Argon plasma coagulation (APC) is a safe and effective technique that is widely used for the treatment of gastrointestinal vascular lesions. It is considered the technique of choice for the management of these lesions.1
Rendu–Osler–Weber disease is a condition characterized by the appearance of arteriovenous malformations of the skin, mucous membranes and various organs, typically presenting gastrointestinal bleeding. APC has proven to be an effective technique for the treatment of these patients.2,3
It has been described that the contact of the probe with the mucosa or the high flow of argon can lead to a gas leak through the gastrointestinal wall or even, in some cases, perforation of a hollow organ.4
The presence of pneumoperitoneum after APC can be asymptomatic and caused by the passage of gas through the gastrointestinal wall. In many cases, the treatment of choice is conservative.5,6
We present a case of massive pneumoperitoneum after APC treated with conservative management with demonstrative imaging studies.
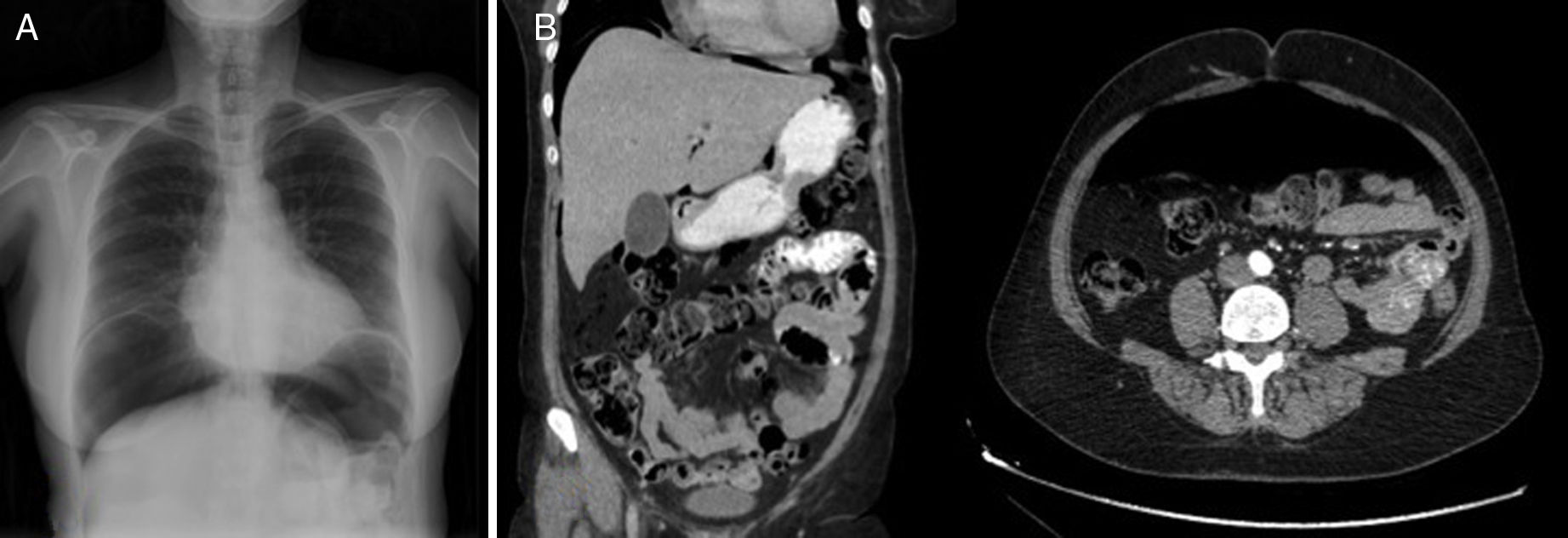
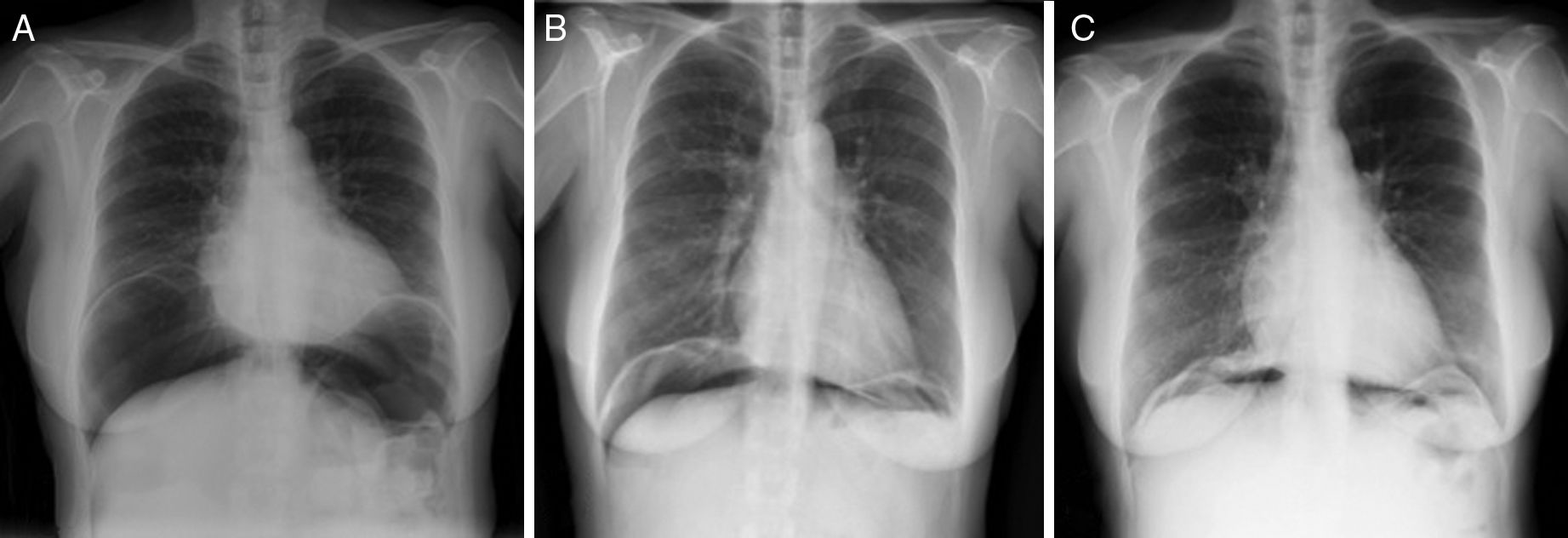
The patient is a 57-year-old woman under treatment with APC (Argon ERBE APC-300) due to gastric and duodenal angiodysplasias secondary to Rendu–Osler–Weber disease. After one session, she came to the emergency room with abdominal distension and epigastric pain that had been progressing for 12h. The patient presented a distended abdomen that was slightly painful, with no signs of peritoneal irritation. Chest radiograph revealed a massive pneumoperitoneum (Fig. 1). Triple-contrast CT detected abundant pneumoperitoneum with no free fluid; no area of dehiscence was observed, and there was no extravasation of water-soluble oral contrast (Fig. 1). While in the emergency department, a series of analyses showed no elevated acute-phase reactants, and the patient remained asymptomatic. In view of the clinical–analytical–radiological findings, we decided on observation and conservative management with nil per os, a nasogastric tube and iv ertapenem 1g/24h. No complications were observed during hospitalization, and the patient was discharged after 6 days. After discharge, a clinical-radiological follow-up showed a gradual reduction of the intra-abdominal air volume (Fig. 2).
In the literature, there are few reported cases of massive pneumoperitoneum after APC: 3 in the management of colon polyps, and 2 in the treatment of angiodysplasias. In 3 of these patients, conservative treatment was used without complications, while in the other 2, exploratory laparotomy was performed initially but no perforation was observed.5–7 There are probably many more cases of pneumoperitoneum after APC than those reported. If systematic abdominal radiographs were performed after each treatment, we would find a high number of cases of asymptomatic pneumoperitoneum.6
When faced with the finding of pneumoperitoneum after APC, we should not automatically assume that there is a perforation of a hollow viscus; instead, it may be caused by the passage of gas through the gastrointestinal wall.5,6 It is essential to identify those patients who have perforated hollow viscus, who will require urgent surgery, and to differentiate them from patients with an accumulation of intra-abdominal air simulating perforation, but which is really caused by the passage of gas through the gastrointestinal wall, and in whom conservative management can be considered.5,6 In the latter, conservative treatment could be the treatment of choice, and urgent surgery should be reserved for patients with signs of peritoneal irritation or clinical-analytical findings of sepsis.6 In series such as those by Kwan et al. or Manner et al., no cases of perforation of hollow viscus associated with APC have been described; however, Grund et al. describe an accidental perforation rate of 0.31%.1,4,7
CT is considered the diagnostic technique of choice when there is a suspicion of perforated hollow viscus.8–10 The main signs of perforation include the identification of a defect in the intestinal wall and the presence of gas or extraluminal oral contrast.8 The time elapsed from the perforation to the completion of the CT does not influence its diagnostic accuracy.9 Extravasation of oral contrast is a direct sign of perforation with high specificity but very low sensitivity. Its absence does not exclude the presence of perforation.8,10
The argon gas is slowly reabsorbed, so the long-term persistence of air at the intra-abdominal level should not cause alarm. Conservative treatment of these patients can be safely maintained as long as the patient remains asymptomatic, with no peritonism, and there is a progressive decrease in intra-abdominal air volume.6,7 The literature describes a reduction in intra-abdominal air volume after one month and complete disappearance after 3 months.5 In the case of our patient, 15 days later there was a notable decrease in intra-abdominal air, which practically disappeared after one month.
In conclusion, in cases of pneumoperitoneum after APC, it is essential to distinguish between that caused by the passage of gas through the gastrointestinal wall and pneumoperitoneum secondary to perforated hollow viscus. As in the case presented, in patients who are scarcely symptomatic, with no signs of peritoneal irritation or sepsis, and no conclusive radiological findings, the treatment of choice is conservative management, which presents no risk for developing complications.
Please cite this article as: Fernández Gómez-Cruzado L, Prieto Calvo M, Alonso Calderón E, Larrea Oleaga J, Marquina Tobalina T. Manejo conservador del neumoperitoneo masivo tras electrocoagulación con argón plasma. Cir Esp. 2018;96:56–58.
This case report was presented as a poster at the 23rd Annual Meeting of the Asociación de Cirujanos del Norte in Laredo, Spain, on May 6, 2016.