To evaluate the clinical outcome of early closure of a protective ileostomy and preoperative stimulation of the efferent limb in a cohort of patients with rectal cancer treated surgically, primarily using the laparoscopic approach.
MethodsWe performed an observational retrospective cohort study in a prospectively recorded series of patients with rectal cancer who underwent laparoscopic surgery with a protective loop ileostomy between 2017 and 2022. Ileostomy closure was programmed for within 3 months after surgery. All patients underwent stimulation of the efferent limb. Primary outcomes were morbidity and mortality, length of stay (LOS), and re-admission.
ResultsBetween 2017 and 2022, 108 patients underwent resection for rectal cancer and protective ileostomy. The laparoscopic approach was performed in 84.3% of patients (n = 91). Permanent ileostomy was performed in 5 patients (4.6%). Ileostomy closure was thus performed in 95.4% of patients (n = 103). Median time to closure was 74.5 days (range 57–113). In 63.1% (n = 65) of patients, reconstructive surgery was performed within 90 days. Prior to closure, efferent limb stimulation was performed in 77.8% (n = 84) of patients. Global morbidity was 26.2% (n = 27) (85.19%, n = 23 Clavien-Dindo I and 7.41%, n = 2 Clavien-Dindo II). The main causes of morbidity were postoperative ileus (10.7%, n = 11) and rectal bleeding (8.7%, n = 9). Anastomosis leakage occurred in 2 patients. Median hospital stay was 6 days (5–7). Readmission was needed in 6.8% (n = 7) of patients.
ConclusionA previous laparoscopic approach, early closure and stimulation of the efferent limb could be a useful strategy to reduce the morbidity and mortality of temporary ileostomy closure.
Evaluar los resultados del cierre temprano de la ileostomía de protección y la estimulación del asa eferente en una cohorte de pacientes operados de cáncer de recto intervenidos inicialmente por laparoscopia.
MétodosEstudio observacional retrospectivo de una cohorte de pacientes afectos de cáncer de recto que han sido intervenidos entre 2017 y 2022 por laparoscopia con ileostomía de protección. El cierre se planteó antes de los tres meses. Todos los pacientes recibieron estimulación del asa eferente. Los objetivos primarios fueron la morbi-mortalidad, la estancia hospitalaria y los reingresos.
ResultadosEntre 2017 y 2022, se intervinieron por cáncer de recto con ileostomía de protección n = 108 pacientes. El 84.3% (n = 91) se realizó por laparoscopia. La tasa de ileostomía permanente fue del 4.6% (n = 5), por lo que se cerraron el 95.4% (n = 103). La mediana de tiempo al cierre fue de 74,5 días (rango 57–113). En el 63.1% (n = 65) la cirugía reconstructiva se realizó antes de los 90 días y la estimulación del asa eferente en el 77,8% (n = 84) de los pacientes. La morbilidad global fue de 26.2% (n = 27) (85.19%, n = 23 Clavien-Dindo I y 7.41%, n = 2 Clavien-Dindo II), siendo el íleo paralítico (10.7%, n = 11) y las rectorragias (8.7%, n = 9) las más frecuentes. La dehiscencia de anastomosis ocurrió en 2 pacientes. La mediana de estancia hospitalaria fue de 6 días (rango 5–7). Reingresó el 6.8% (n = 7) de los pacientes.
ConclusiónEl abordaje laparoscópico previo, el cierre temprano y la estimulación del asa eferente podrían considerarse las tres estrategias para reducir la morbi-mortalidad en el cierre de la ileostomía de protección.
Anastomotic leakage (AL) is the most feared complication after rectal resection. The most accepted preventive measure to date has been the use of a temporary lateral ileostomy. Although this approach does not preclude AL, it significantly reduces the clinical consequences.1–3
Lateral ileostomy is an invasive technique that requires a second surgical procedure for closure. It entails serious morbidity while it is in place4 and also after surgical closure. Furthermore, it has been demonstrated that late closure impairs recovery of sphincter function.5,6
The most accepted indications for placement of an ileostomy are low anastomosis, preoperative chemotherapy (CT), radiotherapy (RT) and intraoperative complications.7
Surgical treatment of rectal cancer has undergone major advances in recent decades. Implementation of the laparoscopic approach, for example, has led to fewer intraoperative adhesions and improved techniques, allowing for the performance of low and ultralow anastomosis.8 A second major advance has been the use of transanal approaches, with more frequent use of coloanal anastomosis.9 And third, findings from new therapeutic oncologic protocols (that include Total Neoadjuvant Therapy (TNT) and RT and longer periods before elective surgery) suggest that outcomes in currently treated patients are not comparable with outcomes from former series.10 It is thus necessary to evaluate the outcomes of the use of ileostomy in this new clinical scenario.
Many studies in recent years have evaluated the possibility of early closure,11–19 but this is not always easy due to postoperative complications, the patient’s clinical situation immediately after surgery, and the need for adjuvant treatments. In a previous retrospective analysis, we observed that the laparoscopic approach and a shorter time lapse before closure were associated with a significant reduction in postoperative morbidity and postoperative stay.20 A concept of interest, albeit not widely used, is stimulation of the efferent limb before closure in order to improve functional bowel recovery and reduce postoperative ileus.21–23
In 2017, we developed a prospective protocol in a series of patients with rectal cancer for management of lateral ileostomy closure based on early closure (within 3 months) and preoperative stimulation following the laparoscopic approach. The aim of this study was to analyze the applicability and clinical outcome of this therapeutic protocol.
MethodsWe performed a retrospective observational cohort study in a prospective series of all patients undergoing rectal cancer resection with temporary ileostomy between January 2017 and December 2022. All data were collected from the hospital’s computerized medical records. The rules of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) were followed.
Starting in 2017, we implemented a prospective protocol for managing ileostomy reversal. This included:
- 1
A postoperative rectosigmoidoscopy to evaluate the integrity of the rectal anastomosis.24
- 2
Stimulation of the efferent limb by stoma-therapist nurses. To this end, a 16–18 French (Ch) Foley catheter was inserted into the efferent limb. A balloon with 2 cc of air was inflated to avoid reflux of the enteral nutrition solution (DIENAT®). During the first session, a stoma-therapist nurse started with 50 mL of product, subsequently increasing 50 mL at each session to a maximum of 200 mL. The number of sessions depended on patient tolerance and started approximately 2 weeks before the second surgery. Two sessions per week were generally given.
- 3
Early closure before three months.
- 1
Rectal adenocarcinoma resection with a temporary ileostomy.
- 2
Patients over 18 years old.
- 1
Rectal resection due to benign pathology (diverticulitis, Crohn disease or endometriosis) or malignancy other than rectal adenocarcinoma.
Primary outcomes were morbidity and mortality measured using the Clavien-Dindo scale, length of stay (LOS) in days, and re-admission to the emergency department or hospital wards.
Secondary outcomesSecondary outcomes were permanent ileostomy rate, time to ileostomy closure, reasons for delay of ileostomy closure, surgical site infection (SSI) and incisional hernia rate.
Other variables recorded were:
- 1
Demographics: age, gender, ASA type, ECOG score and BMI.
- 2
Comorbidities: medical comorbidities and previous abdominal surgeries.
- 3
Features of rectal tumor: distance from the anal verge, TNM stage and neoadjuvant or adjuvant therapy.
- 4
Features of rectal resection: rectal resection technique (laparoscopic, converted or open rectal resection) with partial, Total Mesorectal Excision (TME) or Transanal Total Mesorectal Excision (TaTMe) and type of anastomosis (hand or stapler suture).
- 5
Features of ileostomy closure: type of surgery (elective or emergency), duration, type of anastomosis (stapler, manual, or end-to-end manual), intraoperative complications, use of mesh for repair, and clinical or radiological findings of incisional hernia after one year of follow up.
All variables were expressed in percentages (%) and standard deviation (SD) if they followed normal distribution. If the distribution was not normal, they were expressed as median and range. The descriptive analysis was performed using SPSS® statistical processing.
Standardized surgical technique- 1
Peristomal circular incision.
- 2
Exteriorization of efferent and afferent limbs, without tension.
- 3
Confection of side-to-side anastomosis with a 75-mm linear stapler.
- 4
Closure of the enterotomy with a 75-mm linear (optional with continuous 3/0 barbed suture or absorbable 3/0 Vycril).
- 5
Staple line reinforcement of the enterotomy with continuous 3/0 barbed suture or absorbable 3/0 Vycril.
- 6
Optional placement of mesh.
- 7
Purse-string skin closure with intradermal non-absorbable synthetic suture 3/0 with optional Penrose drain.
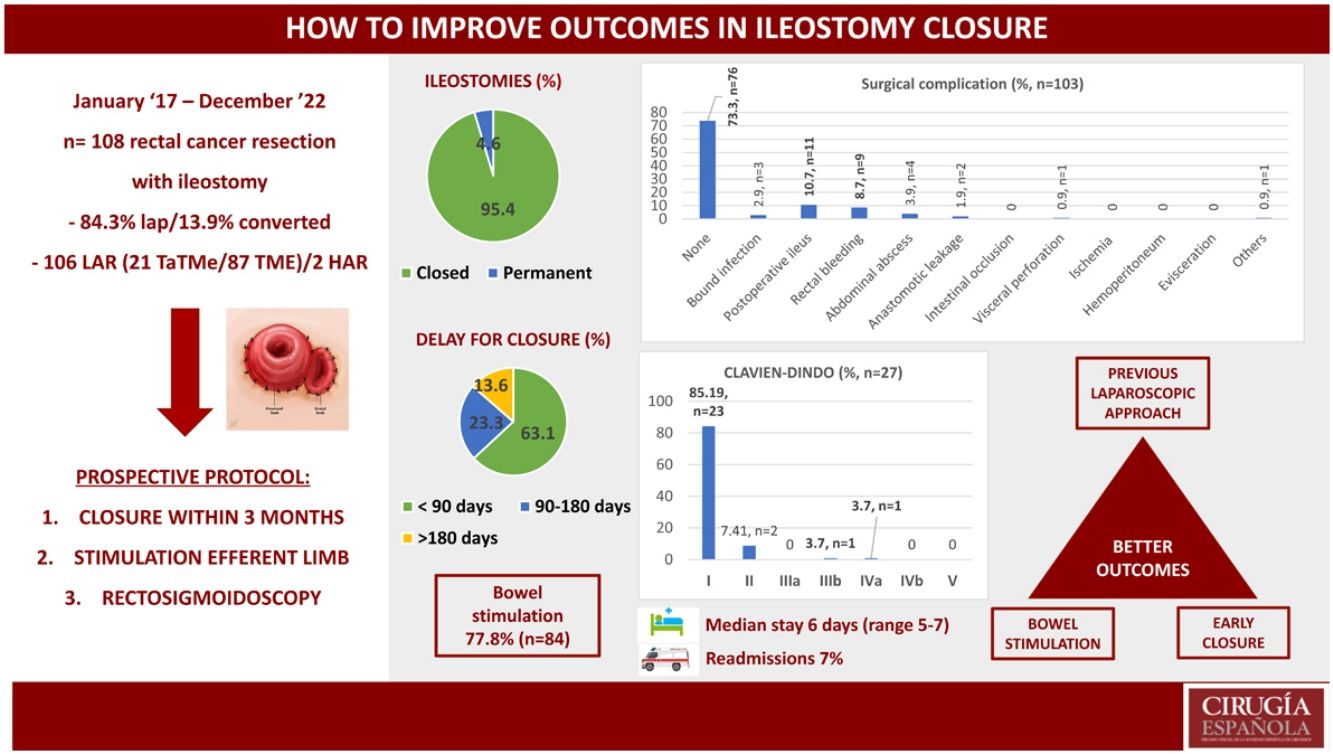
Between 2017 and 2022 we performed 108 rectal cancer resections with associated loop ileostomy. The patients’ demographic characteristics are summarized in Table 1.
Demographic characteristics.
| Sex | Women | 44 (40.7%) | |
| Men | 64 (59.3%) | ||
| Age | 69 years (±10.79 SD) | ||
| ASA | I | 5 (4.6%) | |
| II | 60 (55.6%) | ||
| III | 42 (38.9%) | ||
| IV | 1 (0.9%) | ||
| V | 0 (0.0%) | ||
| BMI (kg/m2) | 25.93 ± (4.54 SD) | ||
| ECOG | 0 | 50 (46.3%) | |
| 1 | 47 (43.5%) | ||
| 2 | 10 (9.3%) | ||
| 3 | 1 (0.9%) | ||
| 4 | 0 (0.0%) | ||
| 5 | 0 (0.0%) | ||
SD, Standard Deviation; ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group.
Sixty-three per cent (n = 68) of the rectal resections were performed to treat tumors located in the middle third of the rectum (5−10 cm from the anal margin), while 22.2% (n = 23) were higher tumors (10−15 cm) and 16% (n = 17) were lower tumors (<5 cm from the anal verge). Rectal resection was attempted by laparoscopy in 84.3% of cases (n = 91), with a 13.9% conversion rate (n = 15). Lower anterior resection (LAR) was performed in 106 cases (98.1%). Conventional laparoscopic TME was done in 87 cases (80.56%), and 21 (19.44%) were treated with TaTMe. Most anastomoses were performed mechanically (95.4%, n = 103).
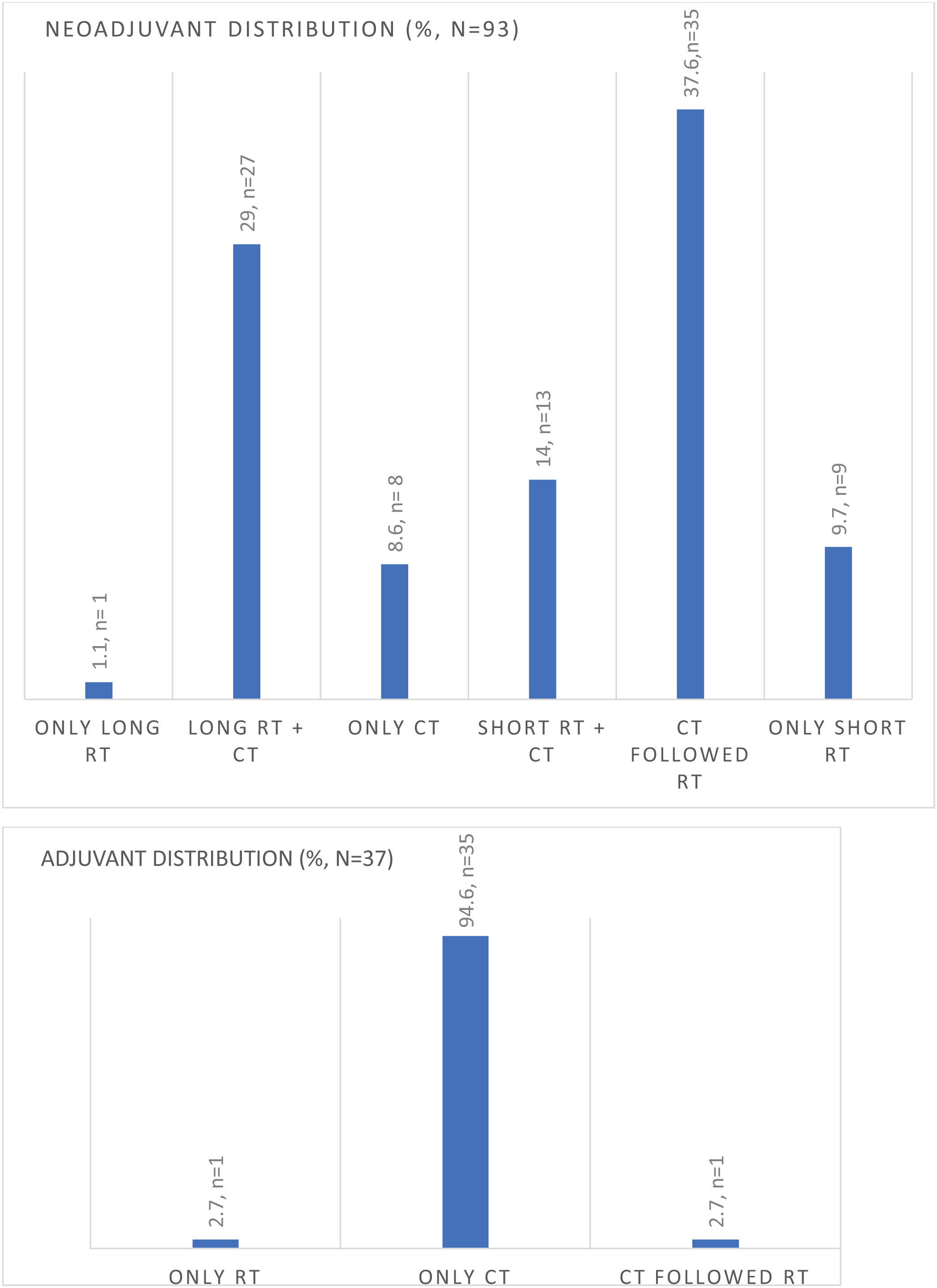
Ninety-three patients (86.1%) received neoadjuvant therapy, while 34.4% (n = 37) of patients received adjuvant therapy after rectal resection. Preoperative RNM TNM stage is summarized in Table 2, and the type of neoadjuvant or adjuvant therapy is shown in Fig. 1.
Ninety-four per cent (n = 103) of the temporary ileostomies were finally closed, with a permanent ileostomy rate of 4.6% (5/108). Reasons for non-closure were progression of cancer with liver metastasis, anastomotic stricture of coloanal anastomosis, laryngeal cancer, rectal ischemia after rectal resection, and death before reversal. One patient underwent urgent surgery for stoma incarceration. The other 99% (n = 102) were closed electively at a median of 74.5 days after rectal resection (range 57–113). The rate of patients closed before 90 days was 63.1% (n = 65), while 23.3% (n = 24) were closed between 90 and 180 days, and 13.6% (n = 14) after 180 days. Reasons for delay after 180 days were diverse: anastomotic leakage or stricture of coloanal anastomosis, chronic pelvic sepsis, local recurrence, urinary fistula, progression of rectal cancer with liver metastasis, prostate cancer, complication of cholecystitis surgery and the pandemic.
Regarding patients who received adjuvant therapy (n = 37), we have observed that the median time of closure was 70 days (range 47–186), compared with patients who did not receive adjuvant therapy (n = 68) and were closed after a median time of 76 days (range 58–101).
Seventy-eight per cent of patients (n = 84) had had preoperative bowel stimulation, with a median of 3 sessions (range 2–5) before the second surgery. The remaining 22% did not have bowel stimulation (n = 24), as 5 patients (4.6%) had a permanent ileostomy, and 19 patients (17.6%) missed the initial months of protocol implementation.
During closureThe medium time of ileostomy reversal was 79.70 min ± 38.87 SD. Eighty-four per cent of patients (n = 89) did not present any type of technical difficulty during operation, and adhesions were the most commonly reported difficulty (8.7%, n = 9). The predominant anastomosis technique was side-to-side with linear stapler, performed in 96.1% of patients (n = 99). Purse-string closure was chosen over skin closure in 92.2% of patients (n = 95).
Incisional hernia was found during ileostomy closure in 7 cases (6.8%). The need for mesh implantation during abdominal wall closure was determined by the surgeon in 12% of cases (n = 13).
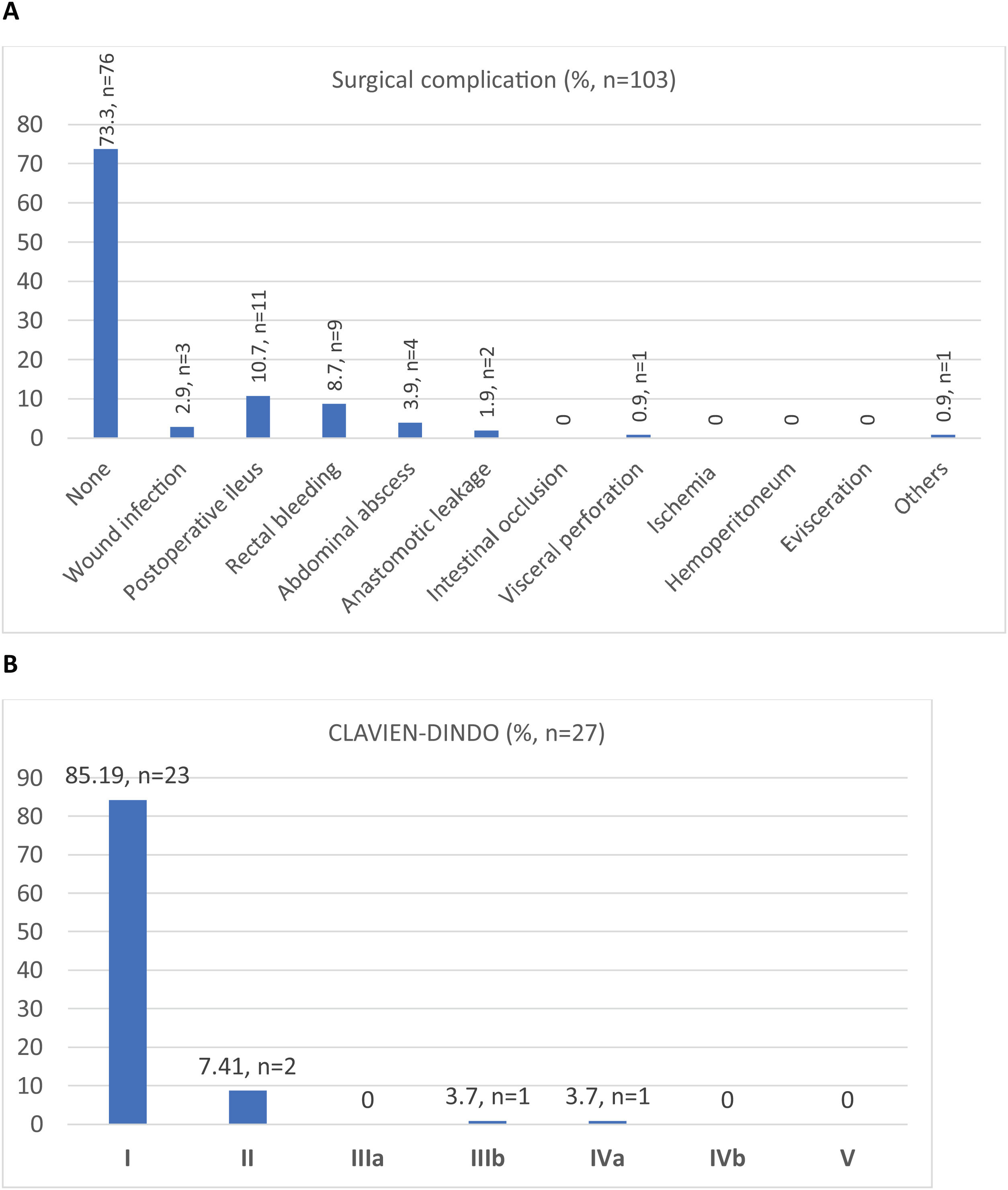
After closureEighty-four percent of patients (n = 88) had no medical complications, and 73.3% of patients (n = 76) had no surgical complications. Urinary tract infection (4.9%, n = 5) followed by catheter bacteriemia (2.9%, n = 3) were the top medical complications.
Out of a total of 103 patients, the surgical morbidity rate was 26.2% (n = 27). The most frequent surgical complication was postoperative ileus (10.7%, n = 11), followed by lower gastrointestinal bleeding from the ileal anastomosis with rectal bleeding in 8.7% (n = 9). There were 2 cases of AL and one of visceral perforation, all 3 of which required a second surgery, with a reoperation rate of 2.8% (n = 3). Regarding SSI, the wound infection rate was 2.9% (n = 3). Morbidity and mortality are summarized in Fig. 2 (A and B).
Median length of stay was 6 days (range 5–7). The re-admission rate was 6.8% (n = 7), due to the following main reasons: paralytic ileus, wound infections, UTI and acute kidney injury, rectal bleeding, intestinal obstruction, and intra-abdominal abscess.
Follow-up one year after closureIncisional hernias were observed clinically in 3 cases (2.9%) at the one-year follow up, and radiological incisional hernias were revealed by the CT scan in 6.8% (n = 8).
DiscussionThe use of a temporary ileostomy as protection for lower rectal anastomosis continues to be a controversial topic due to fear of reduced quality of life during its use and elevated morbidity after its closure. The main finding of interest of our study is that a protocol including early closure, preoperative bowel stimulation and systematized technical closure appears to be feasible in most cases, and the clinical outcome is acceptable.
Clinical interest to reduce or minimize the use of ileostomy is high. Several technical options have been developed, such as ghost ileostomy25,26 and temporary percutaneous ileostomy (ALPPI trial)27 in selected cases, using scores to stratify the risk of anastomotic leakage. The Colon Leakage Score (CLS) is used to decide in which borderline patients ghost ileostomy should be carried out.25,26 However, scientific information to strongly support these techniques is lacking, and ileostomy continues to be recommended by most clinical guidelines for management of rectal cancer. Such a scenario appears to justify attempts to improve the clinical management of ileostomy in order to reduce its morbidity.
Currently, the main topic of discussion appears to be the moment and rate of closure of the ileostomy. The length of time before closure may be affected by several factors, including an uneventful outcome after major surgery, clinical status in aged and frail patients, the need for adjuvant therapy, and the opinion that many surgeons and oncologists prefer to delay closure for fear of morbidity, which would also delay initiation of adjuvant therapy. At our hospital, oncologists started adjuvant therapy after rectal resection, and the ileostomy closure was performed between cycles (1–6). After ileostomy closure was done, adjuvant therapy was continued. This management needed to provide less patient morbidity so that there was no delay to continue adjuvant treatment. Our results showed a similar median of days for ileostomy closure in patients that received adjuvant therapy (70 days, range 47–186) compared to patients without adjuvant therapy (76 days, range 58–101).
In our initial analysis,20 the delay in closure clearly had an impact on the incidence of postoperative morbidity, significantly reducing this from 42% to 23% (P = .012) when closure was achieved within 3 months. Our previous results on morbidity are summarized in Table 3.
Comparison of morbidity of EC vs LC from our previous experience with 191 patients (2004–2016).
| Group | |||
|---|---|---|---|
| Early (≤3 months) | Late (>3 months) | ||
| Complication | 14 (23%) | 54 (42%) | P = .012 |
| Reintervention | 1 (2%) | 18 (14%) | P = .008 |
| Deaths | 0 | 3 (2%) | ns |
| Group | |||
|---|---|---|---|
| Early (≤3 months) | Late (>3 months) | ||
| Timing (months) | 2 (1−2) | 6 (4−10) | |
| First bowel movement (hours) | 28 (12−92) | 36 (12−768) | ns |
| Diet (hours) | 48 (16−192) | 72 (16−768) | P = .007 |
| LoS (days) | 6 (3−15) | 6 (4−96) | P = .001 |
| Group | |||
|---|---|---|---|
| Early (n = 61) | Late (n = 130) | ||
| Clavien | I | 8 (13.1%) | 19 (14.6%) |
| II | 4 (6.6%) | 9 (6.9%) | |
| III | 0 | 4 (3.1%) | |
| IV | 0 | 12 (9.2%) | |
| V | 0 | 3 (2.3%) | |
| Total | 12 (19.7%) | 47 (36.2%) | |
Ns, no significance.
Data contribution by Turrado et al., presented at the 24th EAES Congress, Amsterdam 2016.19
Several high-quality studies have evaluated the feasibility and potential advantages of early closure (less than 15 days after LAR), but results have not been unanimously more successful than standard closure (SC). Wang et al.14 demonstrated that performing ileostomy reversal before 30 days significantly improved total complications versus doing so after 30 days, but this improvement was not significant when compared to the top 5 surgical complications (wound infection, bowel obstruction, fistula, abscess and leakage). Logically, local complications before closure, such as skin irritation, stomal ulcer, and retraction of the stoma, were significantly lower with early closure (EC). In contrast, Clausen et al.15 found no statistically significant differences in terms of AL, reoperations, major complications (Clavien-Dindo grade ≥ III) or global morbidity between very early closure (VEC) (≤3 weeks), EC (≤6 weeks) and SC (>6 weeks). O’Sullivan et al. and Menahen et al.16,17 found similar evidence, with no differences regarding primary outcomes and only more significant results on postoperative ileus in the EC group (≤14 days). A recent meta-analysis found that EC, particularly before 2 weeks, had significantly higher odds of AL than SC, and this timing of closure was discouraged.28 In terms of functionality and quality of life, Podda et al.18 did not find differences between EC (≤30 days) and delayed closure (DC, ≥60 days). Similar results were found in the ESAY trial11 regarding the quality-of-life scale at 12 months follow-up.
In our study, we chose the timing of 3 months as a pragmatic limit for stoma closure. Firstly, this choice was made because, in our previous experience between 2004 and 2016, we had observed that surgical morbidity and mortality, time to initiation of oral intake, and length of stay were reduced when surgery was performed within 3 months.20 Secondly, we needed to consider the time to evaluate the need for chemotherapy (CT), the rectosigmoidoscopy schedule, and the waiting list for surgery. Thus, 3 months was chosen as a reasonable time, considering that ours is a busy hospital and that this was the time limit in our previous cohort for better outcomes. We achieved ileostomy closure before 3 months in a high proportion of patients (63%, n = 65). Only 13% (n = 14) of ileostomies took more than 180 days to close. Moreover, almost all the ileostomies we performed (n = 108) were closed (n = 103).
Another potentially important reason to reduce the interventional lapse is the impact of a defunctioning sphincter on anorectal function and increased LARS rate. Vogel et al.6 found that the risk of developing major LARS was related to a prolonged time with defunctioning ileostomy. Proposed pelvic prehabilitation before ileostomy closure could be an option.
The delay in ileostomy closure12 is considered to play a role in the occurrence of the most frequent complication after ileostomy reversal — paralytic ileus. The time between rectal resection and ileostomy closure affects the functionality of the efferent limb, as demonstrated by the atrophy of the intestinal mucosa of the defunctionalized bowel.21 A common intraoperative finding related to late closure is stenosis of the distal limb due to atrophy. Based on pediatric experiences, preoperative ileum stimulation may favor better postoperative function. Two randomized trials22,23 compared postoperative ileus after stimulation of the distal limb. The findings showed statistically significant superiority in patients who had been stimulated and had shorter hospital stays, earlier time to flatus, and lower ileus rate. The first randomized trial of 2014 had a median time to closure of 185 days (range 75–405) for patients who received stimulation of the efferent limb, while patients who did not receive stimulation had a median time of 168 days (range 58–270, P = .812).22 The second randomized trial of 2022 had a median time to closure of 6.7 months (range 5–11) for patients who had received stimulation of the efferent limb, while patients who did not receive stimulation had a median time of 7.3 months (range 5−10), P = .55.23 In our opinion, both approaches (stimulation of the efferent limb and ileostomy reversal within 3 months) could benefit our patients, especially considering that we work on a busy hospital with a waiting list for surgery.
Bowel stimulation was possible in 78% (n = 84). Global surgical morbidity was 26%, and postoperative ileus was the major contributor (10%).
Although our study is not a comparative analysis, we believe that previous laparoscopic surgery may have a capital influence on the lower number of adhesions observed during ileostomy closure. When comparing our morbidity rate with the most recent metanalyses,14,16–18 our results in postoperative ileus, AL, major morbidity (Clavin-Dindo ≥ 3) and reoperation rate are similar to or even better than other studies. Only one study reported length-of-stay data16 that were similar to ours. Furthermore, ours is the only study to mention re-admissions. Table 4 compares our morbidity data with those of other recent series.
Comparison of our morbidity data with recent series.
| Our results | Wang et al.14 | Podda et al.18 | O'Sullivan et al.16 | Menahen et al.17 | |
|---|---|---|---|---|---|
| Postoperative ileus | 10.7% (11/103) | EC 4 (4.1%) | EC (12/306 = 3.9%) | EC (12/275 = 4.36%) | EC (14/252 = 5.55%) |
| LC 3 (3%) | LC (25/293 = 8.5%) | SC (24/259 = 9.2%) | LC (39/318 = 12.26%) | ||
| Wound infection | 2.9% (3/103) | EC 19 (20.4%) LC 9 (9.7%) | EC (30/275 = 10.9%) LC (10/259 = 3.8%) | MD | EC (39/252 = 14.47) |
| LC (17/318 = 5.34%) | |||||
| Anastomotic leakage | 1.9% (2/103) | EC 5 (3.3%) | EC (10/306 = 3.27%) LC (9/293 = 3.07%) | EC (13/230 = 5.6%) | EC (6/150 = 4%) |
| LC 5 (3.3%) | SC (10/220 = 4.5%) | LC (9/148 = 6%) | |||
| Reoperations | 1.9% (2/103) | EC (8/160 = 5%) LC (4/164 = 2.4%) | EC (22/306 = 7.1%) | EC (23/275 = 8.36%) | EC (14/252 = 5.55%) |
| LC (13/293 = 4.4%) | SC (11/259 = 4.2%) | LC (16/318 = 5.03%) | |||
| Global medical complications | 15 (14.60%) | MD | EC (17/275 = 6.2%) | MD | MD |
| LC (11/259 = 4.24%) | |||||
| Global surgical complications | 27 (26.20%) | MD | MD | MD | MD |
| Global morbidity | 42 (40.80%) | EC (103/324 = 31.7%) | EC (90/306 = 29.41%) | EC (70/275 = 25.45%) SC (56/259 = 21.62%) | EC (55/210 = 26.19%) |
| LC (61/324 = 18.8%) | LC (87/306 = 28.43%) | LC (78/257 = 30.35%) | |||
| Clavien-Dindo ≥ 3 | 2 (1.80%) | EC (12/198 = 6.1%) | EC (27/195 = 13.8%) LC (28/192 = 14.28%) | EC (22/275 = 8%) | MD |
| LC (2/198 = 1%) | SC (12/259 = 4.6%) | ||||
| Length of stay in days (LOS) | 6 days (range 4−23) | MD | MD | Bausys EC 7 days ± 0.75 SD, SC 6 days ± 0.25 SD | MD |
| Danielsen EC 4 days ± 6.25 SD, SC 4 days ± 6.5 SD | |||||
| Klek EC 5 days ± 0.5 SD, SC 5 days ± 0.25 SD | |||||
| Re-admissions | 7 (6.8%) | MD | MD | MD | MD |
EC, Early closure; LC, Late Closure; SC, Standard Closure; MD, missing data.
Regarding SSI, our study had a wound infection rate of 2.9%. It is of note that most surgeries were closed with purse-string skin closure (PSC) (92.2%, n = 95). The advantages of this technique have been demonstrated by Hajibandeh et al.29 in a meta-analysis of 14 studies (n = 1102 patients). They showed the significant superiority of PSC, with a wound infection rate of 2.47%, compared to a rate with linear skin closure (LSC) of 22.08%. A recent meta-analysis by Mirande et al.30 also demonstrated significantly lower rates of SSI with PSC (2.7%) compared to primary closure or primary closure plus drain (6.9% vs 5.7%). In our opinion, the evidence to date strongly supports the recommendation of this skin closure approach.
Regarding incisional hernia, the use of mesh to reinforce the abdominal wall was used at the surgeon’s discretion in 8.7% of cases. The one-year clinical rate of incisional hernia was less than 3%.
The main weakness of our study is that it is not comparative. Nevertheless, we believe its strength lies in the pragmatic application of a prospective homogenous protocol in daily practice.
ConclusionThe laparoscopic approach, early closure and stimulation of the efferent limb could be 3 pillars to reduce morbidity and mortality following ileostomy closure. Further high-evidence studies are required to evaluate LARS and to determine the best time for ileostomy reversal.
Conflict of interestNone declared.
Funding informationThis research study received no specific grants from funding agencies in the public, commercial, or non-profit sectors.
Ethical approvalThis study was reviewed and approved by the Ethics Committee at our medical center.
We would like to thank our stoma-therapist nurse, Mercedes Rubio Vázquez, for all her effort in performing efferent stimulation limb and following up of our patients with stomas.