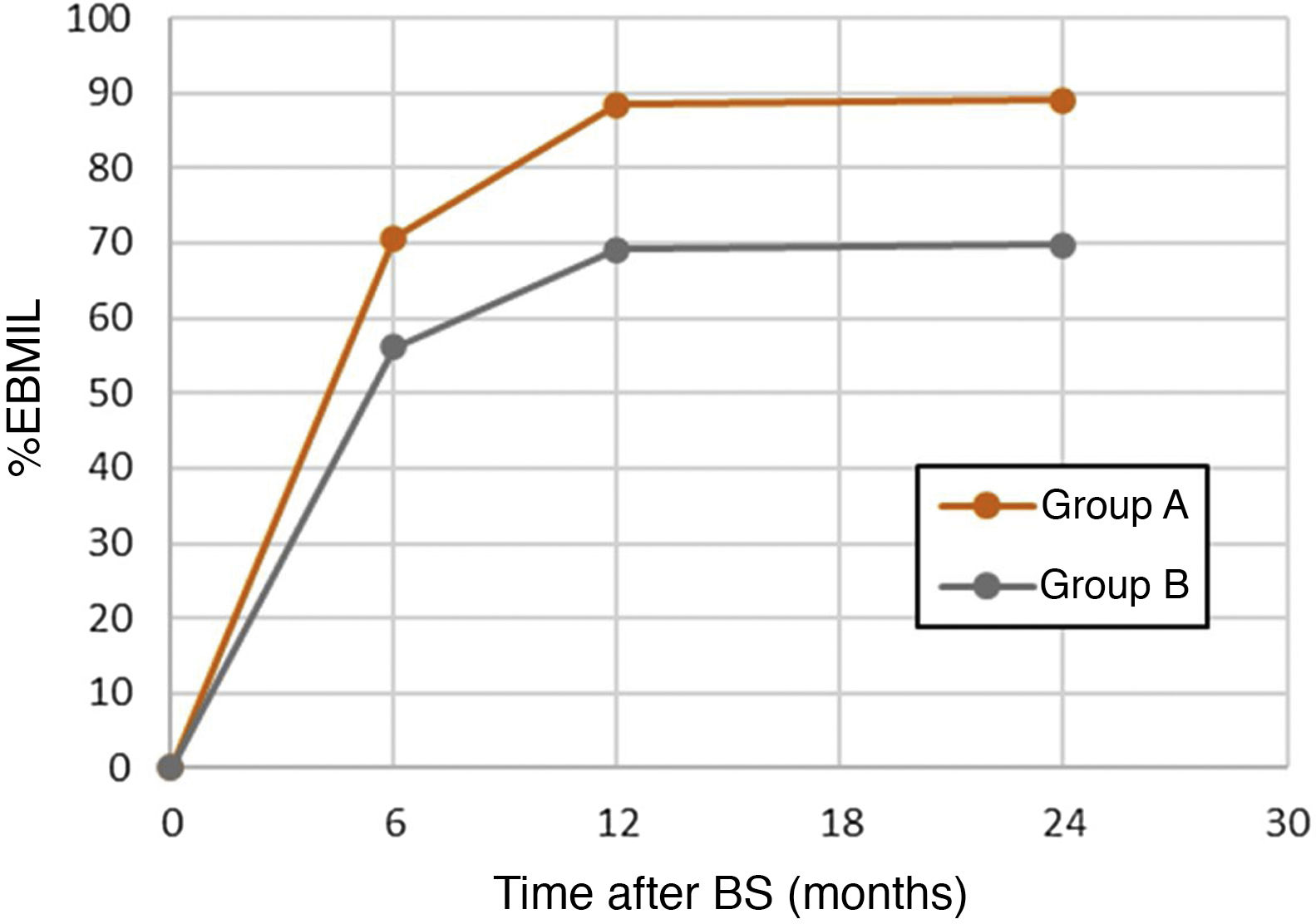
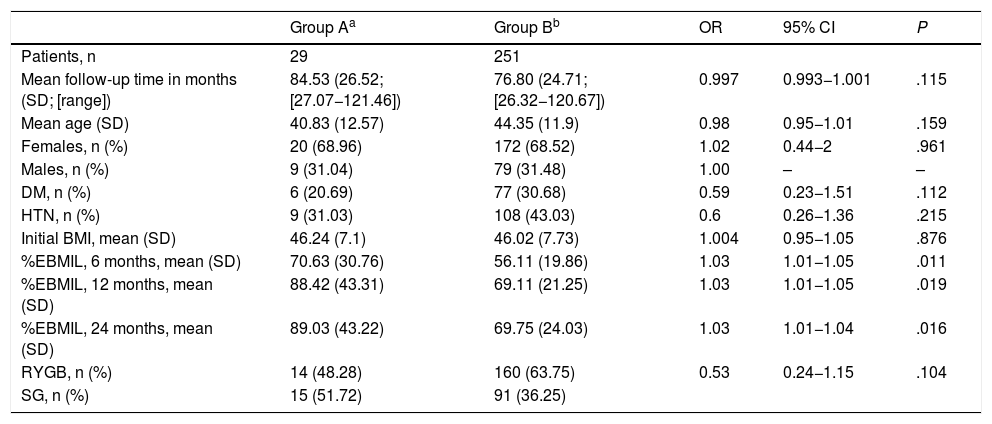
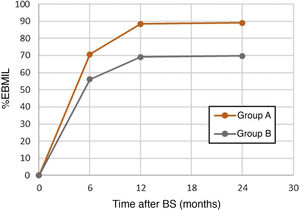
When both groups were compared (Table 1), the univariate analysis did not show statistically significant differences in terms of follow-up time, age, gender, comorbidities, initial BMI or surgical technique, although it did show a certain protective tendency for HTN, DM and RYGB. However, the greater weight loss (%EBMIL) at the 6th, 12th, and 24-month postoperative follow-up visits had a statistically significant correlation with a higher risk of DNSC (P < .05) (Fig. 2).
Comparison of clinical characteristics between the two groups.
| Group Aa | Group Bb | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Patients, n | 29 | 251 | |||
| Mean follow-up time in months (SD; [range]) | 84.53 (26.52; [27.07−121.46]) | 76.80 (24.71; [26.32−120.67]) | 0.997 | 0.993−1.001 | .115 |
| Mean age (SD) | 40.83 (12.57) | 44.35 (11.9) | 0.98 | 0.95−1.01 | .159 |
| Females, n (%) | 20 (68.96) | 172 (68.52) | 1.02 | 0.44−2 | .961 |
| Males, n (%) | 9 (31.04) | 79 (31.48) | 1.00 | – | – |
| DM, n (%) | 6 (20.69) | 77 (30.68) | 0.59 | 0.23−1.51 | .112 |
| HTN, n (%) | 9 (31.03) | 108 (43.03) | 0.6 | 0.26−1.36 | .215 |
| Initial BMI, mean (SD) | 46.24 (7.1) | 46.02 (7.73) | 1.004 | 0.95−1.05 | .876 |
| %EBMIL, 6 months, mean (SD) | 70.63 (30.76) | 56.11 (19.86) | 1.03 | 1.01−1.05 | .011 |
| %EBMIL, 12 months, mean (SD) | 88.42 (43.31) | 69.11 (21.25) | 1.03 | 1.01−1.05 | .019 |
| %EBMIL, 24 months, mean (SD) | 89.03 (43.22) | 69.75 (24.03) | 1.03 | 1.01−1.04 | .016 |
| RYGB, n (%) | 14 (48.28) | 160 (63.75) | 0.53 | 0.24−1.15 | .104 |
| SG, n (%) | 15 (51.72) | 91 (36.25) |
RYGB: Roux-en-Y gastric bypass; SD: standard deviation; DM: diabetes mellitus; SG: sleeve gastrectomy; HTN: hypertension; 95% CI: 95% confidence interval; BMI: body mass index; OR: odds ratio; %EBMIL: percent excess body mass index lost.
To calculate the odds ratio for bother surgical techniques, sleeve gastrectomy was used as the reference value.
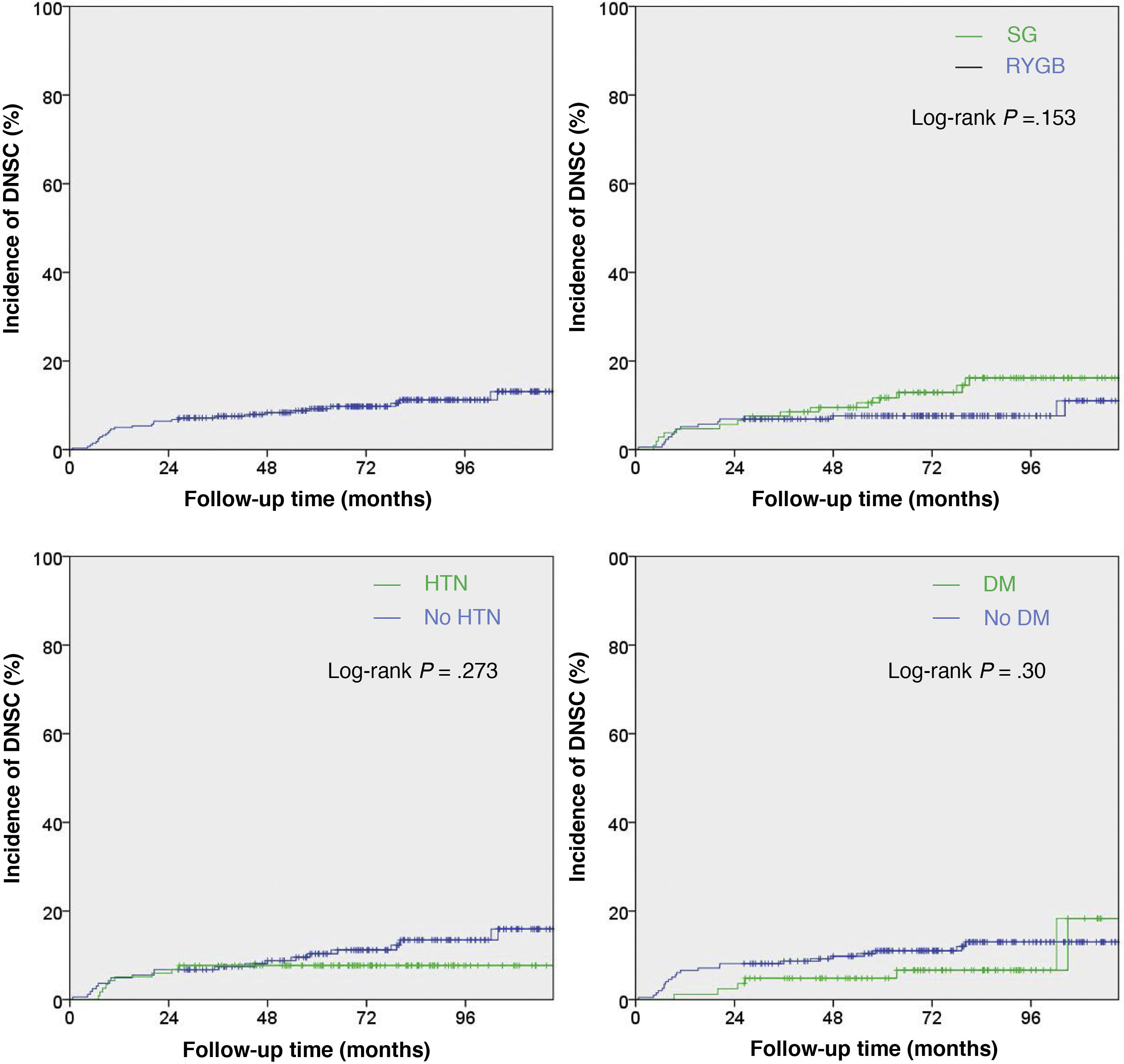
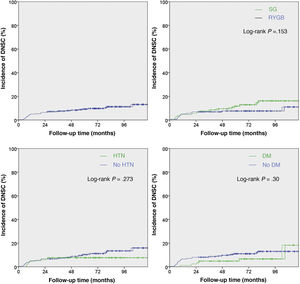
Finally, we used Kaplan–Meier curves to analyze the probability of incidence of DNSC during the follow-up period for the group of patients studied (n = 280), both as a whole and compared according to the type of surgical intervention and prevalence of hypertension and DM (Fig. 3).
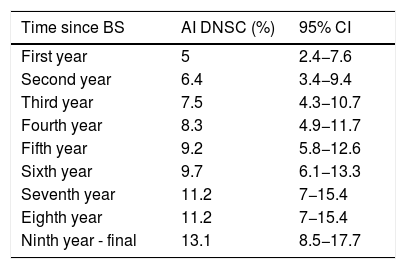
As shown in the first graph of Fig. 3, the increase in cumulative incidence was notably faster during the first year. In this first year, the cumulative incidence was 5% (95% CI 2.4−7.6) (Table 2). Subsequently, the rate of increase decreased, but remained constant. The final cumulative incidence after 10 years of follow-up was 13.1% (95% CI 8.7–17.7). This value is compatible with the calculated mean incidence (10.35%) without taking into account the time from BS to the diagnosis of DNSC.
Kaplan–Meier analysis of the sample studied (n = 280) showing the incidence of de novo symptomatic cholelithiasis and its confidence intervals over time.
| Time since BS | AI DNSC (%) | 95% CI |
|---|---|---|
| First year | 5 | 2.4−7.6 |
| Second year | 6.4 | 3.4−9.4 |
| Third year | 7.5 | 4.3−10.7 |
| Fourth year | 8.3 | 4.9−11.7 |
| Fifth year | 9.2 | 5.8−12.6 |
| Sixth year | 9.7 | 6.1−13.3 |
| Seventh year | 11.2 | 7−15.4 |
| Eighth year | 11.2 | 7−15.4 |
| Ninth year - final | 13.1 | 8.5−17.7 |
BS: bariatric surgery; DNSC: de novo symptomatic cholelithiasis; AI: accumulated incidence; 95% CI: 95% confidence interval.
In terms of hypertension, DM and type of BS, none of the P-values (Log-rank) reached statistical significance.
DiscussionIn the present study, we found that approximately one in 10 patients who underwent BS developed DNSC, the majority of which (75%) appeared during the first 24 months after the procedure and required cholecystectomy in almost all cases. Its development occurs mainly during the period of rapid weight loss, and this incidence is similar to reports from other retrospective studies3,4.
However, 83 patients without biliary symptoms (group B) underwent imaging testing for an unrelated reason, and DNC was found in 15.6%. This indicates that the actual incidence of DNC is higher than what we report.
With regards to the risk factors for developing DNSC after BS, the current evidence is scarce. In the present study, a univariate analysis was carried out to analyze the independent variables and the probability of developing DNSC. A higher %EBMIL significantly increased the risk of DNSC, which coincides with the Coupaye et al.8 and Li et al. study data10.
In our study, patients undergoing gastric bypass showed a lower tendency to develop DNSC, although this difference was not statistically significant. These results coincide with the Coupaye et al. study8, in which the surgical technique was not directly related to the probability of developing DNSC. In addition, there does not seem to be a difference in weight loss between RYGB and SG according to 2 randomized clinical trials published in this regard11,12, so a similar risk for DNSC could be expected after both surgical techniques.
A lower tendency for the development of DNSC was also observed in hypertensive and diabetic patients, and these could be interpreted as possible protective factors, but the results were not statistically significant in any case. Regarding the protective effect of HTN, this has already been previously described by Guzmán et al.13, but, as described in their study, this association could be a consequence of chance since there are no physiological mechanisms to justify it.
In the present study, concomitant cholecystectomy was performed in patients with cholelithiasis who were to undergo BS, since, despite the added morbidity that this could entail, the probability of developing DNC after BS is not negligible13.
On the other hand, in the guidelines for the postoperative management of bariatric patients published by Vilallonga et al.3, the prophylactic use of ursodeoxycholic acid (UDCA) is recommended during the period of rapid weight loss in patients who undergo BS and have had no previous cholelithiasis (level of evidence 1a, recommendation A), since it has been shown to significantly decrease the development of DNC13–19. However, its cost-effectiveness has yet to be determined3, and it is associated with low therapeutic compliance, mainly due to the side effects present in up to 25% of cases in the form of nausea, diarrhea or skin changes, and its dosage (2–3 times a day)20. A recent meta-analysis by Magouliotis et al.21 also recommends its use. Even so, these results should be interpreted with caution, taking into account that few studies are included. Also, the efficacy of UDCA may vary depending on the surgical technique performed, due to the differences that this implies in both the microbiome and in the bile acid profile.
Among the limitations of our study are its retrospective and single-center nature, with a limited number of patients. Furthermore, it is worth noting that postoperative imaging tests were not done systematically, which would have allowed us to determine the real global incidence of DNC. Positive features of the study include the fact that the mean follow-up was 6.4 years and that the loss of patients during this period was minimal. This, together with the fact that no statistically significant differences were found during the follow-up time between the two groups (A and B) and that the results are supported by the Kaplan–Meier curves, gives consistency to the study carried out.
In conclusion, the incidence of de novo symptomatic cholelithiasis and cholecystectomy after BS is relatively high, mainly during the period of rapid weight loss and at higher %EBMIL. The two therapeutic options for the treatment of cholelithiasis in bariatric patients include concomitant cholecystectomy in the case of preoperative cholelithiasis (regardless of symptoms) and the use of UDCA during the period of greatest risk for the development of DNSC. However, randomized studies would be needed to clarify the role of both concomitant cholecystectomy and the use of UDCA in these patients.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Andrés-Imaz A, Martí-Gelonch L, Eizaguirre-Letamendia E, Asensio-Gallego JI, Enríquez-Navascués JM. Incidencia y factores de riesgo para el desarrollo de colelitiasis tras cirugía bariátrica. Cir Esp. 2021;99:648–654.