During the current SARS-CoV-2 virus pandemic, an extensive therapeutic arsenal is being used that includes uncommonly used drugs, so it is important to understand their possible adverse effects.
We present the case of a patient admitted for respiratory infection due to COVID-19, who presented an acute colic perforation after being treated with high doses of corticosteroids and tocilizumab. Similar clinical cases have been reported in patients with autoimmune diseases treated with tocilizumab, although the information about intestinal perforations and COVID-19 infection is very scarce. However, as the clinical spectrum of the virus is apparently broad,1 the possibility that it is the only underlying cause cannot be completely ruled out.
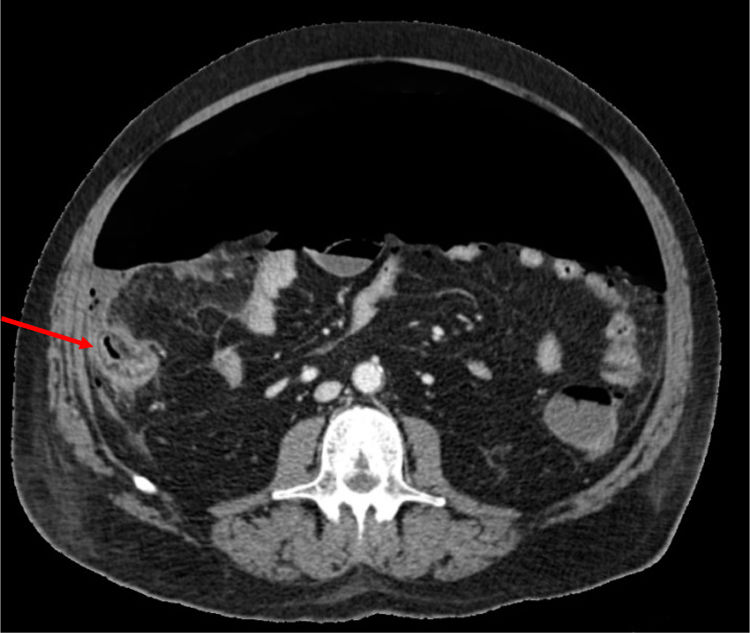
Our patient is a 66-year-old male with metabolic syndrome as the only history of interest, with no regular treatment. In the context of acute respiratory failure secondary to COVID 19, he presented abdominal pain with guarding and increased leukocytosis and associated PCR. Abdominopelvic CT identified abundant pneumoperitoneum secondary to perforation of the right colon. Right colectomy was performed, observing no signs of ischemia or diverticula in the surgical specimen.
Previously, the patient had been treated with methylprednisolone (100 mg/day for 5 days), tocilizumab (single 600 mg dose, corresponding with 8 mg/kg) and 15 days prior to the procedure, azithromycin, hydroxychloroquine and lopinavir/ritonavir. The biopsy revealed an area of necrosis with ulceration of the mucosa measuring 12 mm on the anterior side of the cecum, with no signs of malignancy or other areas of ischemia.
Tocilizumab is a humanized monoclonal antibody that blocks both soluble and membrane receptors of interleukin 6, thereby inhibiting the inflammatory cascade generated by said mediator. It is commonly used in autoimmune diseases as part of the group of disease-modifying biological drugs.
This drug has not yet been approved by the Food and Drug Administration to treat COVID-19 pneumonia. However, its use in severe cases has become widespread in the context of the pandemic.
Its safety was evaluated in the LITHE2 clinical trial, which described serious gastrointestinal complications as uncommon and appearing in patients under concurrent treatment with corticosteroids or NSAIDs (Fig. 1).
Nevertheless, several studies indicate a higher risk of lower intestinal perforation in patients treated with tocilizumab,3–5 with a described incidence of close to 2/1,000 patients/year in whom at least one dose of tocilizumab has been administered,6 especially at 8 mg/kg versus 4 mg/kg.7 Similarly, the risk of intestinal perforation is higher compared to other disease-modulating biological drugs.3,4,8,9 These perforations appear in the first 12 months after treatment, do not increase over time,5,9 and are always more frequent in patients who have received corticosteroids.5,6 A possible explanation could be the role of IL-63 in the intestinal barrier function, as well as a lower intensity in the immediate inflammatory response.5 Finally, it should be noted that mortality after perforation in these cases can reach 46%,3 a fact that would significantly worsen the already uncertain prognosis of patients with COVID-19 infection.10
Please cite this article as: Gonzálvez Guardiola P, Díez Ares JÁ, Peris Tomás N, Sebastián Tomás JC, Navarro Martínez S. Perforación intestinal en paciente COVID-19 en tratamiento con tocilizumab y corticoides. A propósito de un caso. Cir Esp. 2021;99:156–157.