Mixed adenoneuroendocrine carcinomas (MANEC) are characterized by the simultaneous presence of an exocrine glandular component and a neuroendocrine component. They are rare, heterogeneous tumors that present a very variable morphological/clinical profile and prognosis.
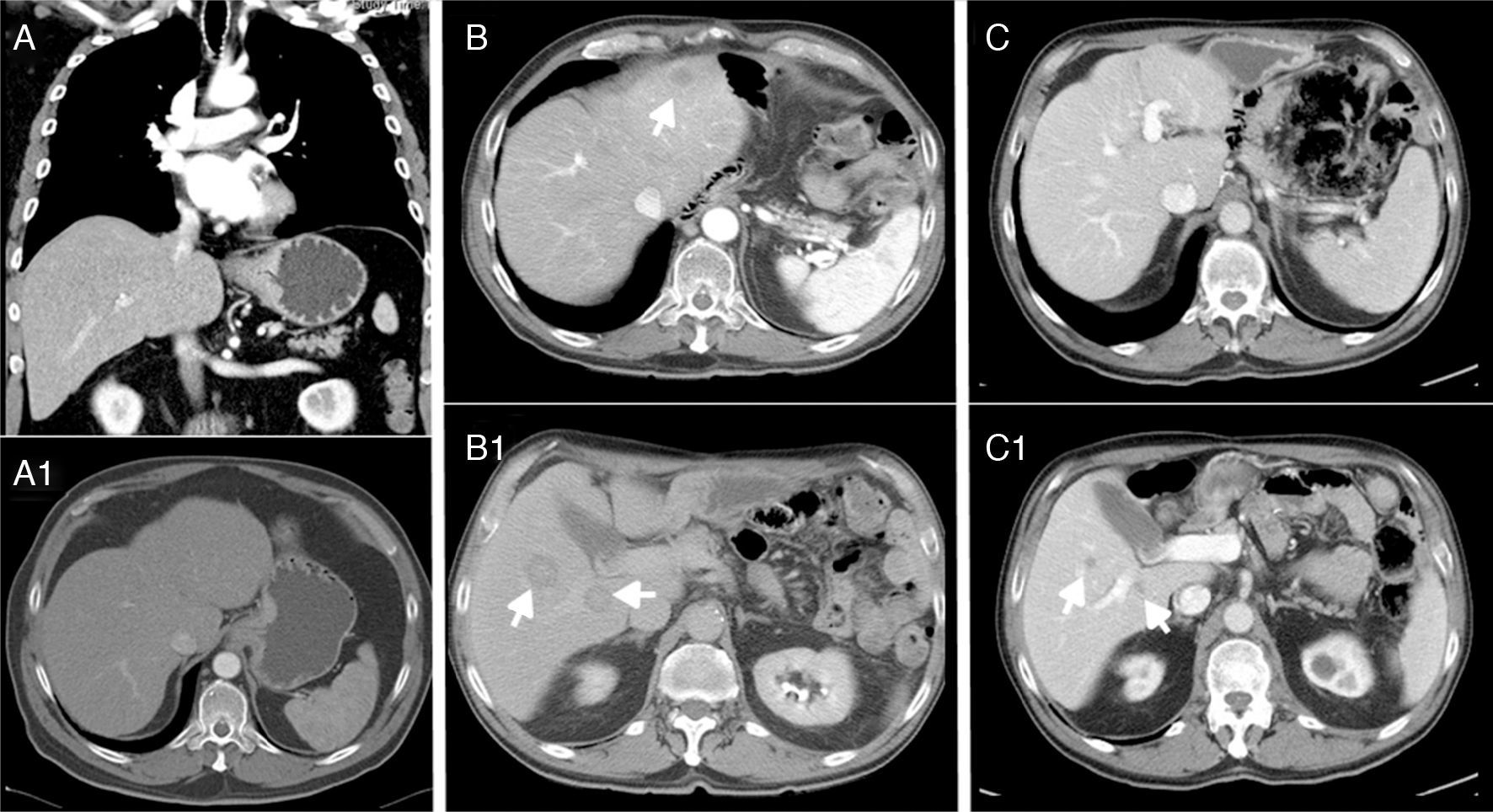
We present the case of a 69-year-old man with controlled hypertension, who reported progressive dysphagia over the previous 4 months and weight loss. Endoscopy revealed a neoproliferative mass in the cardia with stenosis, and biopsy demonstrated a poorly differentiated adenocarcinoma. The CT scan (Fig. 1A) showed mucosal and submucosal thickening of 5cm of the cardia (probable T2–T3), inconclusive lymphadenopathy at the gastrohepatic ligament, but no evidence of distant metastasis. Tumor marker levels were: CEA: 18.7ng/mL and CA 19-9: 32U/mL.
(A-A1) Thickening of the mucosa and submucosa 5cm from the cardia, suspicious lymphadenopathy in the gastrohepatic ligament, with no evidence of distant metastasis; (B-B1) hepatic lesions compatible with metastasis in segments II, VI and VII after 14 months; (C-C1) stable disease with no local recurrence and no growth of the metastases, which are currently smaller than 1cm after 32 months.
During surgery, a mass was identified in the esophagogastric junction that infiltrated the hiatus as well as a peripheral single hepatic metastasis in segment III, but no other tumors were found by intraoperative ultrasound. Since the metastasis was solitary and peripheral, we decided to perform hepatic metastasectomy as it did not imply greater risks for the patient. Subsequently, esophagogastrectomy was performed with esophagojejunal intramediastinal Roux-en-Y anastomosis as a palliative method.
The macroscopic study of the surgical piece showed circumferential involvement of the esophagogastric junction and free margins (proximal margin 4cm).
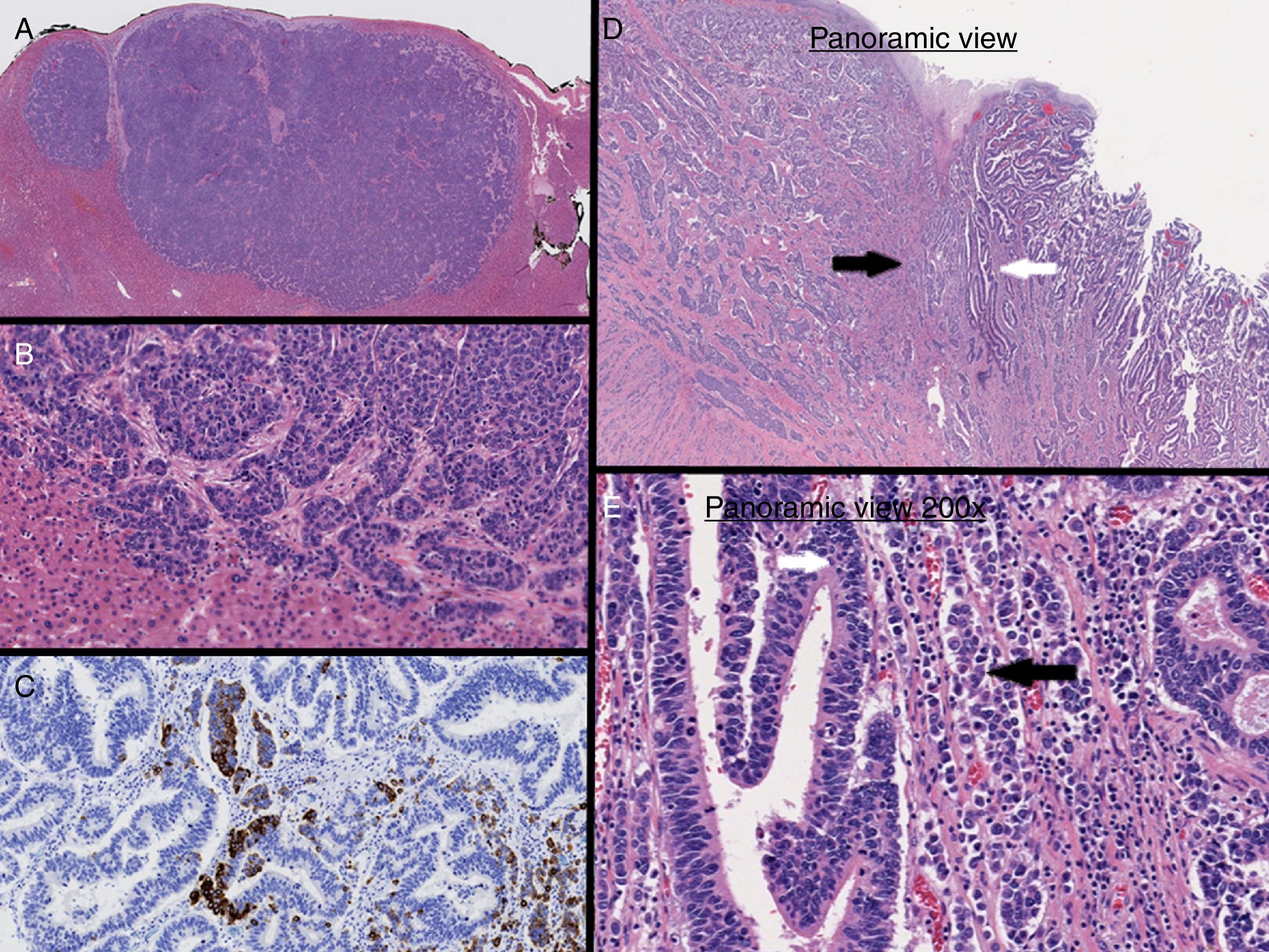
The pathology study identified a grade 3 (WHO 2010) MANEC tumor with Ki-67 index >20%. One infiltrated lymph node was isolated from 28 studied. The liver lesion was positive for MANEC tumor with negative HercepTest™. The final stage was pT3N3M1 (AJCC/UICC TNM 7th Edition), therefore the patient received 6 cycles of adjuvant chemotherapy with carboplatin and etoposide.
After 6 months, CT and SPECT/CT were normal and showed no evidence of recurrence. However, after 14 months, another CT study (Fig. 1B) showed hepatic lesions compatible with metastasis in segments II, VI and VII, therefore another line was initiated with 6 cycles of carboplatin+5-fluorouracil. The patient presented a very favorable response (RECIST criteria), with a decrease in the size of the metastases greater than 50%. The 32-month CT scan showed stable disease with no local recurrence and no growth of the metastases, which are currently smaller than 1cm (Fig. 1C).
MANEC are characterized by a neuroendocrine component, marked by the expression of synaptophysin (75%–90%), chromogranin (60%–70%) and CD56 (50%), and an exocrine glandular component, identified by its structure with positivity for cytokeratins 7 and 20 as well as CEA. The exocrine component generally has an adenocarcinoma histology, but cases of squamous cells have also been described in tumors of the esophagus and anorectal region.1,2
In our case, immunohistochemical staining of the liver lesion showed positivity for CDX2, synaptophysin and chromogranin A (Fig. 2A–C). Based on these results, it was concluded that the liver lesion was a metastatic MANEC lesion of the esophagogastric junction (Fig. 2D and E) that also presented the 2 components simultaneously, similar to the primary tumor.3,4
At a molecular level, MANEC can arise independently from 2 different precursor cells in a synchronous manner or be derived from a multipotent stem cell. Furlan analyzed polymorphic microsatellite markers in MANEC tumors and observed a close genetic relationship between the 2 different histological components. This finding suggests that the mechanism of monoclonal tumorigenesis (cell capacity for neuroendocrine and glandular differentiation) is the most frequent and probable genetic event.5,6
The prognosis is related with size, invasion and degree of differentiation. Those that are poorly differentiated with adenocarcinoma components, as in the case of MANEC, have a poorer prognosis and, given their rarity, the 5-year overall survival rate is difficult to predict.7,8
Treatment is controversial, both due to the particularities of the tumor and the absence of relevant studies to give recommendations. Adjuvant therapy is used universally in all cases as it increases survival. Although it is a chemosensitive tumor in which there is a very good initial response, recurrence or progression are frequent. Among the drug therapies, the combination of cisplatin and etoposide is the most widely used at present.
Surgery is reserved for localized disease with curative intent. In disseminated disease, surgery is used for palliative purposes.
In the literature, there are few publications about MANEC of the esophagogastric junction. Our patient presented a preoperative biopsy of gastric adenocarcinoma, and surgery showed a solitary synchronous metastasis, so we decided on radical surgery and chemotherapy as the best way to palliate a suspected Siewert type 3 gastric adenocarcinoma. The alternative would have been not to operate and place an endoprosthesis endoscopically to treat the dysphagia. However, this last option would not have given the precise diagnosis of MANEC tumor. Furthermore, surprisingly, from our review it can be deduced that the cases of MANEC tumors that have reported the longest survival included patients who received radical therapy, including esophagectomy or gastrectomy with chemotherapy or radiotherapy, although the role of the latter is controversial and used in cases of localized non-metastatic tumors.9,10
The optimal and protocolized management of these tumors is unknown due to their low frequency and few reports in the literature, which are limited to small series of patients.
When treatment is planned, the most aggressive component is what determines the approach. In general, tumors with a poorly differentiated neuroendocrine carcinoma component should be treated as neuroendocrine carcinomas, and tumors composed of an adenocarcinoma together with well-differentiated neuroendocrine tumors should be treated as adenocarcinomas.
We present a unique case of metastatic MANEC tumor of the esophagogastric union treated with radical surgery (leaving no macro or microscopic disease – R0, with modified D2 lymphadenectomy without including the splenic hilum – level 10), since it was considered palliative surgery, and several lines of chemotherapy. The case highlights the great response to the carboplatin and 5-fluorouracil regimen and the possibility of resecting the tumor to obtain the definitive diagnosis of MANEC tumor, with the consequent benefit of administering the most appropriate chemotherapy, resulting in increased survival.
Please cite this article as: Monteiro de Melo Santos D, López-Tomassetti Fernández E, Sánchez Ramos M, Hernández Hernández JR. Resección paliativa con quimioterapia para carcinomas mixtos adenoneuroendocrinos de la unión gastroesofágica con metástasis hepática sincrónica. Cir Esp. 2018;96:58–61.