One-anastomosis gastric bypass has now become the third most commonly performed bariatric technique worldwide. However, as a consequence of the configuration of this surgery, it can present some chronic complications (anastomotic mouth ulcers and biliary reflux) that physicians must come to better understand and assess. In this narrative review, we aimed to update our knowledge of both the diagnosis and treatment of these two complications in the context of bariatric surgeries. We concluded that a series of pre-, intra-, and postoperative preventive strategies should be considered by surgeons to help reduce the appearance of these complications.
El bypass de una sola anastomosis se ha convertido, a-ctualmente, en la tercera técnica bariátrica más realizada a nivel mundial. Sin embargo, como consecuencia de su conformación, presenta una serie de complicaciones crónicas (úlcera de boca anastomótica y reflujo biliar) que debemos conocer y valorar. Con esta revisión narrativa, se pretende realizar una puesta al día, tanto en el diagnóstico como en el tratamiento de cada una de ellas. Hay una serie de estrategias preventivas pre, intra y postoperatorias que deben tenerse en cuenta para disminuir, en lo posible, su aparición.
Obesity has become a major global health problem that has been increasing in recent years.1,2 Bariatric surgery (BS) is considered the most appropriate treatment for obese patients with a BMI >35Kg/m2 when conservative measures (diet, exercise, and behavioral changes) fail, and it should also be considered in people with metabolic disease and a BMI of 30–34.9 kg/m2.3 BS has been shown to improve weight results in the medium and long term, while also improving patient comorbidities.3–5
Rutledge published the first mini-gastric bypass (MGB) series with 1274 cases, which showed it to be a safe technique with shorter operating time and a short hospital stay.6 In 2005, Carbajo et al. published their first series of 205 patients,7 which was later followed by a second series of 1200 patients8 that included some modifications made to the previous technique (measurement of the entire intestine, as well as a continuous suture between the ascending jejunal limb and the reservoir of about 8 cm to reduce bile reflux), which was called the one-anastomosis gastric bypass (OAGB).
Since then, the technique has grown in popularity and has been approved by the IFSO as a stand-alone bariatric procedure.9 In 2020, the IFSO Consensus Statement (resulting from a modified Delphi study)10 was published, which expanded on the results of the previous Consensus Statement,9 particularly with respect to technical factors of the procedure and comparisons with others, especially those related to Roux-en-Y gastric bypass (RYGB).
Although this technique presents some controversy, its popularity has been increasing in recent years. In fact, the IFSO registry for 2021 showed it is the third most performed technique worldwide (OAGB 7.6%, RYGB 36.9%, sleeve gastrectomy 50.2%).11 This controversy stems from its advantages and disadvantages. In terms of the advantages, the single anastomosis implies shorter surgical time and less risk. Also, most authors currently defend that it is not necessary to close mesenteric defects to avoid internal hernias. Furthermore, the results in terms of weight loss and improved comorbidities are unquestionable.6–8 However, it is still a Billroth II type technique, where it is unknown whether bile reflux may lead to possible consequences in the future.
The following is a narrative review of 2 typical chronic complications of this technique, giving special interest to both their prevention and treatment.
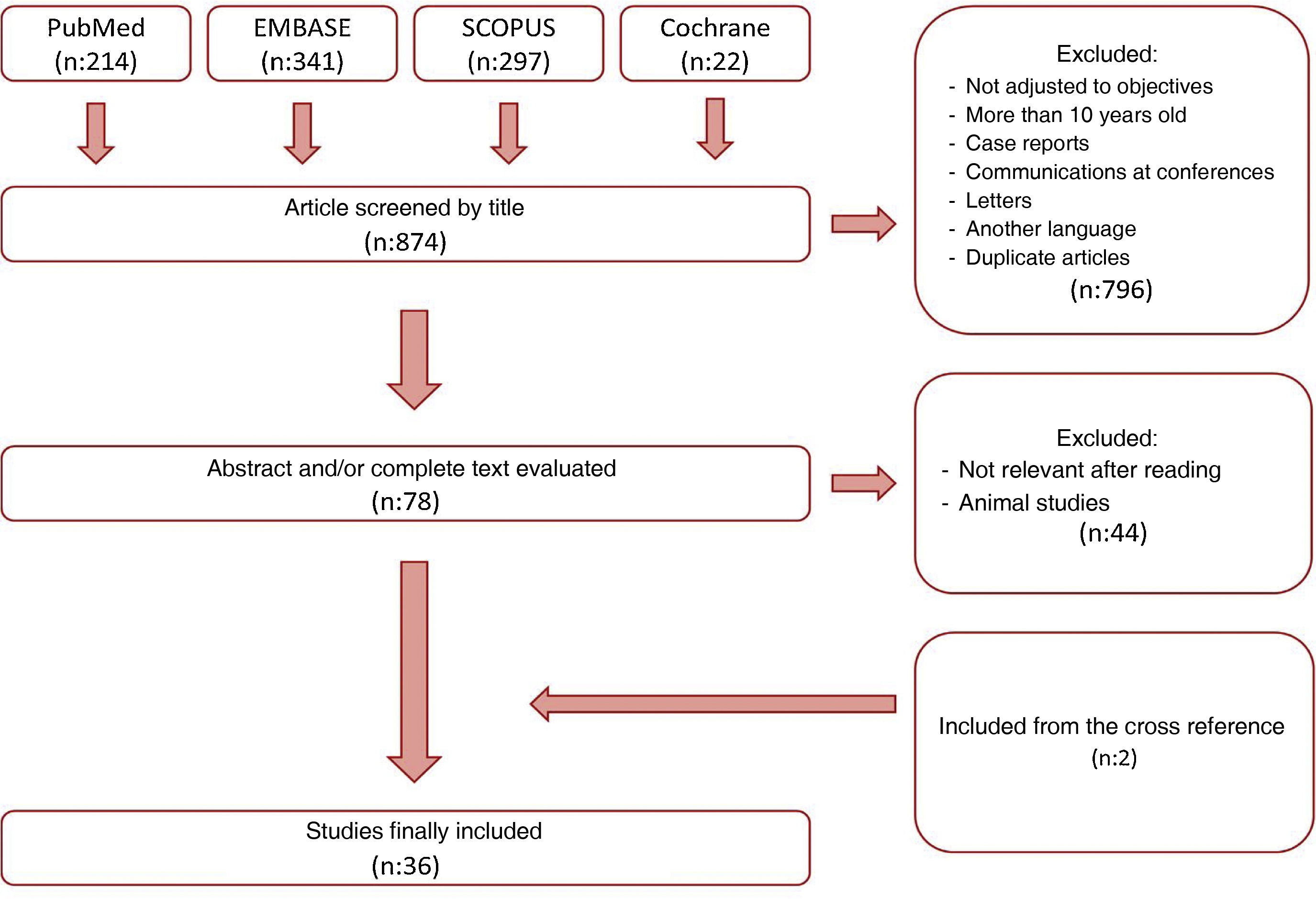
MethodsFor this narrative review, we conducted a search on the PUBMED, EMBASE, MEDLINE and Cochrane Library platforms using the keywords “one-anastomosis gastric bypass” (with its variations “OAGB”, “mini-gastric bypass”), “marginal ulcer” and “bile reflux”, following the strategy described in Fig. 1. The search was carried out in April 2022 and included all publications from the last 10 years. Two independent reviewers (MFM and MGR) assessed all the studies. Among the articles found, we excluded those that did not meet the objectives of our review, isolated clinical cases, as well as those written in a language other than English or Spanish, subsequently evaluating the abstracts and/or full texts of the remaining articles. Studies that were not considered relevant (animal studies, etc.) were excluded. The narrative review was carried out with the texts of the articles that had not been excluded, prioritizing those with higher methodological quality. This process was repeated with some of the cross references of these studies, which were added to the narrative review when they were considered relevant for our paper.
ResultsFig. 1 summarizes our search process. A total of 874 documents were found after a search using the aforementioned keywords. From among all the articles found, we excluded those that did not meet the selection criteria, articles published more than 10 years ago, case reports, articles written in another language, and duplicates. After reading the abstracts and/or full texts, we excluded the articles that were not relevant to our review as well as studies carried out in animals. Two articles were added from the cross references. In the end, a total of 36 articles were selected for thorough analysis.
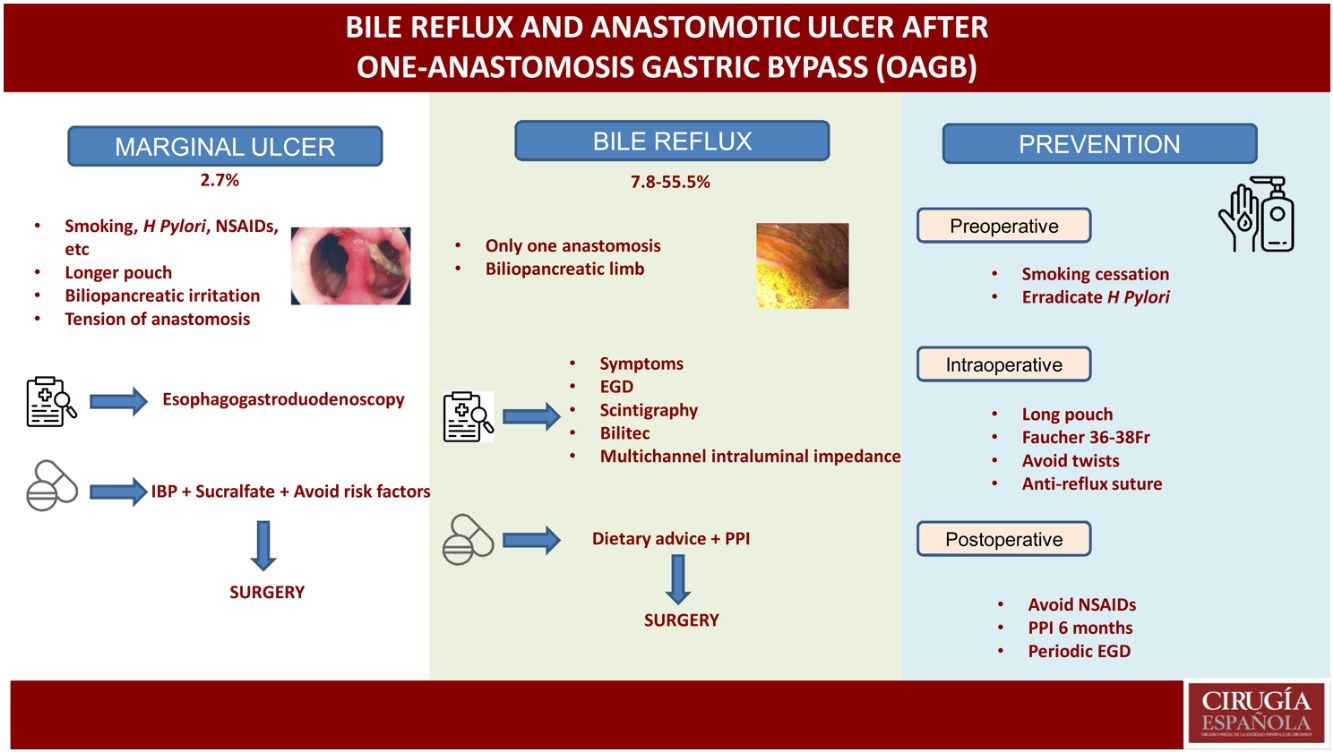
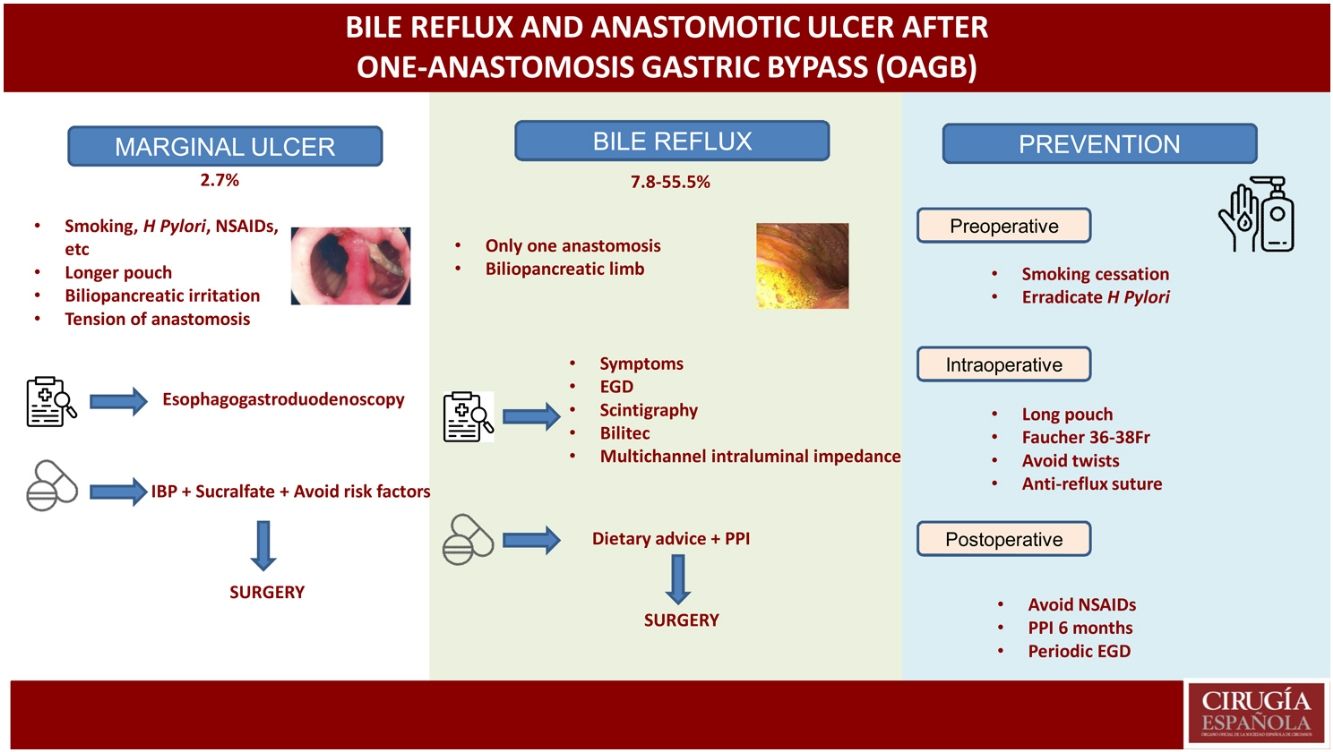
Marginal ulcersMarginal or anastomotic ulcers can appear after gastric bypass for the surgical treatment of obesity and are described in the first days or even many years after surgery. Therefore, this entity should be included in the differential diagnosis of abdominal pain after bariatric surgery (mainly when it appears months or years later).12 Vital signs (heart rate, temperature, and blood pressure) and acute phase reactants (leukocyte formula, C-reactive protein) are not always indicative, so clinical suspicion should remain high.
Generally, these ulcers are caused by the presence of a foreign body (suture thread) in the suture line, reduced blood supply to the gastrojejunal mucosa, compression, or edema in the anastomosis. To date, there are no studies that assess the risk factors for marginal ulceration in patients treated with OAGB, although most authors believe that the risk factors are very similar to patients treated with RYGB (smoking, untreated positive Helicobacter Pylori, chronic use of NSAIDs, no proton pump inhibitors after surgery).12–15 However, there are some details that can specifically facilitate their presence after the OAGB technique, such as:
- •
Presence of a longer pouch: Compared to conventional bypass, the longer reservoir of OAGB means that the anastomosis may be exposed to greater acid secretion (more parietal cells in the gastric pouch).7,8,13
- •
Biliopancreatic irritation: Despite being 200−250 cm from the angle of Treitz, the single anastomosis created is continually exposed to the irritation of pancreatic and bile juices, creating an ulcerogenic condition. This maintained situation could even create a chronic inflammatory process, and the ulcer could lead to a possible perforation. This hypothesis is supported by the fact that conversion and Roux after an intractable ulcer leads to its successful treatment.12,14,15
- •
Tension of the anastomosis: Although there are surgeons who argue that the longer pouch can reduce tension on the anastomosis, others believe the opposite to be true. Unlike the Roux-en-Y configuration, in which the alimentary limb is divided from the biliopancreatic limb (allowing the biliopancreatic limb to move downward with gravity, without tension), in the OAGB setup, both sides of the loop pull downward, potentially creating higher tension.12 In any case, there are no studies to date analyzing the difference in tension between these two procedures.
Musella et al. published data from a multicenter study with 974 consecutive cases, where the anastomotic ulcer rate was 1.7% (4/14 of which required surgical treatment).16 In the systematic review published by Parmar et al. in 2018 with a total of 12 807 patients from 22 medical centers, the percentage of marginal ulcers varied between 0% and 10%. However, out of the 22 centers, only in 4 was the percentage higher than 5%, and the study average was 2.7%.17 Robert et al. published a clinical trial in 2019 comparing the efficacy and safety of OAGB versus RYGB, in which no statistically significant differences were observed for marginal ulcer between the 2 techniques (RYGB n = 3; 13% vs OAGB n = 2; 5%).18
Abdominal pain is the most common symptom of this type of patient. Nausea and vomiting may be present, but significant bleeding is uncommon.19,20 However, up to 28% of patients with a marginal ulcer are asymptomatic and are discovered accidentally during routine esophagogastroduodenoscopy (EGD).
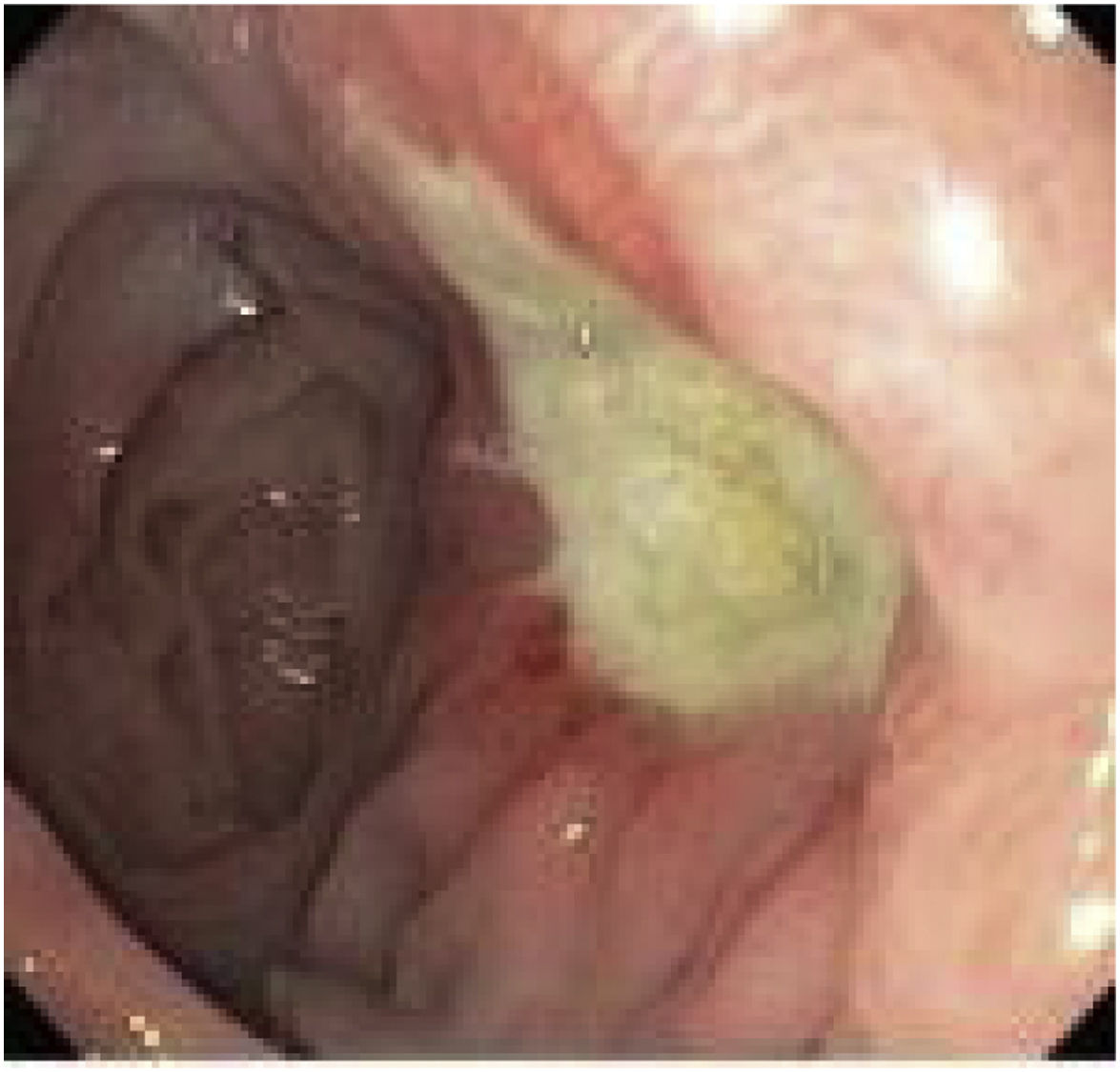
The diagnosis is basically endoscopic (Fig. 2). The endoscopist can remove sutures when they are visible in the lumen and if they are associated with a marginal ulcer.21 This approach has the potential to facilitate healing and thus alleviate abdominal pain. Ulcers should be treated with proton pump inhibitors (PPI) associated with sucralfate, and healing time may vary from 8 weeks to 6 months.22 Calmar et al. proposed a treatment algorithm,23 as follows: recommend and insist on smoking cessation in patients who smoke; study and eradicate the presence of Helicobacter Pylori; avoid chronic NSAID use after the intervention. Treatment consists of a PPI together with sucralfate, with follow-up endoscopy 3 months later. If healed, the authors recommend PPI for life, as well as avoiding risk factors. If not healed, the endoscopy should be repeated in another 3 months. If still not healed, surgical conversion should be considered.15,23,24 Previously reported surgical treatment options include: primary repair of the perforation (with or without debridement) together with omentoplasty; conversion to Roux-en-Y with resection of the distal pouch (technique advocated by most authors); reversion of the OAGB to “normal anatomy”.12
Bile refluxThe main controversy that OAGB presents as a surgical technique is precisely that it presents a single anastomosis and, therefore, can cause chronic biliopancreatic reflux that may have consequences in the medium to long term. In the review published by Keleidari et al.,25 the percentage of reflux varies enormously (between 7.8% and 55.5%), which may be due to the wide variability in both the surgical details of the technique and its diagnosis.
Regarding the technique, there are differences in terms of the size of the reservoir, the length of the biliopancreatic loop, the size and type of anastomosis, the height of the gastrojejunal suture to prevent reflux, etc, which make it difficult to compare techniques. As for diagnosis, there are large differences between studies (from those that only diagnose symptomatic patients, to those that routinely perform scintigraphy and EGD in all patients). For instance, in their first series of patients treated with OAGB,7 Carbajo et al. reported a reflux rate of 0%. In the second published series, with a total of 1200 patients,8 the published reflux rate was 2% (pH-metry and EGD were only performed in the first 20 patients). In contrast, Saarinen et al. reported a reflux rate of 39.5% in their series, taking into account asymptomatic patients and routinely performing EGD with biopsy in all, together with reflux scintigraphy. The authors reported bile reflux in the gastric pouch in approximately one-third of patients, with one case of esophageal bile reflux.26 Keleidari et al. conducted another cohort study in 122 patients in 2019 that attempted to compare the bile reflux incidence rates between OAGB and RYGB 12 months after surgery (7.8% after OAGB and 3.4% after RYGB), concluding that there was no significant difference in bile reflux between the 2 techniques.27
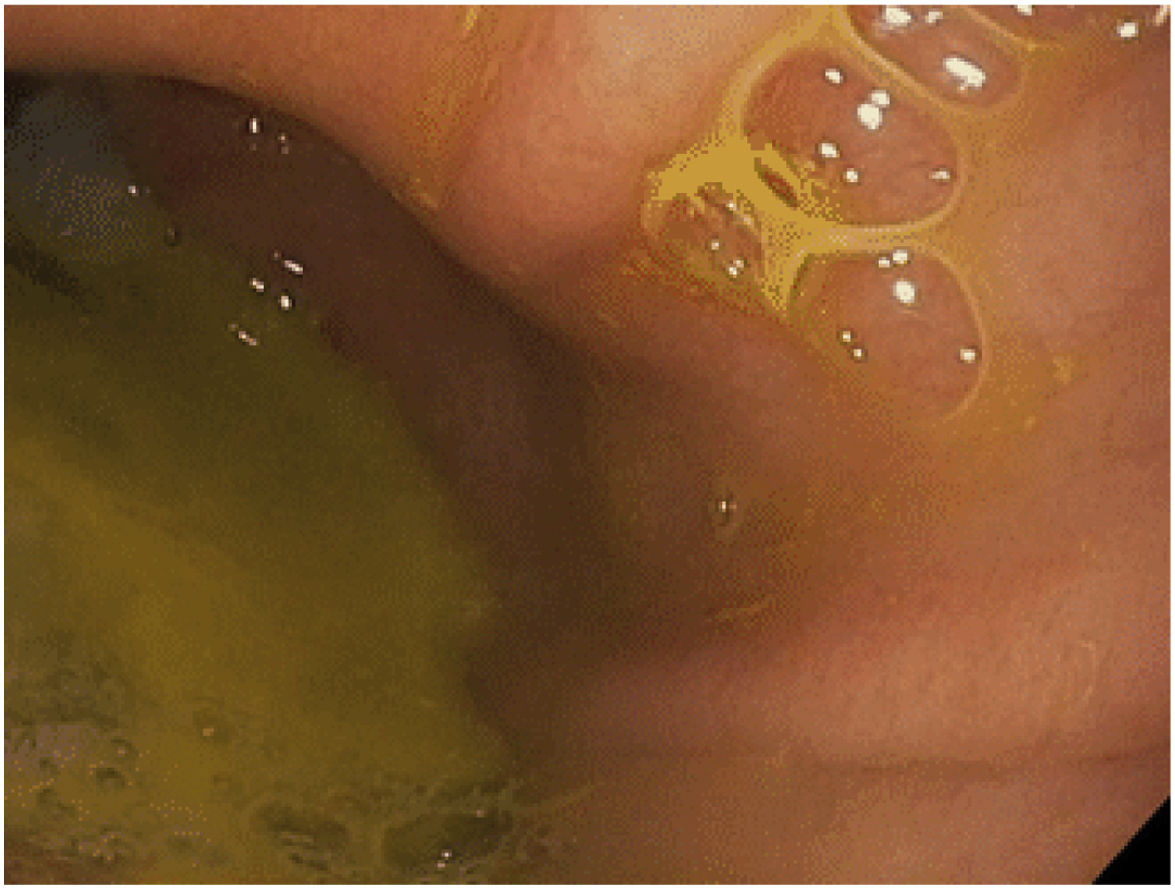
The clinical diagnosis of bile reflux after OAGB remains difficult, except when the patient presents bile regurgitation and/or vomiting (mainly at night). Other less typical symptoms may include nausea, abdominal fullness, etc. The first diagnostic test in these patients should be an EGD, where the presence of traces of bile in the gastric pouch and/or esophagus can be observed, as well as the existence of some mucosal changes that should be biopsied28 (Fig. 3). Other procedures can assist the diagnosis, although they are not very common in routine practice. Hepatobiliary iminodiacetic acid scintigraphy, combined with technetium-99 m (Tc-99 m), is a non-invasive technique as valid as gastric fluid aspiration in the detection of bile reflux in the fasting state.28,30Bilitec is a system that uses a fiber optic spectrophotometry camera to measure the absorption of light at a wavelength of 470 nm. The tube is inserted transnasally into the distal esophagus in the fasting patient and left in situ for 24 h.29,31 It is, however, a system whose clinical acceptance is limited. Direct aspiration of gastric and esophageal fluid provides for chemical analysis of the concentration and composition of the fluid and determination of the presence of bile acids.29,32Multichannel intraluminal impedance monitoring detects the passage of a bolus through the esophagus. It is usually combined with pH tests to assess for acid and non-acid reflux. Combined multichannel intraluminal pH-impedance is highly sensitive and reproducible for all types of reflux, regardless of acidity or composition.29,33
As for the future development of carcinoma after OAGB, there are various hypotheses for and against the risk of degeneration after the procedure.34,35 First, biliopancreatic reflux has been shown to be directly responsible for esophageal adenocarcinoma in an animal model;42 furthermore, according to some authors, excessive bile reflux can cause intestinal metaplasia, Barrett's esophagus, and gastric/esophageal cancer in humans.42,43 However, other authors are convinced that there is no causal relationship between OAGB and cancer based on the involvement of other factors in the genesis of gastric cancer (eating habits, tobacco, Helicobacter Pylori infection), as well as reported cases of cancer occurring in the remnant stomach after RYGB (14 cases) and vertical banded gastroplasty (n = 9).34,36,37 Only 2 cases of gastroesophageal carcinoma have been published which, due to their characteristics, must be analyzed with great caution. The first of these38 is from a 52-year-old man with a BMI of 52 kg/m2 who presented grade C esophagitis in the preoperative study, yet no biopsy was performed. He started to experience dysphagia 2 years after surgery (OAGB) and after EGD he was diagnosed with adenocarcinoma (ADC) in the esophagogastric junction. The second case39 is a 54-year-old man, a smoker and moderate drinker, who underwent OAGB with a BMI of 46.1 kg/m2, in whom preoperative EGD had not even been performed. The patient did not complete follow-up after surgery, and 2 years later he was diagnosed with esophageal ADC after a study for dysphagia and vomiting. Both cases developed 2 years after bariatric surgery in patients who had not been well studied preoperatively; therefore, it may not be appropriate to attribute the cause of tumor development to the surgical technique.
Treatment should be started conservatively, using PPI, sucralfate, with or without bile acid sequestrants, elimination of risk factors, as well as certain dietary advice.40 When this fails, a new surgery should be considered, and most authors advocate conversion to Roux-en-Y.41–44 If the reason for bile reflux is a short pouch, it is questionable whether revision of the gastrojejunal anastomosis is mandatory to effectively treat bile reflux, or whether it might be sufficient to perform a jejunojejunal anastomosis distally in the efferent limb, after dividing the afferent loop along with the gastrojejunal anastomosis. In fact, this second option can reduce the risk of the overall procedure, since the native gastrojejunal anastomosis would be left intact.36 Other authors advise performing a Braun jejunojejunal anastomosis.7 However, prior to revision, it is important to know the size of the limbs before deciding whether to redo the gastrojejunal anastomosis, or simply create the enteroenterostomy to form the "Y" of the Roux-en-Y. The simple addition of an enteroenterostomy can cause or exacerbate malnutrition if the length of the loops is unknown. Another less performed option is reversion to the original anatomy, which may show benefits in terms of weight recovery.45 However, this option can be technically challenging and may be associated with increased risk of stricture and gastro-gastric anastomotic leak. In the systematic review on the surgical management of reflux disease after OAGB published in 2022 by Lee et al., 1.6% of the patients required revision surgery, converting to Roux-en-Y in 91.7% of cases and Braun enteroenterostomy in 2.6%.40
PreventionIn recent years, there has been a lack of evidence related with OAGB perioperative practices,46 although the IFSO consensus documents published in 20189 and 202010 have discussed several aspects related to said practices. We must consider a series of pre- and postoperative strategies as well as intraoperative technical details that can help avoid these chronic complications.
In the preoperative period, smokers should be urged to quit smoking. A study for Helicobacter Pylori should be conducted and, if positive, it should be eradicated. Following IFSO recommendations, this type of surgery should be avoided in patients with Barrett's esophagus or a large symptomatic hiatal hernia.10
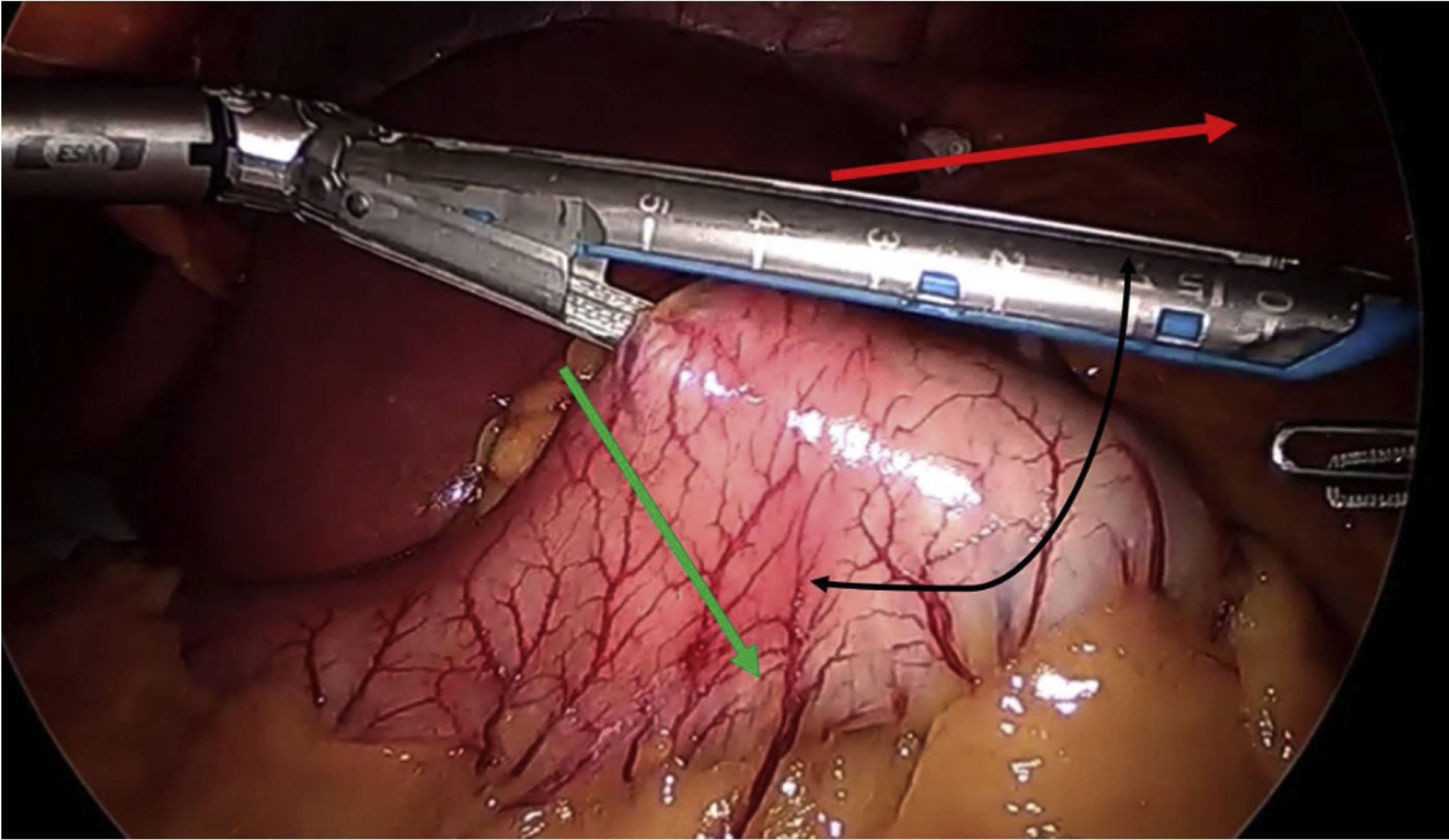
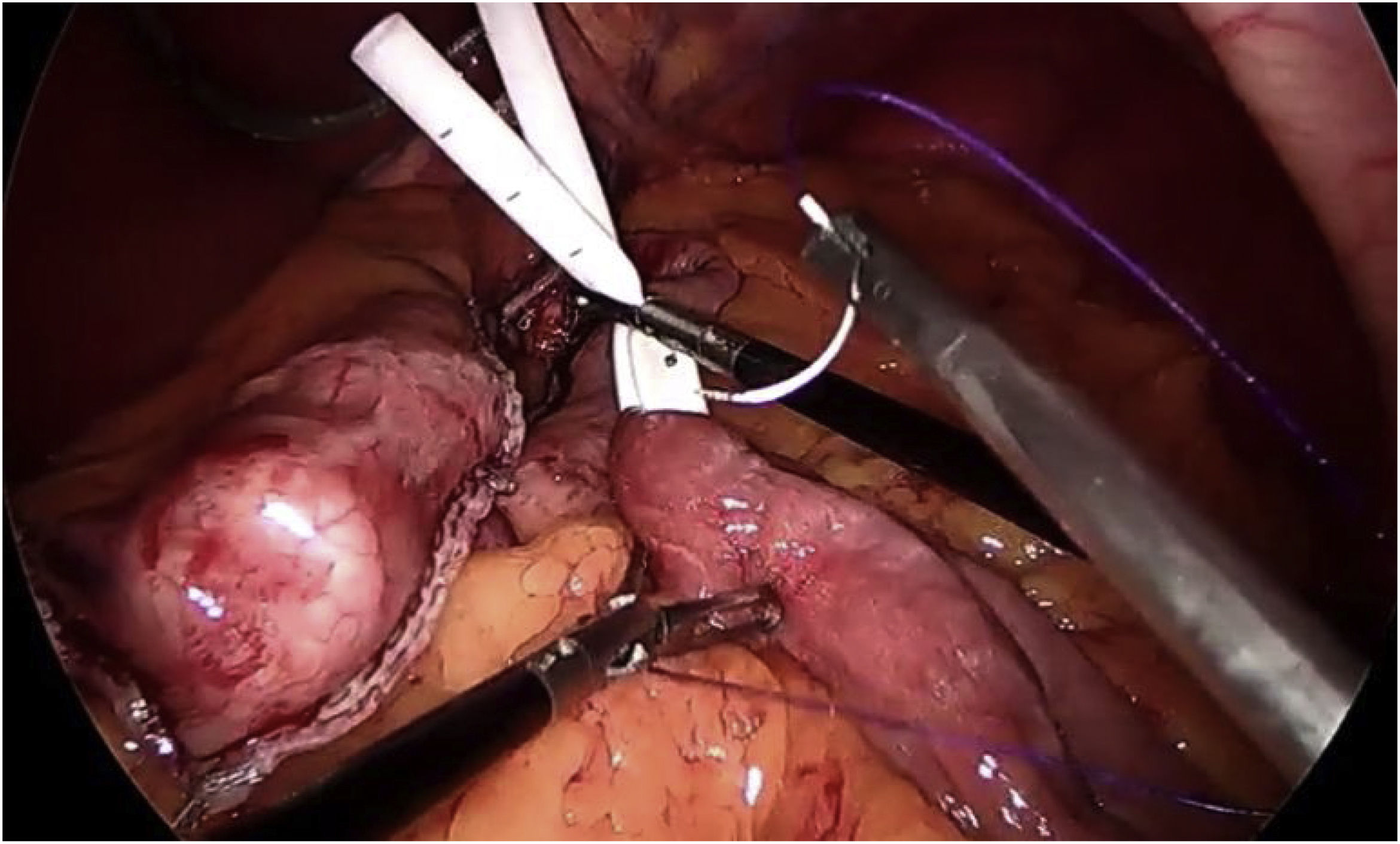
In terms of the technical details,23 a long pouch should be created, performing the division below the “goose foot”, and introducing the first endostapler perpendicular to the entry in the lesser curvature (thus, between 1 and 2 cm of length are gained) (Fig. 4). Most authors advocate using a Faucher probe between 36Fr and 38Fr10 and performing the division while avoiding a twisted pouch. Dr. Carbajo describes a gastrojejunal suture that ascends between 8 and 10 cm from the anastomosis to avoid bile reflux7,8 (Fig. 5). It is also advisable to dissect the lesser curvature in a controlled manner to make the first cut, thereby preventing the contralateral corner of the pouch from having a precarious vascularization.
After surgery, and following the recommendations of the consensus document, chronic use of NSAIDs should be avoided. Most experts advocate taking PPI for 6 months and performing periodic follow-up endoscopies.10
ConclusionsOAGB is a technique that has gained popularity in recent years, becoming the third most performed bariatric technique worldwide. However, as a consequence of its configuration, it presents a series of chronic complications (anastomotic ulcer and bile reflux) that we must be aware of and assess. Pre-, intra- and postoperative preventive strategies must be taken into consideration to reduce the appearance of these complications.
Conflict of interestThe authors declare that they have no conflict of interest.