Journal Information
Share
Download PDF
More article options
Methodological letter
Available online 13 September 2024
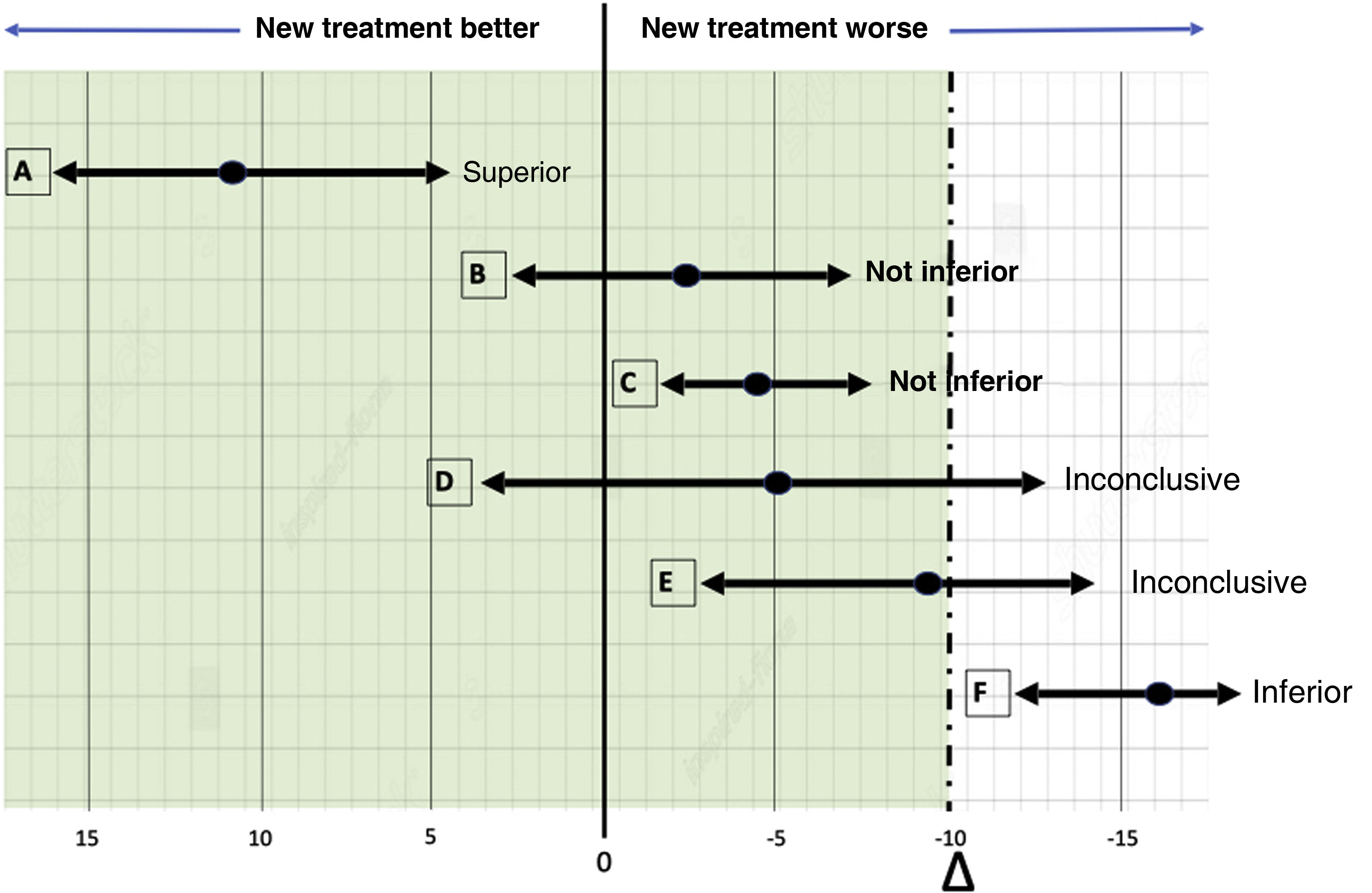
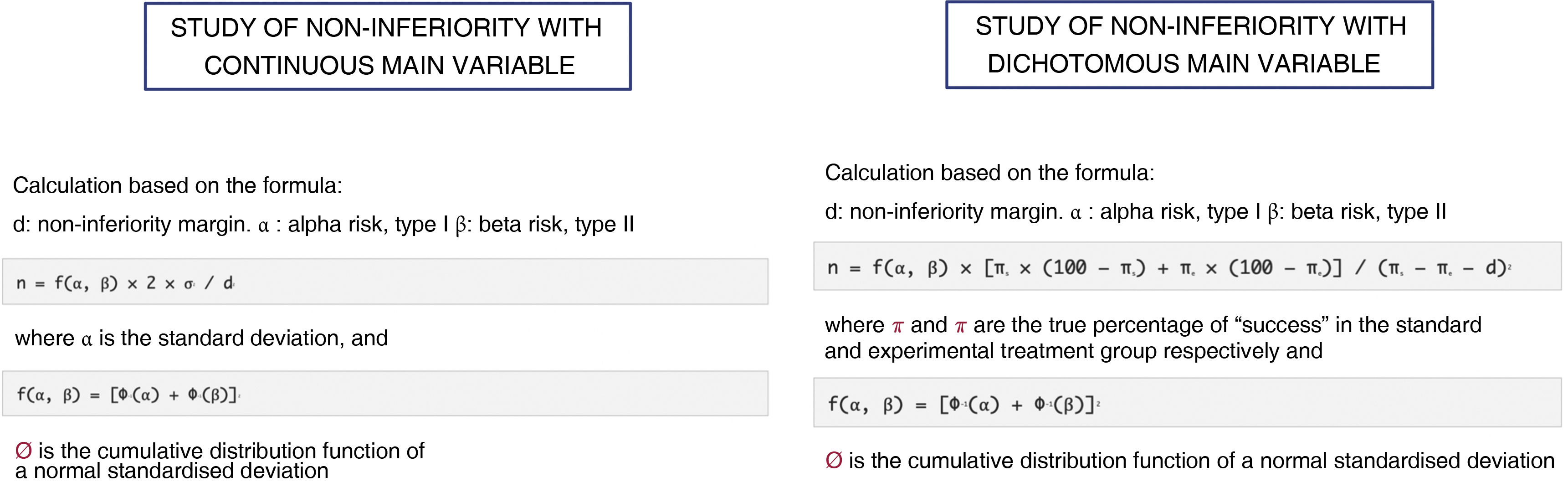
Non-inferiority clinical trial. Features and practical considerations
Ensayo clínico de no inferioridad. Características y consideraciones prácticas
Visits
1
a Consorci Corporació Sanità ria Parc Taulí CCSPT, Institut d’Investigació i Innovació Parc Taulí I3PT-CERCA, Departamento de Cirugía, Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain
b Responsable de la Unitat de Suport a la Recerca (USR), Institut d’Investigació i Innovació Parc Taulí I3PT-CERCA, Sabadell, Barcelona, Spain
This item has received
Article information
These are the options to access the full texts of the publication Cirugía Española (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail