Negative pressure wound therapy (NPWT) is widely known in surgical practice. The initial indications for NPWT were chronic wounds, especially diabetic foot, vascular and decubitus ulcers, and infected traumatic wounds. Nowadays, the use has been widely increased. Although in the field of abdominal wall surgery, it has mainly been used in the treatment of surgical wound complications after hernia repair, other indications have been added after years of experience in the management of NPWT.
Therefore, the aim of this article is to analyze and review the main indications of NPWT in abdominal wall surgery, as well as the advantages obtained with its application.
La terapia de presión negativa para el tratamiento de las heridas (TPN) es ampliamente conocida en la práctica quirúrgica. Las indicaciones iniciales de la TPN fueron las heridas crónicas, sobre todo úlceras de pie diabético, vasculares y por decúbito, y las heridas infectadas traumáticas. En la actualidad, el uso se ha diversificado ampliamente. Aunque en el campo de la cirugía de pared abdominal se ha utilizado principalmente en el manejo de las complicaciones de la herida quirúrgica tras la reparación herniaria, otras indicaciones han sido añadidas tras adquirir la experiencia durante años en el manejo de la TPN.
Por ello, el objetivo de este artículo es analizar y revisar las principales indicaciones de la TPN en la cirugía de pared abdominal, así como las ventajas que se obtienen con su aplicación.
Negative pressure therapy for wound treatment (NPWT) is widely known in surgical practice, and was first introduced in its current form in 1997 by Dr Argenta and Dr Morykwas.1
The basic system consists of a porous sponge, usually polyurethane, which fills the wound cavity to be treated, together with an adhesive plastic occlusive dressing and a tube that connects to the sponge and transmits the suction generated by an electronic device.2 All devices currently in existence are based on this original design, although some include some improvements such as the instillation of liquids into the wound or changes in the material for application in different situations.2,3
The initial indications for NPWT were chronic wounds, especially diabetic foot, vascular and decubitus ulcers, and infected traumatic wounds. Nowadays, the use has diversified widely. Although in the field of abdominal wall surgery it has been mainly used in the management of surgical wound complications (SWC) after hernia repair, other indications have been added after years of experience in NPWT management.
Therefore, the aim of this article is to analyze and review the main indications of NPWT in abdominal wall surgery, as well as the advantages obtained with its application.
Negative pressure therapy mechanism of actionThe therapeutic effects of NPWT are macro-deformation of the wound edges, micro-deformation of the sponge-wound interface, elimination of exudate and maintenance of a moist and stable environment for proper wound healing.2 This is in addition to microscopic effects such as cell proliferation, angiogenesis and granulation tissue formation.3
Macro deformation is the ability of vacuum application to contract the edges of the wound, decreasing its area, depending on the elasticity of the tissue where the sponge is placed. esponja2. Factors modifying macro deformation are the level of suction, volume and type of sponge.4–6 Micro deformation is related to the pores of the sponge (typically 400–600 µm); the vacuum pressure is transmitted to the wound cells, which lose their spherical shape, promoting division and proliferation.7 This effect is what mainly induces cell proliferation and angiogenesis.3 It is important to note that open-pore sponges, as most systems use, effectively transmit vacuum and contract by up to 80% at 125 mmHg; it is therefore important to adapt and cut the sponge to fit the wound perfectly when replacing it.8–10 Suction also helps to reduce tissue oedema, improves tissue perfusion,6 removes excess fluid from the wound, helping to create an ideal environment for healing.
Indications for negative pressure therapy in abdominal wall surgeryThe main indications for NPWT in abdominal wall surgery are:
- –
Temporary closure of the abdominal cavity due to complications arising from hernia repair: support of surgical wound closure by secondary intention or delayed closure, and management of the open abdomen with fascial traction
- –
In the context of chronic prosthetic infection (CPI), with the aim of attempting to salvage the surgical mesh.
- –
Prophylaxis of SWC after hernia repair or incisional NPWT.
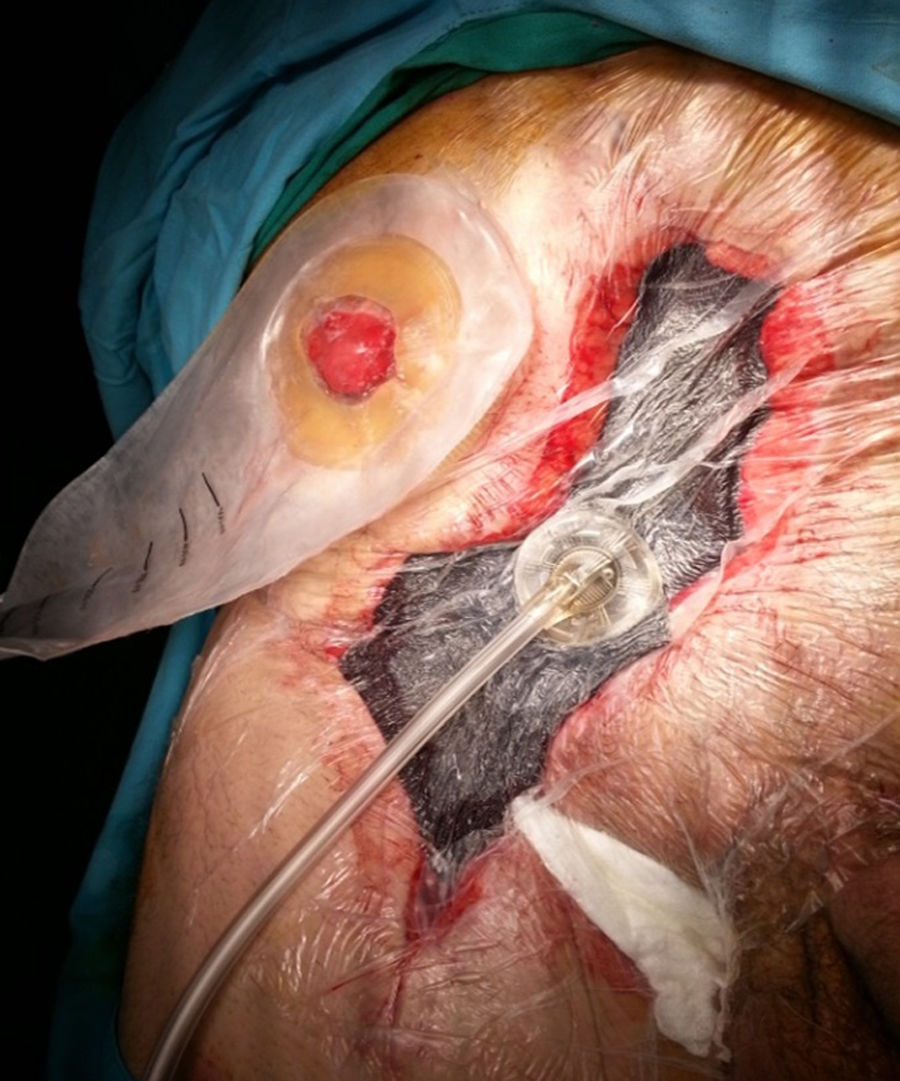
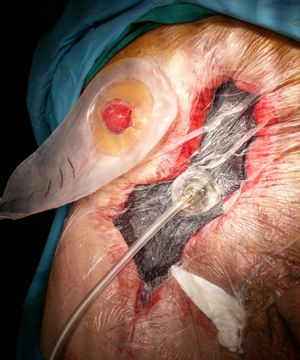
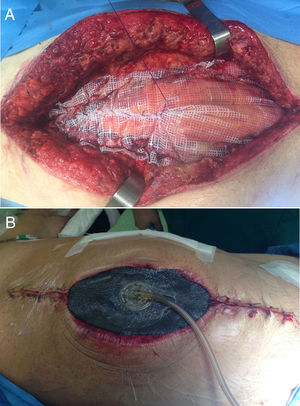
It is the most frequently used application in the context of abdominal wall surgery (Fig. 1). The device is useful in cases of SWC, mainly in situations of infection (SWI), dehiscence or necrosis of its edges. There are factors associated with the appearance of SWC, depending on the patient (age, diabetes, immunosuppression, obesity, bronchopathy, etc.), and others related to the surgical technique, such as open versus laparoscopic repair, wide tissue dissection, complex abdominal wall repair, especially anterior component separation (ACS) or panniculectomies, etc.11 NPWT can also be combined with the application of skin grafts in order to reduce the healing time of the surgical wound.2
Although the replacement technique in NPWT is well known in the scientific community, it is worth remembering that changes should be performed every 2–3 days, especially in wounds that have a large amount of exudate or contamination. If the wound has little discharge and detritus or slough has been controlled, and only edge granulation is awaited, changes could be spaced out to a maximum of 4–5 days. Finally, the anterior aponeurosis, subcutaneous cellular tissue and muscle are the structures where the wound granulates best; in this regard, this factor of NPWT should be taken into account, because in cases of its use on the posterior fascia of the rectum or on the peritoneum (e.g., when a hernial sac is present) granulation is minimal or much slower.12
Management of the open abdomen with negative pressureThe NPWT system classically has its main use in traumatic abdomens. Along with damage control, visceral oedema and sepsis control, temporary closures of the abdominal cavity with this device have been shown to better manage fluids, maintain low levels of intestinal fistulisation and allow faster and complete fascial closures of the abdominal cavity.13–15
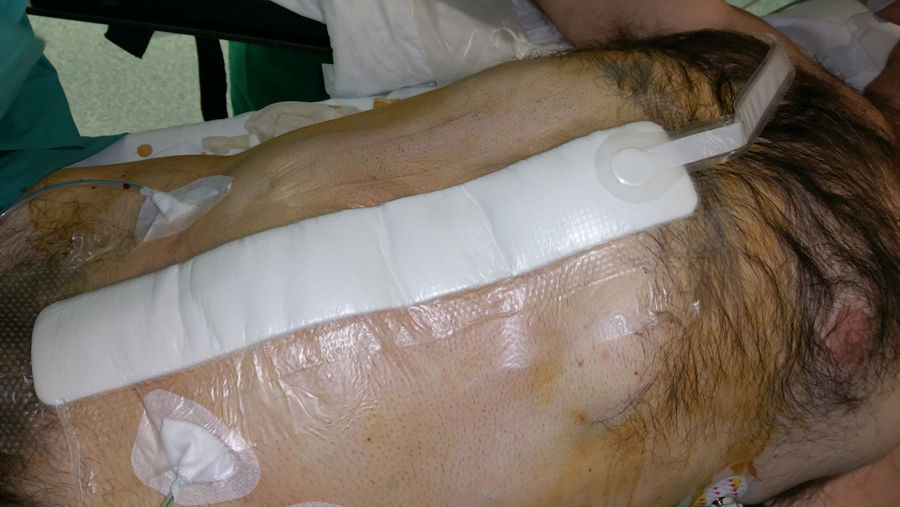
In the field of abdominal wall surgery, this application in these situations is performed together with the use of fascial traction (or Leppäniemi technique), especially in cases of evisceration after laparotomy or in cases where neither primary fascial closure nor bridging with the prosthesis can be performed16 (Fig. 2).
The first studies on fascial traction-mediated closure in patients with open abdomen were published in 2007.16,17 This novel technique at that time was justified due to the high percentages of "planned ventral hernias" caused by damage control surgery in the management of abdominal trauma.18 Thus, once the dressing that will separate the viscera from the mesh and the sponge is in place, the prosthesis is attached to the edges of the fascia. The mesh, usually polypropylene (PPL), although sometimes a composite mesh can be used, is adjusted to the size of the defect and fixed with a non-absorbable or slowly resorbing monofilament. Finally, the device sponges are placed over the prosthesis in the conventional manner.16
Facial traction in this context has shown a higher percentage of complete fascial closure and a lower incidence of residual incisional hernia, reducing the rate of complications and the dreaded enteroatmospheric fistula, although in this case, some series have shown an increased incidence.19,20
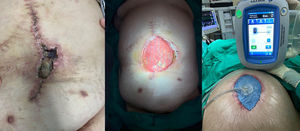
Conservative management of chronic prosthesis infectionCPI is a potentially devastating complication of hernia repair; its incidence ranges from .7%– 25.6%, depending on several factors, such as patient comorbidity, surgical technique or type of prosthesis used.21 If an infected mesh has to be removed, the patient faces the disadvantages of hernia recurrence and morbidity related to additional surgical procedures, with the risk of enterotomies, bleeding or intestinal fistulae related to the mesh removal process. In addition, this procedure may leave a complex open wound, with an underlying abdominal wall weakness or defect, which may be a greater clinical problem than the original hernia.22
The type of infected prosthesis influences the difficulty of solving the clinical problem. Thus, a large-pore PPL mesh in an extraperitoneal position can be managed routinely without explantation, whereas multifilament, microporous and composite (e.g., expanded polytetrafluoroethylene or PTFE) meshes require removal in almost all cases.22,23 Partially absorbable meshes appear to be more advantageous in the success of conservative management of CPI compared to non-absorbable meshes. 23 Finally, the finding of methicillin-resistant Staphylococcus aureus (MRSA) in the culture of exudate obtained at the site of infection also presents a predictive factor for the need for prosthesis removal.24
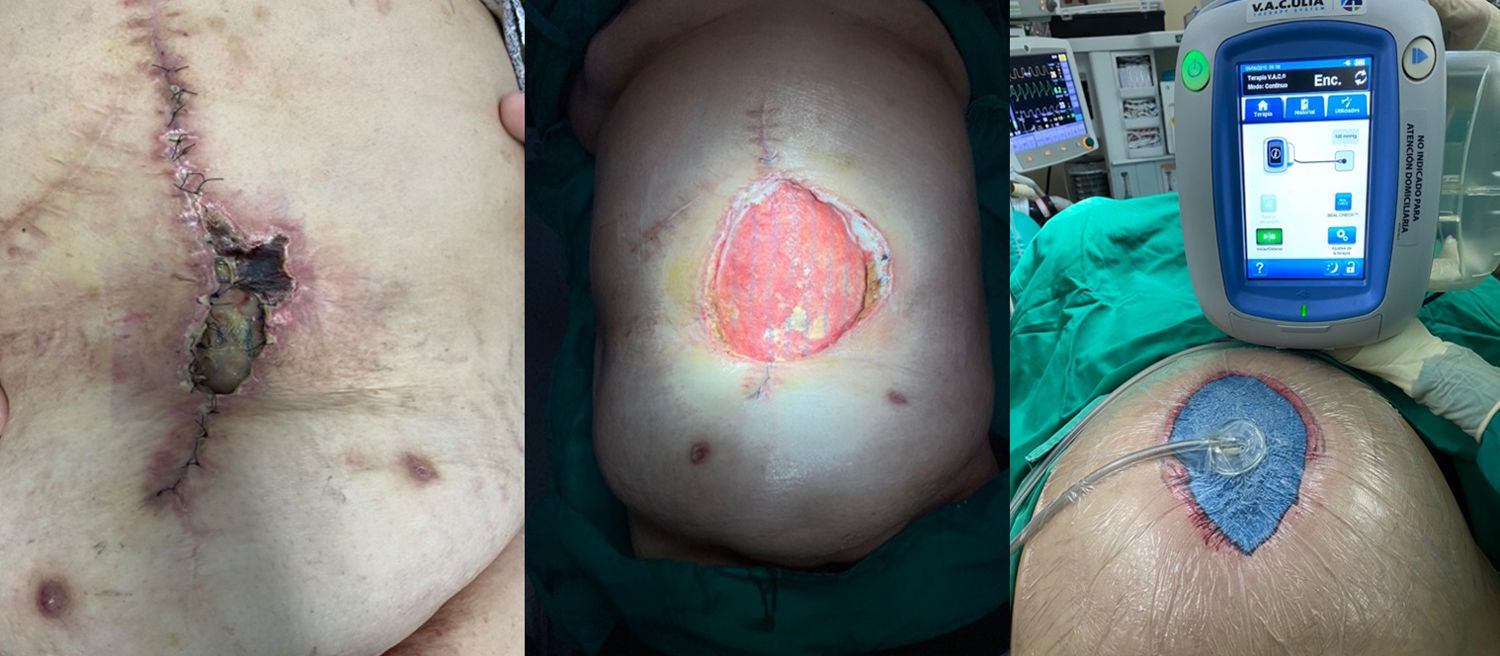
CPI management in each patient should be individualised, in most cases attempting to salvage the mesh after close monitoring and before condemning the patient to the morbidity associated with its removal. This conservative treatment can be achieved by several methods, with varying success rates. An accepted strategy includes the application of NPWT, especially when SWI occurs after hernia repair, which may lead to CPI a posteriori25 (Fig. 3). NPWT contributes to the growth of granulation tissue through the mesh, which may protect it from bacterial presence in the surgical wound.26 Thus, a large prosthesis pore size (2−5 mm) allows for better incorporation and migration of both leukocytes and macrophages from underlying tissues compared to the pore size of heavy-weight meshes.
Several authors have demonstrated good results with the use of NPWT in the salvage of infected meshes. Greenberg27 evaluated the treatment of infected composite meshes in 11 patients, finding that in 4 cases complete removal was necessary, although none of them developed hernia recurrence. Stremitzer et al.28 used NPWT in patients with infected exposed mesh, saving 17 out of 31 infected meshes (55%) with this therapy; however, only 20% of infected PPL meshes and 23% of infected composite meshes could be saved. The authors suggested that conservative treatment should be applied in cases of absorbable prosthesis infection, while non-absorbable meshes should require early surgical removal.
Baharestani and Gabriel29 reviewed 21 patients with an infected exposed mesh; in 18 of them the wound was successfully closed, but 13 of the patients required total or partial mesh removal. The authors concluded that the most important factor for successful treatment of infected biological or synthetic meshes is adequate debridement with early initiation of NPWT.
Berrevoet et al.30 reported 63 patients treated with NPWT due to an infectious complication after retromuscular and intraperitoneal hernia repair. The rate of IPC was 8.7%, but all retromuscular meshes were spared, giving an overall success rate of 95% with this therapy. Similar results were obtained by Boettge et al.23 and Nobaek et al.31 in series of 48 and 30 patients treated for IPC after supraaponeurotic and retromuscular repairs, respectively, with a conservative treatment success of 92% and 84%.
The advantages of NPWT have been combined with the topical effect of antiseptic solutions added to the device with instillation. García Ruano et al.32 reported positive results with such a device in 45 patients, with partial removal of the mesh being necessary in only one patient (8.3%).
As potential drawbacks of conservative management of CPI with NPWT, the possibility of development of intestinal fistulas has been mentioned as one of the most serious complications associated with the therapy.29 However, with the use of different pressure gradients and appropriate sponge types to regulate the aggressiveness of local treatment, this complication can be minimised. Furthermore, other groups report high costs associated with prolonged NPWT to obtain a positive outcome. However, when compared to the potential costs associated with additional hospital days and complex surgery to remove the mesh, it could be argued that the seemingly most cost-effective solution is conservative treatment.30 It is also important to note that restoration of the integrity of the abdominal wall muscle fascia may be incomplete with this treatment, which may explain the slightly higher incidence of incisional hernia in the groups that underwent this treatment to salvage the infected mesh.33
Incisional negative pressure therapy for prophylactic use in abdominal wallPatients undergoing complex abdominal wall repair are at high risk for a number of wound complications.33 In these procedures, the incidence of SSI, haematoma, seroma or wound dehiscence ranges from 15%–46%.28 Overall, SWCs pose a substantial threat to patients' health and may result in costly or invasive interventions, necessitating the use of appropriate prevention practices.
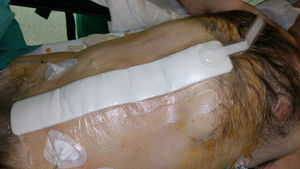
Since 2010, the application of NPWT to closed surgical incisions has been proposed as a means to reduce SWC, especially SWI.2 This physical barrier associated with the advantages of vacuum on the wound may promote a favourable healing environment. The mechanical unloading of tension at the wound site may also facilitate apposition of the wound edges and may therefore be of particular importance in wide abdominal incisions associated with complex abdominal wall reconstruction.
Incisional NPWT has been the focus of new research aimed at preventing immediate postoperative complications in closed surgical incisions (Fig. 4). Although the literature is replete with data on the use of incisional NPWT, few studies, mostly retrospective descriptive, have focused on its prophylactic application after abdominal wall herniated prosthetic repair (Table 1).
Published studies on prophylactic incisional negative pressure therapy in abdominal wall surgery.
| Author | Type of study | Dressing(pressure mmHg/days) | Cases of NPWT | Conventional dressing cases | Hernia repair performed | Mesh used | Conclusions |
|---|---|---|---|---|---|---|---|
| Conde-Green et al.34 | R | ADAPTIC® (J&J) and VAC® (KCI)−125/5 d | 23 | 33 | ACS |
|
|
| Pauli et al.42 | R | ADAPTIC® (J&J) y VAC® (KCI)−75/7 d | 49 | 70 |
|
|
|
| Olona et al.41 | R | PREVENA™ (KCI)−125/7 d | 5 | 37 | Chevrel | synthetic (PPL) |
|
| Gassman et al.38 | R | ADAPTIC® (J&J) y VAC® (KCI)−125/7 d | 29 | 32 |
|
|
|
| Soares et al. (2014) | R | VAC® (KCI)−125/3 d | 115 | 84 | ACS |
|
|
| de Vries et al.35 | R | PREVENA™ o VAC® (KCI)−100/5−10 d | 32 | 34 |
|
|
|
| Diaconu et al.40 | R | PREVENA™ (KCI)−125/5 d | 62 | 42 |
|
|
|
| Hopkins et al.36 | R | PREVENA™ (KCI)−125/5−7 d | 34 | 51 |
|
|
|
| Licari et al.37 | R | PREVENA™ (KCI)−125/7 d | 70 | 110 |
| Synthetic |
|
| Bueno-Lledó et al.(2020) | RCT | PICO® (SN)−80/7 d | 72 | 74 |
| Synthetic (PPL or PVDF)BIO-A® (in TAR) |
|
ACS: Anterior component separation; dif.: differences; IPOM: Intraperitoneal onlay mesh; J&J: Johnson and Johnson; NPWT: Negative pressure wound therapy; PPL: Polipropylene; PVDF: Polyvinylidene difluoride; R: Retrospective; RCT: Retrospective clinical trial; SN: Smith and Nephew; SSC: Surgical site complication; SWI: Surgical wound infection; TAR: Tranversus abdominis release.
Conde-Green et al.34 studied 56 patients undergoing ACS, in 23 of whom incisional NPWT dressings were applied prophylactically, and another 33 received conventional dressings. Their results suggest that incisional NPWT after complex abdominal wall repair could significantly improve SWC and skin dehiscence rates, frequently associated with this approach. Other authors35–37 reach the same conclusion, with a lower incidence of SWI at 30 days and a cost-effectiveness analysis that would justify its routine use in high-risk populations. The same results were demonstrated by Gassman et al.38 and Soares et al.,39 especially in the overall SWI rate and hernia recurrence rate, although they observed that after a 90-day follow-up, the most severe wound complications requiring reoperation occurred 4 weeks after reconstruction. These findings suggest that incisional NPWT may improve the incidence of SWI in the short term, but may not translate into better long-term outcomes.
Diaconu et al.40 suggested that the use of incisional NPWT in wall repair associated with panniculectomy, decreases non-infectious SWC and consequent reoperation in a patient population at high risk of SWC. Finally, Olona et al.41 apply the benefit of instillation to the incisional NPWT device, preventing local postoperative complications and resulting in a shorter hospital stay.
Although prophylactic NPWT reduces wound morbidity in some surgical populations, it does not appear to offer this advantage in wall reconstruction in contaminated fields.42 A recent meta-analysis43demonstrated that incisional NPWT is cost-saving when used in high-risk patients. The study concludes that in 829 patients undergoing complex wall reconstruction (260 with NPWT dressing and 569 with standard dressings), this therapy resulted in an estimated cost saving of $1542 and could be a cost-effective option, more so when the estimated rate of SWI in this risk population may be higher than 16%.
Lastly, Bueno-Lledó et al.44 provide the only clinical trial in this regard, concluding that the use of prophylactic incisional NPWT dressing after ACS, transversus abdominis release (TAR) and Rives-Stoppa repairs significantly reduced the overall incidence of SWC and SWI at 30 days postoperatively. However, no significant differences in seroma, haematoma, wound dehiscence or length of hospital stay were observed between the groups.
In some cases, prophylactic management of incisional NPWT may have the added benefit of a theoretical reduction in hernia recurrence45; but most of the studies reviewed conclude that this therapy does not substantially influence the occurrence or not of hernia recurrence. Thus, some patients who experienced SWI and subsequent CPI required complete mesh excision for resolution, which may have led to an increased likelihood of developing a recurrence of their original hernia.45 This hypothesis requires future studies to clarify the differences in this respect between the incisional NPWT and groups of patients with conventional dressings.
As final conclusions, the role of NPWT in the field of abdominal wall surgery should be highlighted. As we have seen, this therapy constitutes a support in the temporary closure of the abdominal cavity after complications derived from hernia repair, especially in cases of the need for surgical wound closure by second intention, or in the management of the open abdomen with fascial traction. Finally, we must underline the support that this therapy provides to the general surgeon in complications as relevant as CPI, with the possibility of trying to save the surgical mesh, and in the prophylaxis of SWC after abdominal wall repair.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Bueno-Lledó J, Martínez-Hoed J, Pous-Serrano S. Terapia de presión negativa en cirugía de la pared abdominal. Cir Esp. 2022;100:464–471.