To investigate magnetic resonance imaging (MRI) accuracy for determining the location of rectal tumors with respect to the peritoneal reflection (PR) and its potential involvement.
MethodsProspective study of 161 patients ongoing surgery for rectal cancer. A double-ink method has been aplied to examine surgical specimen, orange ink for the serosal surface and indian ink for the mesorrectal margin, and assess preoperative MRI accuracy.
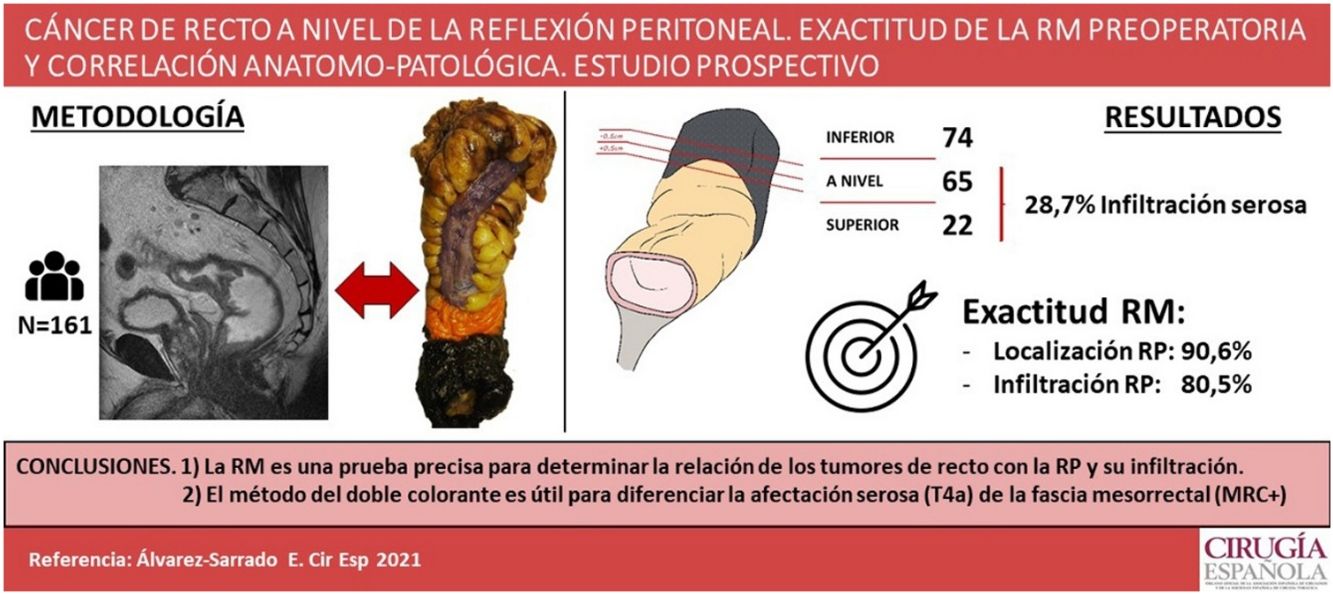
ResultsTwenty-two tumors were located above, 65 at and 74 below PR. MRI accuracy was 90.6% for determining tumor’s location with respect to the PR and 80.5% for defining peritoneal involvement. For classifying tumors according to their intra or extraperitoneal location an accuracy of 92.5% was set for MRI. Histophatologic peritoneal involvement was found in 28.7% of tumors located above or at the PR.
ConclusionsMagnetic resonance imaging accurately predicts the location of rectal tumors with respect to the PR and its potential involvement. The double-ink method is useful to assess serosal involvement (pT4a) and to distinguish mesorrectal fascia from the peritonealized surface.
Establecer la exactitud de la resonancia magnética (RM) para determinar la localización de los tumores rectales en relación a la reflexión peritoneal (RP) y su potencial afectación.
MétodosEstudio prospectivo de 161 pacientes intervenidos por cáncer de recto. Las piezas quirúrgicas han sido analizadas mediante un método de doble tinción, superficie serosa con colorante naranja y grasa mesorrectal con tinta china, para comparar los resultados con la RM preoperatoria.
ResultadosVeintidós tumores se localizaron por encima, 65 a nivel y 74 por debajo de la RP. La RM clasificó la localización del tumor respecto a la RP de manera correcta en el 90,6% y fue capaz de detectar el 80,5% de los casos con infiltración de la RP. La RM presentó una exactitud del 92,5% para clasificar el tumor como intra o extraperitoneal. El 28,7% de los tumores por encima y a nivel de la RP presentaba anatomopatológicamente infiltración de la serosa peritoneal.
ConclusionesLa RM es una prueba precisa para determinar la localización de los tumores de recto en relación con la reflexión peritoneal y su posible afectación. En el tallado macroscópico, el método de doble colorante es eficaz para determinar la afectación serosa (pT4a) y diferenciarla de la fascia mesorrectal.
In rectal cancer surgery, circumferential resection margin (CRM) involvement is considered the most important factor related to local and systemic recurrence1–5. Magnetic resonance imaging (MRI) is essential in attempting to reduce the rate of CRM involvement in the preoperative planning of mesorectal excision and determining the indication for neoadjuvant chemoradiotherapy (CRT), primarily due to the potential involvement of the mesorectal fascia1,4.
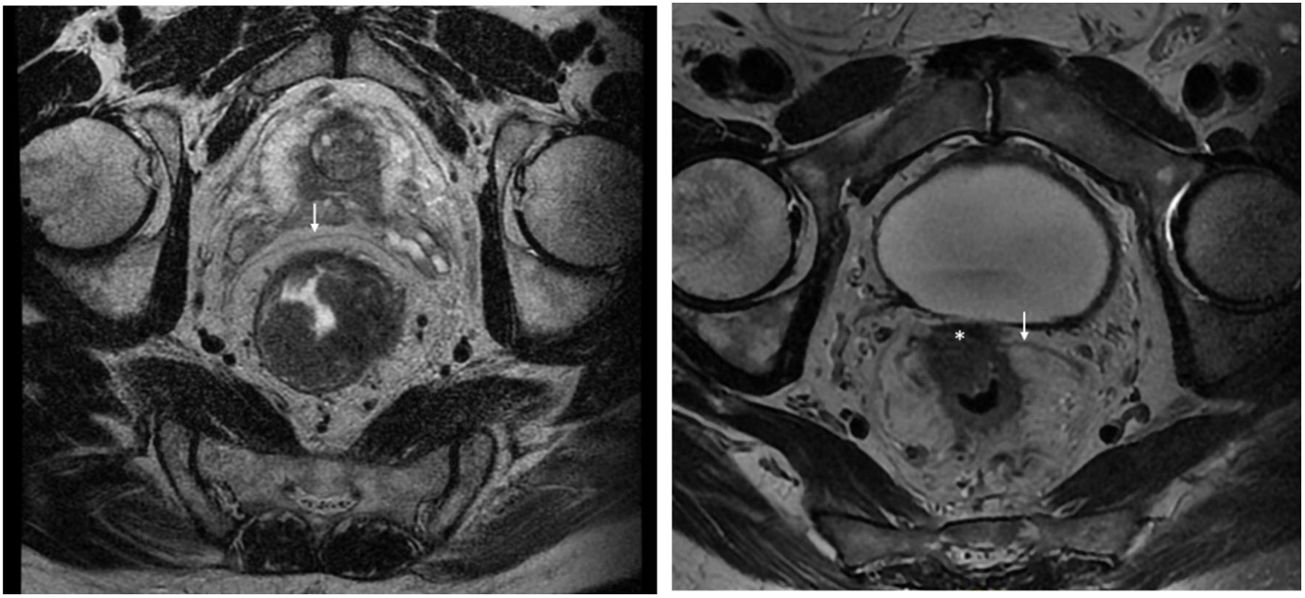
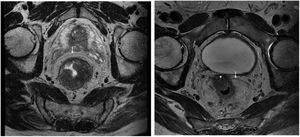
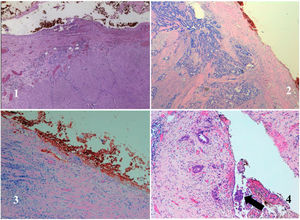
Rectal tumours located above the peritoneal reflection (PR) and presenting with anterior serosal infiltration should be classified as T4a and CRM should not be considered6. Anatomically, the mesorectum is very thin anteriorly, and therefore tumours located at the level of the PR could potentially involve both the mesorectal fascia and the peritoneal serosa. In this regard, the MERCURY study highlights that involvement of the PR should be reported on MRI and histopathologically considered CRM negative, as the tumour is in contact with the peritoneal cavity (Fig. 1)7. Preoperative staging by MRI to differentiate between CRM involvement and infiltration of the PR is a potentially important factor in selecting the type of neoadjuvant treatment. However, this distinction regarding tumour location with respect to the PR and its infiltration has not been specifically analysed in published results for upper third rectal cancer8,9. The ESMO guidelines state that these tumours should be treated as a sigmoid colon tumour, and only recommend neoadjuvant CRT in upper rectal cancer when extension to neighbouring structures or CRM involvement is detected by imaging studies1,10.
However, studies published by Shepherd have shown that T4a tumours involving the peritoneal serosa are associated with tumour recurrence and have an increased risk of peritoneal carcinomatosis11,12. This involvement can be quantified histopathologically by establishing 4 progressive grades that have been related to overall survival.
Therefore, it seems important to improve the anatomopathological assessment of rectal cancer in these locations, in relation to CRM, PR and the grade of histopathological involvement of the visceral peritoneum in T4a tumours. The aim of this study is to evaluate the accuracy of MRI in determining the location of rectal tumours in relation to the PR and its infiltration using the anatomopathological study of the surgical specimen as a reference method.
MethodsA prospective, observational study of 161 patients who underwent surgery for rectal neoplasia with curative intent between 2016 and 2019. The study was assessed and approved by the research committee and the centre’s ethics committee.
All patients over 18 years of age diagnosed with a rectal neoplasm and undergoing mesorectal excision with local curative intent were included. Patients with tumours other than adenocarcinoma and patients undergoing surgery for local recurrence after previous resection were excluded. Epidemiological variables were collected, such as age, sex and comorbidities, and physical examination data, such as tumour height by means of rigid rectoscopy.
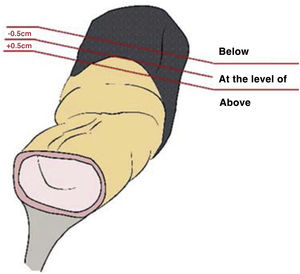
Staging MRI for rectal cancer was performed with a 1.5T phased-array coil (General Electric Healthcare España). The protocol for rectal cancer staging involves T2-weighted images in the 3 planes and an axial diffusion-weighted image, and they were assessed by expert radiologists and discussed in the multidisciplinary group. The PR was assessed in the 3 planes (axial, sagittal and coronal) and tumours located at ±5 mm from the line determined as PR were classified as tumours at the level of the PR. MRI was used to determine the height of the tumour, its circumferential location, depth of penetration, CRM status, location with respect to the PR and its infiltration, the presence of adenopathies, or EVI, among other elements.
The criteria for the indication of neoadjuvant treatment were as follows: T3cd-T4b tumours, threatened or involved CRM, presence of extramural venous invasion, N2 or extramesorectal adenopathies and involvement of the levator or sphincters.
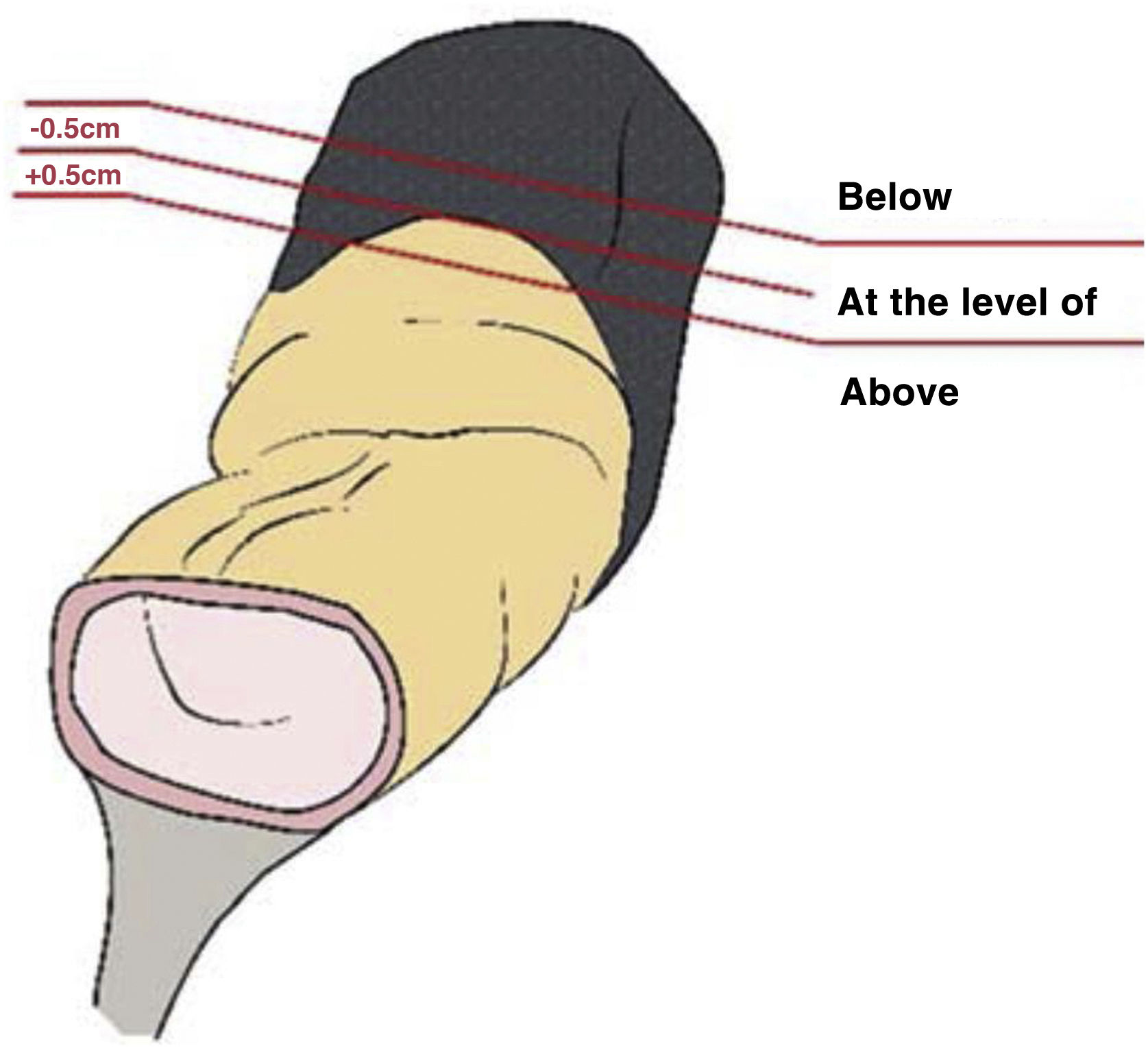
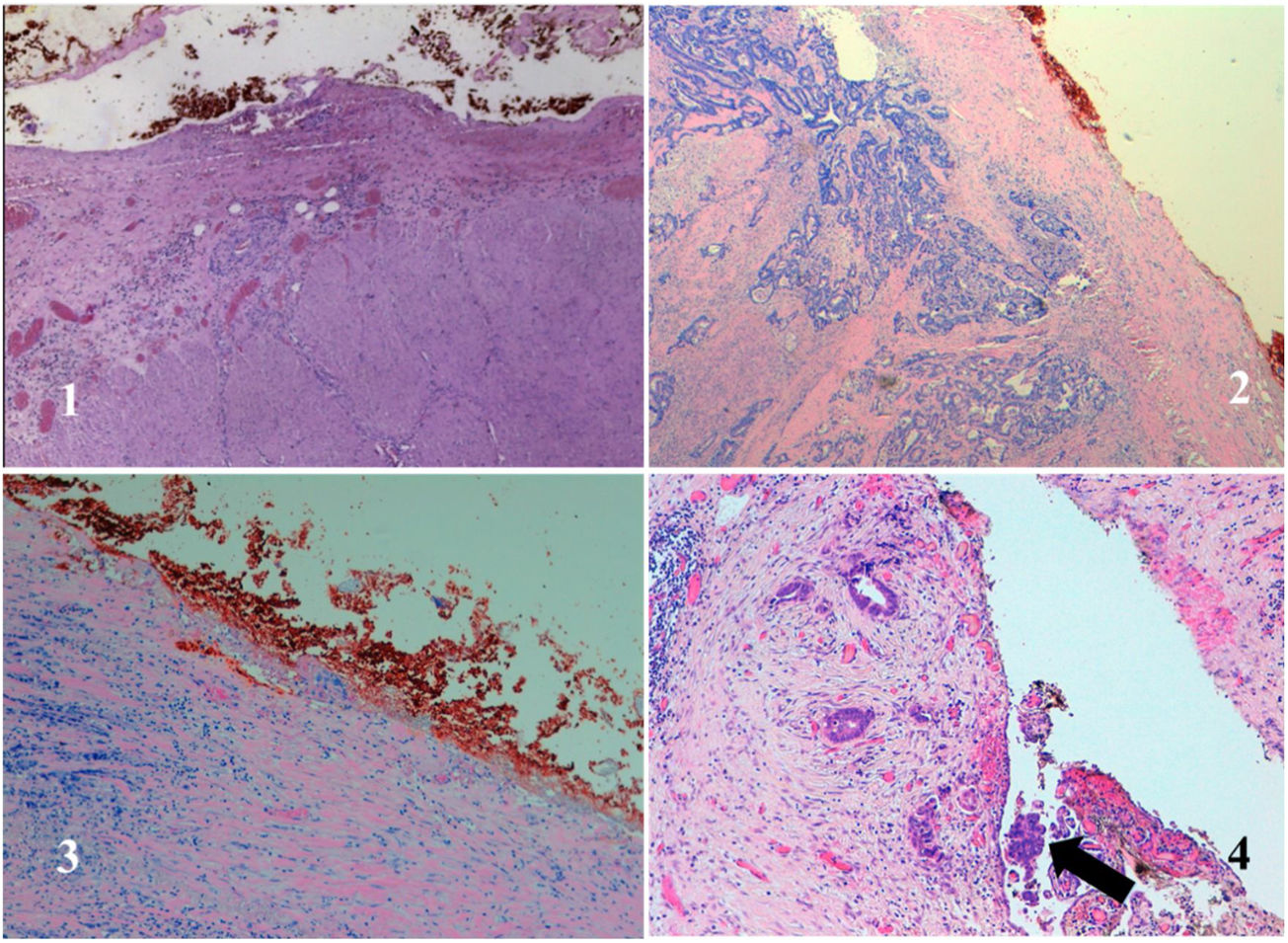
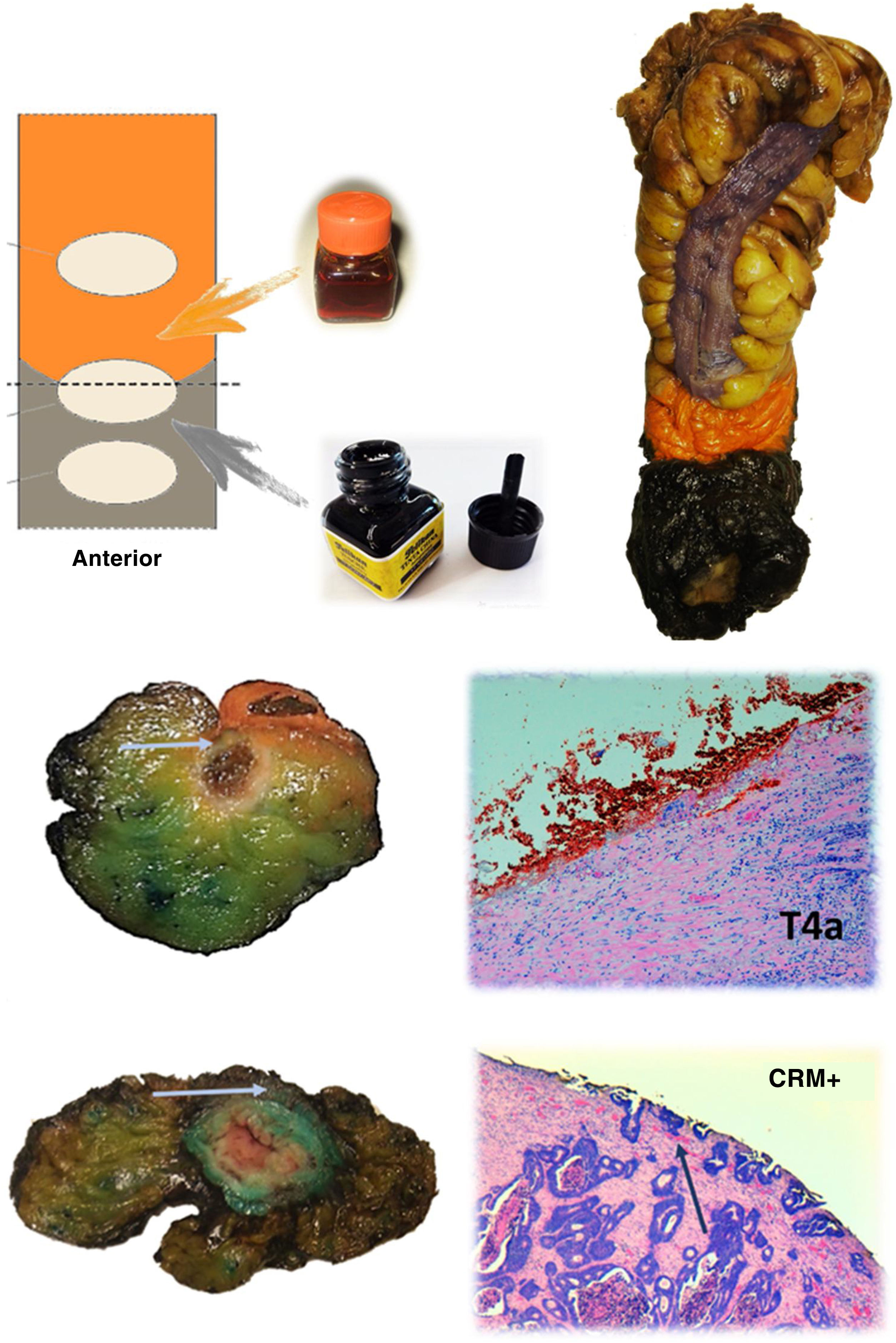
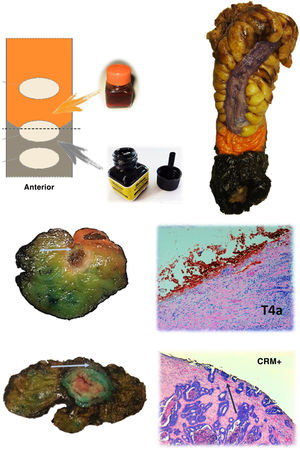
The anatomopathological study of the surgical specimen was performed by expert pathologists attached to the multidisciplinary group on colorectal tumours. To analyse the macroscopic relationship between the tumour and the PR, the mesorectal margin was painted with India ink and the PR with orange ink, and the specimens were systematically photographed. Tumours located at the level of the PR or within 5 mm of it were considered level for the purposes of this study, as detailed in Fig. 2. Once the blocks were carved and stained, serosal infiltration was specifically analysed as per the 4 grades described by Shepherd. Grades 1 and 2 were considered free of infiltration and grades 3 and 4 as infiltration (Fig. 3)11. The anatomopathological study includes macroscopic data, such as the size of the tumour, its location with respect to the PR and distal margin, and microscopic data, such as the depth of infiltration, CRM status, the presence of lymph node metastases or EVI, and infiltration of the PR determined using Shepherd’s grades.
SPSS v22 was used for statistical analysis. Categorical variables were compared using the χ2 statistic and Fisher’s exact test, while continuous variables were compared using the non-parametric Mann–Whitney U test. The non-parametric Kruskal–Wallis test was used to compare several groups. The accuracy of the MRI was assessed by means of contingency tables expressing the degree of agreement using the Kappa index (KI): weak 0–.4; moderate .41–.6; good .61–.8; very good .81–1 (Fig. 4).
Protocol for staining the surgical specimen. The mesorectal fat is stained with India ink and the serosal surface with orange dye. In the lower part, axial sections of 2 different surgical specimens and histological sections showing involvement of the resection border at the level of the orange dye (T4a) and the India ink (CRM involvement). The arrows point to the tumour front in both macroscopic and microscopic sections.
The median age of the patients was 65 years (range 36–89) and 56.5% were male. Forty-four tumours were located in the upper third (27.3%), 63 in the middle third (39.1%), and 54 in the lower third (33.5%) (Table 1).
Descriptive data of the series.
| Variable | N | % |
|---|---|---|
| Median age | 65 | |
| Sex (M/F) | 91/70 | 56.5/43.5 |
| Location of the tumour (MRI) | ||
| Upper third | 44 | 27.3 |
| Middle third | 63 | 39.1 |
| Lower third | 54 | 33.5 |
| Adenopathies (MRI+) | ||
| Locoregional | 90 | 55.9 |
| Extramesorectal | 19 | 11.8 |
| Distant metastases (cM1) | 11 | 6.8 |
| CRM (MRI) | ||
| Free | 102 | 63.4 |
| Threatened | 29 | 18 |
| Involved | 30 | 18.6 |
| Neoadyuvant | ||
| CRT | 61 | 37.9 |
| CT | 3 | 1.9 |
| Intervention performed | ||
| LAR | 43 | 26.7 |
| ULAR | 55 | 34.2 |
| Extraelevator APE | 36 | 22.4 |
| Intersphincteric APE | 10 | 6.2 |
| Pelvic exenteration | 7 | 4.3 |
| Hartmann | 8 | 5 |
| TaTME | 2 | 1.2 |
| Laparoscopy | 103 | 62.1 |
| Quality of the mesorectum | ||
| Satisfactory | 126 | 78.3 |
| Partially satisfactory | 19 | 11.8 |
| Unsatisfactory | 16 | 9.9 |
| Type of resection | ||
| R0 | 146 | 92.7 |
| R1 | 15 | 9.3 |
At diagnosis, 90 patients (55.9%) had suspicious locoregional adenopathies and 19 patients had extramesorectal adenopathies (11.8%). On preoperative MRI the CRM was considered threatened in 29 patients (18%), and involved in 30 (18.6%). Sixty-four patients received neoadjuvant treatment, 61 of them pelvic CRT. The quality of mesorectal excision was satisfactory in 78.3%, and partially satisfactory in 11.8% of the cases.
After anatomopathological study of the surgical specimen, 22 tumours were located above the PR, 65 at its level and 74 below. Ninety-seven point seven percent of the tumours of the upper rectum, 65.1% of those of the middle third and 5.6% of those of the lower third were located at the level of or above the PR. Most tumours (65.2%) were moderately differentiated. Lymphatic embolization was found in 40 cases (24.8%), vascular in 24 (14.9%), and neural in 34 (21.1%). Of these, all 3 types of invasion coexisted in 20 cases. In addition, serous involvement was demonstrated in 28.7% of the tumours at the level of or above the PR: 17.2% Shepherd's grade 3 and 11.5% Shepherd’s grade 4. The CRM was involved in 14 pieces (8.7%), while the distal border was involved in one case (.6%).
Correlation MRI-anatomopathological studyMRI correctly classified the location of the tumour with respect to the PR in 86.3% of cases (IK .77), considering the whole series, and 90.6% if we consider only the cases without neoadjuvant treatment (IK .81). Likewise, for classifying the tumour as intraperitoneal or extraperitoneal, MRI showed an accuracy of 92.5%, with an IK of .87 (Table 1).
For tumours at the level of and above the PR, the accuracy of MRI for determining serosal involvement was 80.5%, with an IK of .51. Sensitivity and specificity were 64% and 87.1%, respectively. Specifically regarding cases with serosal involvement, MRI detected 60% of Shepherd grade 3 cases and 70% of grade 4 cases.
The CRM was involved in 14 specimens (8.7%). Of these, the quality of mesorectal excision was satisfactory in 64% and partially satisfactory in 36%. The accuracy of MRI for determining CRM status was 92.7%, with an IK of .42. Sensitivity and specificity were 42.9% and 96.6%, respectively (Table 2).
Accuracy of MRI in different parameters taking the anatomopathological study of the surgical specimen as a reference.
| Variables | MRI | AP | Overall accuracy | Kappa Index |
|---|---|---|---|---|
| Relationship with PR | 86.3% (90.6% without CRT) | .77 (.81 without CRT) | ||
| Above | 20 | 22 | ||
| At the level of | 69 | 65 | ||
| Below | 72 | 74 | ||
| Location | 92.5% | .87 | ||
| Intraperitoneal | 37 | 38 | ||
| Intra-extra | 38 | 36 | ||
| Extraperitoneal | 86 | 87 | ||
| Serosal involvement | 80.5% | .51 | ||
| No: Shepherd 1 | 63 | 52 | ||
| Shepherd 2 | 10 | |||
| Yes: Shepherd 3 | 24 | 15 | ||
| Shepherd 4 | 10 | |||
| T stage | 59.8% | .38 | ||
| 32 | 83 | |||
| T3 | 82 | 59 | ||
| T4a | 11 | 20 | ||
| T4b | 36 | 15 | ||
| N stage | 54% | .1 | ||
| N0 | 71 | 99 | ||
| N1 | 51 | 37 | ||
| N2 | 33 | 25 | ||
| CRM | 92.7% | .42 | ||
| Free | 102 | 141 | ||
| Threatened | 29 | |||
| Involved | 30 | 13 |
Definitive pathological T-staging classified 67 pieces as pT2 or lower, 59 as pT3, and 35 as pT4. The accuracy of MRI was 59.8%, with an IK of .38. Overstaging occurred in 34.8% of cases, mainly due to classifying tumours as T3 that had ultimately not reached the mesorectal fat.
There was a median of 21 isolated nodes (range 2–69). Tumour adenopathy was identified in 38.5% of the patients. The accuracy of MRI for detecting pathological adenopathy was 54%, with an IK of .1.
DiscussionThe present study was designed, within the multidisciplinary group, to prospectively analyse the location by MRI of rectal cancer with respect to the PR, and to establish a correlation with anatomopathological characteristics.
Location of the PR varies greatly; it can even be located in the middle third of the rectum, and is an important factor in staging locally advanced rectal cancer. In the present study, 97.7% of the tumours of the upper rectum, 65.1% of the tumours of the middle rectum, and 5.6% of the tumours of the lower rectum were located at the level of or above the PR. Several studies have established, by intraoperative rigid rectoscopy or MRI, the distance from the PR to the anal margin to be between 5–16 cm, generally shorter in women13–15. This implies that tumours at the same distance from the anal margin may be located intraperitoneally or extraperitoneally, depending on the depth of the PR. However, this aspect has been virtually ignored and there is little scientific evidence on the prognostic implications of rectal cancer in relation to the location of the PR.
As early as 1995, Shepherd established four grades of peritoneal involvement and their possible relationship with recurrence and survival in rectal neoplasms11. The same group published long-term results in 2010, showing that PR involvement is a predictive factor for survival in patients undergoing surgery for rectal tumours12. According to the present study, the double dye method allows very precise analysis of tumour location with respect to the PR. In the present series, up to 60% of the tumours were located at or above the PR. Of these, 28.7% had serous involvement (11.5% grade 4), data similar to those published by Shepherd11.
MRI is now a fundamental tool in the preoperative local staging of rectal cancer, as it can determine with high precision T stage, CRM, extramural venous infiltration and, with less precision, lymph node involvement.7,16–19 In the present study, the accuracy of MRI in determining CRM involvement in patients who did not receive CRT was 92.7%, similar to data published in the MERCURY study and by other authors7,17.
Several studies have evaluated the ability of MRI to identify the PR20–22. However, the present study is the first to evaluate the relationship of the tumour with the PR by comparing MR images with the anatomopathological study of the surgical specimen, using a double staining method. The accuracy of MRI was 90.6% for determining tumour location with respect to the PR in patients without neoadjuvant therapy, and 92.5% for classifying it as intraperitoneal or extraperitoneal, with an excellent Kappa correlation. MRI was able to correctly determine peritoneal serosal involvement in tumours above and at the level of the PR in 80.5% of cases, with a moderate Kappa correlation. This correlation is mainly due to T4a and Shepherd’s grade 3 tumours with serosal retraction, which MRI is unable to detect as they do not present peritoneal nodules.
There is controversy about the indication for neoadjuvant CRT in upper rectal tumours. Marinello et al. specifically analysed this issue and concluded that most upper rectal tumours can be treated by subtotal excision of the mesorectum without the need for preoperative CRT, with a local recurrence rate of 4.9%, similar to that of sigmoid and rectosigmoid junction tumours9. The ESMO consensus currently states that the benefit of neoadjuvant CRT in tumours above the PR is marginal and they should therefore be treated as colon cancer with a level of evidence 1A1. Therefore, using MRI to determine the location of the tumour with respect to the PR is fundamental to discern between intra- or extraperitoneal tumours, since, in tumours above the PR, even if there is involvement of the peritoneal serosa, it is not appropriate to refer to anterior CRM involvement and there is no indication for neoadjuvant RT.
However, a possible alternative in tumours that affect the serosa at the level of the PR would be neoadjuvant CT, in accordance with the indications of the FOxTROT study23. Furthermore, as several authors have suggested, involvement of the peritoneal serosa has been associated with worse survival and higher rates of local recurrence, and should therefore be taken into account in the indication for adjuvant treatment and oncological follow-up11,24,25. In this regard, the PROPHYLOCHIP-PRODIGE 15 study failed to demonstrate the superiority of revision surgery and hyperthermic intraperitoneal chemotherapy over conventional follow-up alone in patients undergoing surgery for colorectal neoplasia with localised and resected carcinomatosis or tumour perforation during surgery26.
The present study demonstrates that preoperative MRI is a reliable test to determine the location of rectal tumours in relation to the PR with an overall accuracy of more than 90%. Furthermore, it allows differentiation between involvement of the mesorectal fascia or the peritoneal serosa, confirmed by double dye test. Finally, the present study is an excellent opportunity to assess in the near future the oncological prognostic implications on local recurrence, carcinomatosis and distant metastasis, conditioned by the existence of a T4a or positive CRM in tumours involving the PR.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Álvarez Sarrado E, Giner Segura F, Batista Domenech A, Garcia-Granero García-Fuster Á, Frasson M, Rudenko P, et al. Cáncer de recto a nivel de la reflexión peritoneal. Exactitud de la RM preoperatoria y correlación anatomopatológica. Estudio prospectivo. Cir Esp. 2022;100:488–495.