Pancreatic hamartomas (PH) represent less than 1% of all hamartomas. They are composed of disorganized acinar, islet, and ductal cells1,2. Given their low incidence, it is difficult to differentiate them from other low-grade benign or malignant tumors, requiring pathological and immunohistochemical studies of the surgical specimen for definitive diagnosis2. We present the first case of PH described in Spain.
A 41-year-old male patient was evaluated at our hospital for an incidental finding of abdominal magnetic resonance imaging (MRI) of a 17 × 12 × 15 mm pancreatic mass. Blood levels of amylase, bilirubin, carcinoembryonic antigen, Ca 19-9, gastrin, and neuron-specific enolase were normal, as were 5-hydroxyindoleacetic acid levels.
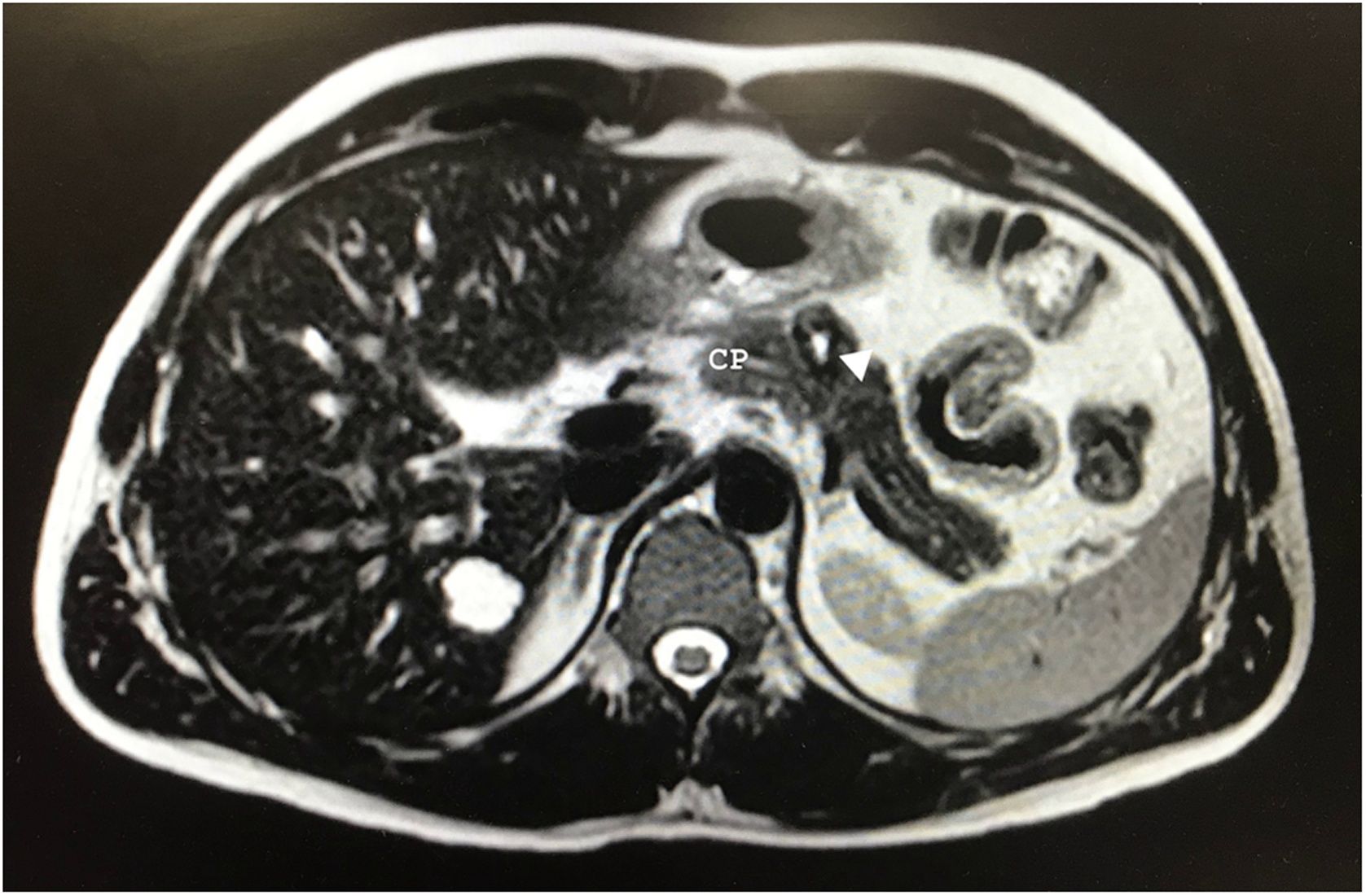
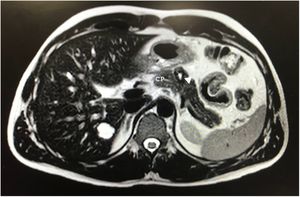
After 10 months of follow-up, the abdominal MRI revealed a lesion in the pancreatic body measuring 20 × 18 × 17 mm that was hyperintense on T2, suggestive of a cystic component vs central necrosis, and slightly hypointense in the periphery with progressive enhancement in the portal and late phase (Fig. 1). Given the presence of a solid component, malignancy could not be ruled out. We suspected a neuroendocrine tumor and requested an octreotide scan, which was normal.
Endoscopic ultrasound revealed a 19 mm lesion in the body of the pancreas that was hypoechogenic, well defined and solid with a central cystic area. Fine-needle aspiration biopsy showed a columnar epithelium with mucinous cells and no atypia. The patient was initially diagnosed with a mucinous tumor (not intraductal mucinous papillary tumor), ruling out solid pseudopapillary tumor (SPT) or a neuroendocrine tumor.
Given the patient’s age, the limited growth of the lesion and the inability to clarify its nature, we performed open spleen-preserving distal pancreatectomy (DP). Macroscopically, the mass was firm and whitish, measuring 1.8 × 1.8 cm. Microscopically, it consisted of randomly distributed ductal, acinar, and neuroendocrine structures embedded in a fibrocellular stroma, with no significant inflammatory infiltrates. Immunohistochemically, the stroma was positive for ß-catenin; negative for IgG, IgG4, synaptophysin, chromogranin, actin, CD34, S100, and Bcl2. In the end, the patient was diagnosed with PH.
The patient developed a type A pancreatic fistula, which was treated conservatively, and he was discharged on the 7th postoperative day with a pancreatic drainage tube, which was removed on the 10th postoperative day. After 5 months of follow-up, he has not presented recurrence of the disease.
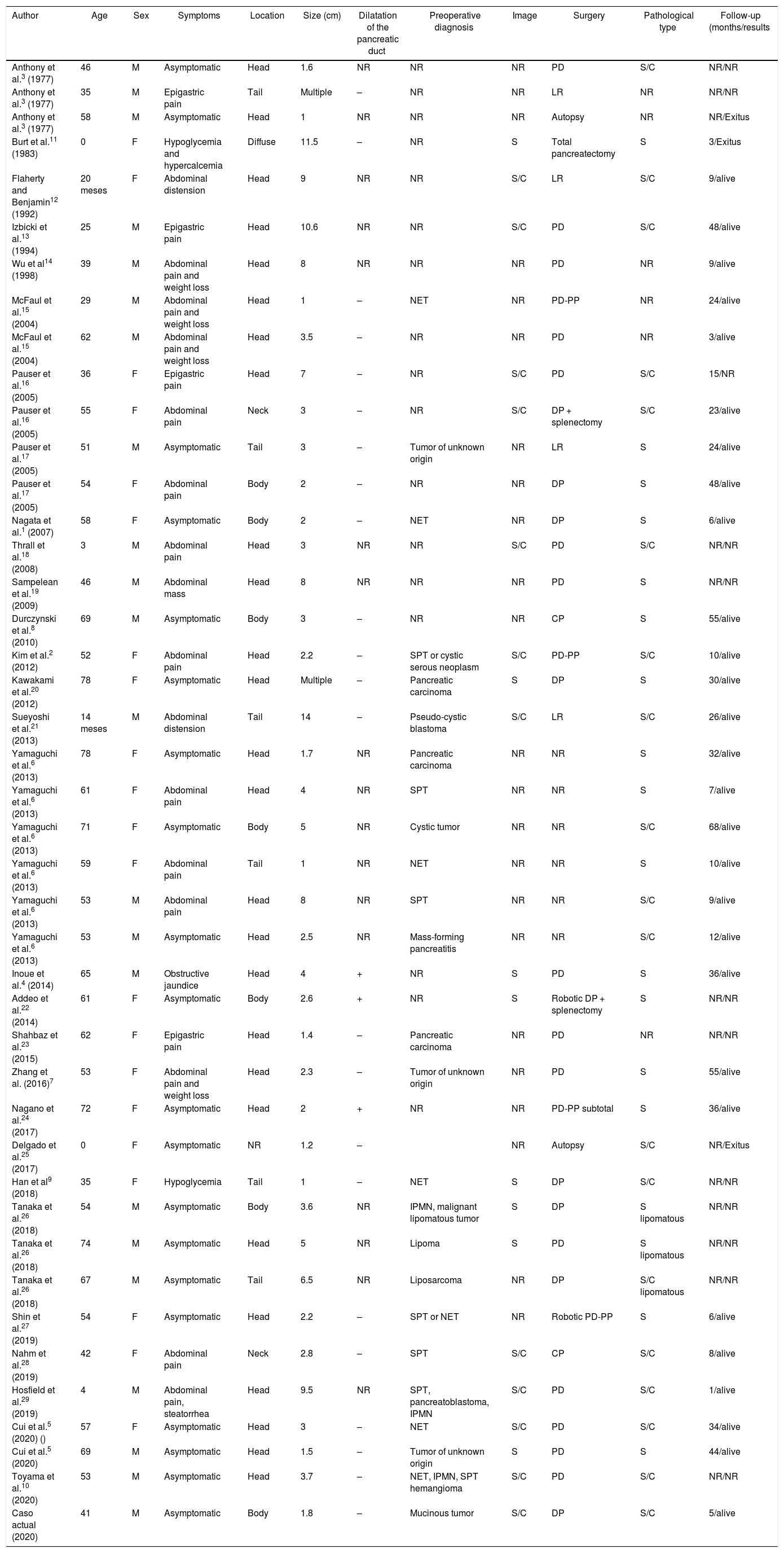
PH are extremely rare. Described for the first time in 1977 by Anthony et al.3, 43 cases have been published since, including the present one (Table 1). They can appear at any age, although the average age is 40–60 years. There is no tendency for PH to affect either sex. Most are either diagnosed incidentally or present with nonspecific signs and symptoms, such as abdominal pain or weight loss. Only one case has been reported that began with obstructive jaundice4. PH can appear anywhere in the pancreas, although most frequently in the head, with a size of 1.0–14.0 cm5.
Clinical-pathological characteristics of pancreatic hamartomas described in the literature (n = 43).
| Author | Age | Sex | Symptoms | Location | Size (cm) | Dilatation of the pancreatic duct | Preoperative diagnosis | Image | Surgery | Pathological type | Follow-up (months/results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthony et al.3 (1977) | 46 | M | Asymptomatic | Head | 1.6 | NR | NR | NR | PD | S/C | NR/NR |
| Anthony et al.3 (1977) | 35 | M | Epigastric pain | Tail | Multiple | – | NR | NR | LR | NR | NR/NR |
| Anthony et al.3 (1977) | 58 | M | Asymptomatic | Head | 1 | NR | NR | NR | Autopsy | NR | NR/Exitus |
| Burt et al.11 (1983) | 0 | F | Hypoglycemia and hypercalcemia | Diffuse | 11.5 | – | NR | S | Total pancreatectomy | S | 3/Exitus |
| Flaherty and Benjamin12 (1992) | 20 meses | F | Abdominal distension | Head | 9 | NR | NR | S/C | LR | S/C | 9/alive |
| Izbicki et al.13 (1994) | 25 | M | Epigastric pain | Head | 10.6 | NR | NR | S/C | PD | S/C | 48/alive |
| Wu et al14 (1998) | 39 | M | Abdominal pain and weight loss | Head | 8 | NR | NR | NR | PD | NR | 9/alive |
| McFaul et al.15 (2004) | 29 | M | Abdominal pain and weight loss | Head | 1 | – | NET | NR | PD-PP | NR | 24/alive |
| McFaul et al.15 (2004) | 62 | M | Abdominal pain and weight loss | Head | 3.5 | – | NR | NR | PD | NR | 3/alive |
| Pauser et al.16 (2005) | 36 | F | Epigastric pain | Head | 7 | – | NR | S/C | PD | S/C | 15/NR |
| Pauser et al.16 (2005) | 55 | F | Abdominal pain | Neck | 3 | – | NR | S/C | DP + splenectomy | S/C | 23/alive |
| Pauser et al.17 (2005) | 51 | M | Asymptomatic | Tail | 3 | – | Tumor of unknown origin | NR | LR | S | 24/alive |
| Pauser et al.17 (2005) | 54 | F | Abdominal pain | Body | 2 | – | NR | NR | DP | S | 48/alive |
| Nagata et al.1 (2007) | 58 | F | Asymptomatic | Body | 2 | – | NET | NR | DP | S | 6/alive |
| Thrall et al.18 (2008) | 3 | M | Abdominal pain | Head | 3 | NR | NR | S/C | PD | S/C | NR/NR |
| Sampelean et al.19 (2009) | 46 | M | Abdominal mass | Head | 8 | NR | NR | NR | PD | S | NR/NR |
| Durczynski et al.8 (2010) | 69 | M | Asymptomatic | Body | 3 | – | NR | NR | CP | S | 55/alive |
| Kim et al.2 (2012) | 52 | F | Abdominal pain | Head | 2.2 | – | SPT or cystic serous neoplasm | S/C | PD-PP | S/C | 10/alive |
| Kawakami et al.20 (2012) | 78 | F | Asymptomatic | Head | Multiple | – | Pancreatic carcinoma | S | DP | S | 30/alive |
| Sueyoshi et al.21 (2013) | 14 meses | M | Abdominal distension | Tail | 14 | – | Pseudo-cystic blastoma | S/C | LR | S/C | 26/alive |
| Yamaguchi et al.6 (2013) | 78 | F | Asymptomatic | Head | 1.7 | NR | Pancreatic carcinoma | NR | NR | S | 32/alive |
| Yamaguchi et al.6 (2013) | 61 | F | Abdominal pain | Head | 4 | NR | SPT | NR | NR | S | 7/alive |
| Yamaguchi et al.6 (2013) | 71 | F | Asymptomatic | Body | 5 | NR | Cystic tumor | NR | NR | S/C | 68/alive |
| Yamaguchi et al.6 (2013) | 59 | F | Abdominal pain | Tail | 1 | NR | NET | NR | NR | S | 10/alive |
| Yamaguchi et al.6 (2013) | 53 | M | Abdominal pain | Head | 8 | NR | SPT | NR | NR | S/C | 9/alive |
| Yamaguchi et al.6 (2013) | 53 | M | Asymptomatic | Head | 2.5 | NR | Mass-forming pancreatitis | NR | NR | S/C | 12/alive |
| Inoue et al.4 (2014) | 65 | M | Obstructive jaundice | Head | 4 | + | NR | S | PD | S | 36/alive |
| Addeo et al.22 (2014) | 61 | F | Asymptomatic | Body | 2.6 | + | NR | S | Robotic DP + splenectomy | S | NR/NR |
| Shahbaz et al.23 (2015) | 62 | F | Epigastric pain | Head | 1.4 | – | Pancreatic carcinoma | NR | PD | NR | NR/NR |
| Zhang et al. (2016)7 | 53 | F | Abdominal pain and weight loss | Head | 2.3 | – | Tumor of unknown origin | NR | PD | S | 55/alive |
| Nagano et al.24 (2017) | 72 | F | Asymptomatic | Head | 2 | + | NR | NR | PD-PP subtotal | S | 36/alive |
| Delgado et al.25 (2017) | 0 | F | Asymptomatic | NR | 1.2 | – | NR | Autopsy | S/C | NR/Exitus | |
| Han et al9 (2018) | 35 | F | Hypoglycemia | Tail | 1 | – | NET | S | DP | S/C | NR/NR |
| Tanaka et al.26 (2018) | 54 | M | Asymptomatic | Body | 3.6 | NR | IPMN, malignant lipomatous tumor | S | DP | S lipomatous | NR/NR |
| Tanaka et al.26 (2018) | 74 | M | Asymptomatic | Head | 5 | NR | Lipoma | S | PD | S lipomatous | NR/NR |
| Tanaka et al.26 (2018) | 67 | M | Asymptomatic | Tail | 6.5 | NR | Liposarcoma | NR | DP | S/C lipomatous | NR/NR |
| Shin et al.27 (2019) | 54 | F | Asymptomatic | Head | 2.2 | – | SPT or NET | NR | Robotic PD-PP | S | 6/alive |
| Nahm et al.28 (2019) | 42 | F | Abdominal pain | Neck | 2.8 | – | SPT | S/C | CP | S/C | 8/alive |
| Hosfield et al.29 (2019) | 4 | M | Abdominal pain, steatorrhea | Head | 9.5 | NR | SPT, pancreatoblastoma, IPMN | S/C | PD | S/C | 1/alive |
| Cui et al.5 (2020) () | 57 | F | Asymptomatic | Head | 3 | – | NET | S/C | PD | S/C | 34/alive |
| Cui et al.5 (2020) | 69 | M | Asymptomatic | Head | 1.5 | – | Tumor of unknown origin | S | PD | S | 44/alive |
| Toyama et al.10 (2020) | 53 | M | Asymptomatic | Head | 3.7 | – | NET, IPMN, SPT hemangioma | S/C | PD | S/C | NR/NR |
| Caso actual (2020) | 41 | M | Asymptomatic | Body | 1.8 | – | Mucinous tumor | S/C | DP | S/C | 5/alive |
PD: pancreaticoduodenectomy; PD-PP: PD with preservation of the pylorus; F: female; M: male; NR: no reported; CP: central pancreatectomy; DP: distal pancreatectomy; LR: local resection; S: solid; S/C: solid and cystic; NET: neuroendocrine tumor; IPMN: intraductal papillary mucinous neoplasms; SPT: solid pseudopapillary tumour.
11Pediatr Radiol. 1983;13:287-9.
12Hum Pathol. 1992;23:1309-12.
13Am J Gastroenterol. 1994;89:1261-2.
14Histopathology. 1998;33:485-7.
15Pancreatology. 2004;4:533-8.
16Am J Surg Pathol. 2005;29:797-800.
17Mod Pathol. 2005;18:1211-6.
18Pediatr Dev Pathol. 2008;11:314-20.
19J Gastrointest Liver Dis. 2009;18:483-486.
20World J Gastrointest Oncol. 2012;4:202.
21Int J Surg Case Rep. 2013;4:98-100.
22Surg (United States). 2014;156:1284-5.
23Am J Gastroenterol. 2015;110:S109.
24BMC Gastroenterol. 2017;17(1):146.
25Autops Case Reports. 2017;7(4):26-29.
26Am J Surg Pathol. 2018;42(7):891-7.
27Ann Hepato-Biliary-Pancreatic Surg. 2019;23:286.
28ANZ J Surg. 2019;89:E265-E267.
29J Pediatr Surg Case Reports. 2019;48:101258.
Two types of PH are distinguished according to the macroscopic findings: solid type and solid-cystic type6. On ultrasound, they appear to be hypoechoic masses with well-defined margins4,5,7,8.
On computed tomography scans, PH are usually well defined, with a slightly unequal density in solid lesions, hypo- or isodense, with progressive heterogeneous enhancement in late phases. The margins are typically well defined after contrast enhancement1,2,5,9. Contrarily, pancreatic adenocarcinomas are characterized by low enhancement and the invasion of adjacent structures, while NET show clear enhancement in the initial phases after contrast5,9. SPT are similar but usually present encapsulation and peripheral calcifications10. Therefore, PH should be included in the differential diagnosis of pancreatic incidentalomas.
On MRI, the contour is regular, with well-defined edges in T2. The intensity of the interior of the lesion is slightly heterogeneous, hypointense on T1 and iso- or hyperintense on T25,7,9.
Microscopically, PH are composed of a variable proportion of acinar, ductal, and endocrine cells arranged in a disorganized manner, so fine-needle aspiration is not effective. The solid component consists of fibroadipose tissue, while the cystic component is made up of dilated pancreatic ducts. They present differentiated acinar cells, without forming well-organized lobules1,6,7, and characteristically lack 3 structures: concentric elastic fibers around the pancreatic ducts, peripheral nerves, and well-formed Langerhans islets. This distinguishes PH from chronic pancreatitis and hamartoma of the pancreatic duct, where these 3 structures are well preserved6.
Although PH is a benign disease, most patients undergo surgical resection because it is impossible to rule out malignant disease. Given the higher frequency of malignancy or premalignancy in pancreatic incidentalomas, surgery is the indicated treatment, be it pancreaticoduodenectomy, DP, or conservative surgery (central pancreatectomy or enucleation), even though this may be too aggressive in the case of benign tumors8.
Some authors suggest that conservative surgery should be performed to preserve the integrity of the gastrointestinal tract and the endocrine and exocrine function of the pancreas and spleen due to the indolent nature of this tumor7. We opted for spleen-preserving DP due to the initial suspicion, and we decided against performing parenchyma-sparing surgery due to the risk of fistula, given the proximity of the tumor to the main pancreatic duct (Fig. 1).
Please cite this article as: Santana Valenciano Á, Molina Villar JM, Barranquero AG, Sanjuanbenito Dehesa A, Fernández Cebrián JM. Hamartoma pancreático: una causa benigna y poco frecuente de incidentaloma pancreático. Cir Esp. 2022;100:251–255.