The Charcot Foot (CF) consists of a progressive deterioration of the bones and joints, most common in diabetic patients with advanced neuropathy. The great problem is that can be confused with other processes, delaying the diagnosis and specific treatment. The aim is to analyse the cases of CF diagnosed in our hospital and especially to highlight the role of the general surgeon.
Material and methodsRetrospective study of all registered cases diagnosed of CF between the diabetic population of our Department of Health. A review of the literature was performed.
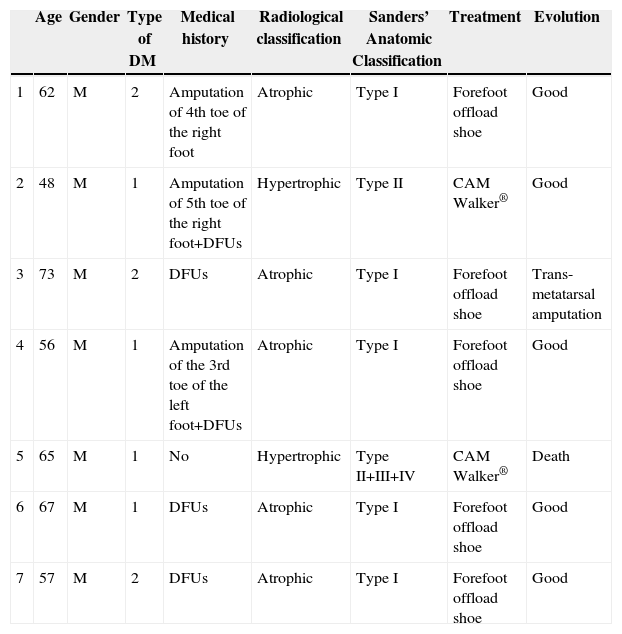
ResultsFrom 2008 to 2012, there are 7 cases of CF were diagnosed (prevalence 1:710). Two of the patients were diagnosed erroneously of cellulitis. The average time of delay in the diagnosis was 10 weeks (minimum 1, maximum 24). The initial treatment was immobilisation of the extremity. Once the oedema was eliminated, an offload orthesis was placed according to Sanders's anatomical classification. Evolution was favourable in 5 patients, 1 patient needed amputation, and other one died of acute cardiac pathology.
ConclusionsThe CF is a more frequent pathology than we believe. The general surgeon is the fundamental prop in the diagnosis and initial treatment. Before the presence of inflammation and oedema of the foot in a patient with diabetes and severe neuropathy, once cellulitis, osteomyelitis, and TVP are ruled out, Charcot neuroarthropathy should be considered.
El pie de Charcot (PC) consiste en un progresivo deterioro de los huesos y articulaciones, sobre todo en pacientes diabéticos afectos de neuropatía grave. El gran problema es que se puede confundir con otros procesos, retrasando el diagnóstico y tratamiento adecuados. El objetivo es analizar los casos de PC diagnosticados en nuestro hospital y, sobre todo, resaltar el papel del cirujano general.
Material y métodosEstudio retrospectivo donde se registran los casos diagnosticados de PC entre la población diabética de nuestro Departamento de Salud y se hace una revisión de la literatura.
ResultadosDesde 2008 hasta 2012 se han diagnosticado 7 casos de PC (prevalencia de 1:710). Dos de los pacientes fueron diagnosticados erróneamente de celulitis. El tiempo medio de demora en el diagnóstico ha sido de 10 semanas (mínimo 1, máximo 24). El tratamiento inicial fue inmovilización de la extremidad. Una vez desapareció el edema, se colocó ortesis de descarga según el tipo anatómico de la clasificación de Sanders. La evolución ha sido favorable en 5 pacientes, un paciente precisó amputación transmetatarsiana y otro fue exitus por enfermedad cardíaca aguda.
ConclusionesEl PC es una enfermedad más frecuente de lo que creemos. El cirujano general es el pilar fundamental en el diagnóstico y tratamiento inicial. Ante la presencia de inflamación y edema del pie en un paciente con diabetes y neuropatía severa, una vez descartadas fundamentalmente la celulitis, la osteomielitis y la trombosis venosa profunda (TVP), se debe pensar en una neuroartropatía de Charcot.
Charcot Foot (CF) or Charcot's neuroarthropathy (CN) consists of a progressive deterioration of bones and joints, mainly in the ankle and foot, especially in patients with a severe neuropathy. Diabetes mellitus (DM) is currently the most frequent cause of neuropathic arthropathy.1 The great problem is that it can be confused with other processes, delaying proper diagnosis and treatment. This encouraged us to perform a retrospective study to record all diagnosed cases of CF amongst the diabetic population, and to highlight the importance of the role of the general surgeon in early detection and initial treatment.
Material and MethodsA retrospective study was performed where the diagnosed cases of CF amongst the diabetic population of our Department of Health were recorded and the literature is reviewed. We collected data regarding age, gender, medical history, type of diabetes, surgical history and prior ailments of the foot, symptoms, average time of evolution, physical examination, vital signs, complementary tests, radiological information, anatomical classification, applied treatment, hospital stay and follow-up.
ResultsFrom January 2008 to December 2012, 4965 patients were treated in the Emergency Room with a diagnosis of diabetic foot. During this period, 7 cases were diagnosed with CF (Table 1), representing a prevalence of 1:710. The average age of the patients was 61.1 years old (minimum 48, maximum 73). As for gender, 100% of patients were male. All patients had DM for more than 10 years, 4 patients were type I and 3 patients were type II. In addition, 4 patients had high blood pressure, COPD, heart disease and dyslipidaemia. In all cases the right foot was affected, except for one patient (left foot). Three patients had surgical history of a toe amputation due to gangrene, and 6 of them also suffered from or had suffered from diabetic foot ulcers (DFUs). 100% of patients went to the Emergency Room. The symptoms for which all patients consulted were light pain and swelling of the foot. During clinical exploration, 4 patients showed oedema and erythema of the foot. In addition, 3 patients evidenced great deformity of the foot or toes along with DFUs. All patients showed signs of severe neuropathy in the lower limbs. Two of the patients had already been treated in the Emergency Room a month before, and they were diagnosed erroneously with cellulitis and treated with anti-inflammatory medication, antibiotics and rest for the limb. The average time of delay in diagnosis was 10 weeks (minimum 1, maximum 24). In the Emergency Room, all patients had a simple X-ray (Rx) of the foot done in 2 projections, blood tests and vital signs taken. No patient had leukocytosis, neutrophilia, or fever. Once admitted, magnetic resonance imaging (MRI) scans and blood tests were performed. None had altered parameters of C-reactive protein or erythrocyte sedimentation rate. The glycosylated haemoglobin (HbA1c) levels had increased in 100% of patients, the medium being 10.8% (minimum 7.9%; maximum 13.2%). The Endocrinology department was consulted to adjust blood sugar levels and the Orthopaedics department was consulted to agree on the type of orthosis. The most frequent radiological formation was the atrophic (5 patients) and then the hypertrophic (2 patients). The anatomical classification was recorded according to Sanders’ classification: 5 patients type I, one patient type II and a case simultaneously involving affecting zones II, III and IV. The initial treatment consisted of the rest in bed with the limb elevated and immobilisation of the limb using a Denis Browne splint. For getting up, the patient used crutches or a wheelchair. Once the oedema decreased, a method of offload was used, according to the type of anatomy. Patients with Sanders’ CF type I used an orthopaedic shoe with offload in the forefoot, and for the remaining patients (Sanders’ CF types II, III or IV) a controlled ankle movement (CAM) Walker® orthosis was placed. To compensate, an adapted shoe was placed in the other foot and crutches were used to facilitate mobility. The average stay was 14.8 days (minimum 7, maximum 33). The average follow-up time was 30 months (minimum 3, maximum 54). During the consultation, the physician carried out a physical examination and took the temperature of the feet with a thermometer, and X-rays or MRI scans were periodically requested to confirm the bone consolidation of the joint. The evolution was favourable in 5 patients, with consolidation of the joint and the maintenance of the stability of the foot with orthopaedic shoes; one patient required trans-metatarsal amputation due to a deterioration and another patient died due to acute heart disease.
Cases diagnosed with Charcot foot (from January 2008 to December 2012).
| Age | Gender | Type of DM | Medical history | Radiological classification | Sanders’ Anatomic Classification | Treatment | Evolution | |
|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | 2 | Amputation of 4th toe of the right foot | Atrophic | Type I | Forefoot offload shoe | Good |
| 2 | 48 | M | 1 | Amputation of 5th toe of the right foot+DFUs | Hypertrophic | Type II | CAM Walker® | Good |
| 3 | 73 | M | 2 | DFUs | Atrophic | Type I | Forefoot offload shoe | Trans-metatarsal amputation |
| 4 | 56 | M | 1 | Amputation of the 3rd toe of the left foot+DFUs | Atrophic | Type I | Forefoot offload shoe | Good |
| 5 | 65 | M | 1 | No | Hypertrophic | Type II+III+IV | CAM Walker® | Death |
| 6 | 67 | M | 1 | DFUs | Atrophic | Type I | Forefoot offload shoe | Good |
| 7 | 57 | M | 2 | DFUs | Atrophic | Type I | Forefoot offload shoe | Good |
DM: diabetes mellitus; DFUs: diabetic foot ulcers; M: male.
The CF was first described by Charcot2 in patients with Tabes dorsalis due to neurosyphillis, but the link between CF and diabetes was made by Jordan,3 which is today the most frequent cause, although other neurological processes can present it, such as siringomielia, leprosy, heavy metal deposits or peripheral nerve injury.4 Diabetic foot, together with its complications, like CF, is a multidisciplinary disease that must be handled by different specialists, but the general surgeon plays a very important role, since the patient goes to the Emergency Room whenever he/she has any problem, and there, the general surgeon assesses and performs a diagnosis for the initial treatment of the patient, before irreversible complications are caused, which may involve amputation of the limb. In fact, there are authors who define CF as a “medical emergency”.5 When we reviewed the literature, we noticed that CF publications are in medical journals of diabetes, Rheumatology, Rehabilitation, Traumatology, Vascular Surgery, and so on, but they are not in journals of General Surgery. All of this encouraged us to perform a retrospective study of all patients diagnosed with CF in our hospital in the last 5 years, and above all, to try to disseminate it in a General Surgery publication.
Despite the fact that the incidence and prevalence of CF is unknown, because patients are erroneously or belatedly diagnosed, it is estimated that CF affects 0.8%–8% of diabetic patients. The incidence is 3–11.7/1000 patients a year.6 From 2008 to 2012, 7 cases have been diagnosed at our centre, which represent a prevalence of 1/710, approximate to the literature. Up to 25% of the cases are bilateral7; in our series, 100% are unilateral. It is more common in type I diabetic patients in their 50s or 60s who have had DM for more than 10 years.6,7 In our study, the average age of the patients was 61.1 years; 4 suffered from DM type I and 3 suffered from DM type II. The pathogenesis is unknown but it is probably a combination of traumatic and vascular factors due to peripheral neuropathy. There are 2 hypotheses: neurovascular (French) and neurotraumatic (German) which, although they differ in the initial cause, they agree that the changes in the bone structure determine an anomalous load distribution that favours the appearance of ulcers, fractures, and calluses. Inflammatory Cytokines that stimulate the formation of osteoclasts1,6–9 have recently been incorporated. In terms of gender, there are no differences between men and women,6,10 although our series featured all males. Having reviewed the literature, we found that some patients attended the Emergency Room,4,11 others to offices for other reasons12 and in most cases, there is no evidence.10,13–15 All our patients went to the Emergency Room, none went to outpatient clinics, which means that all patients were assessed by the general surgeon on call.
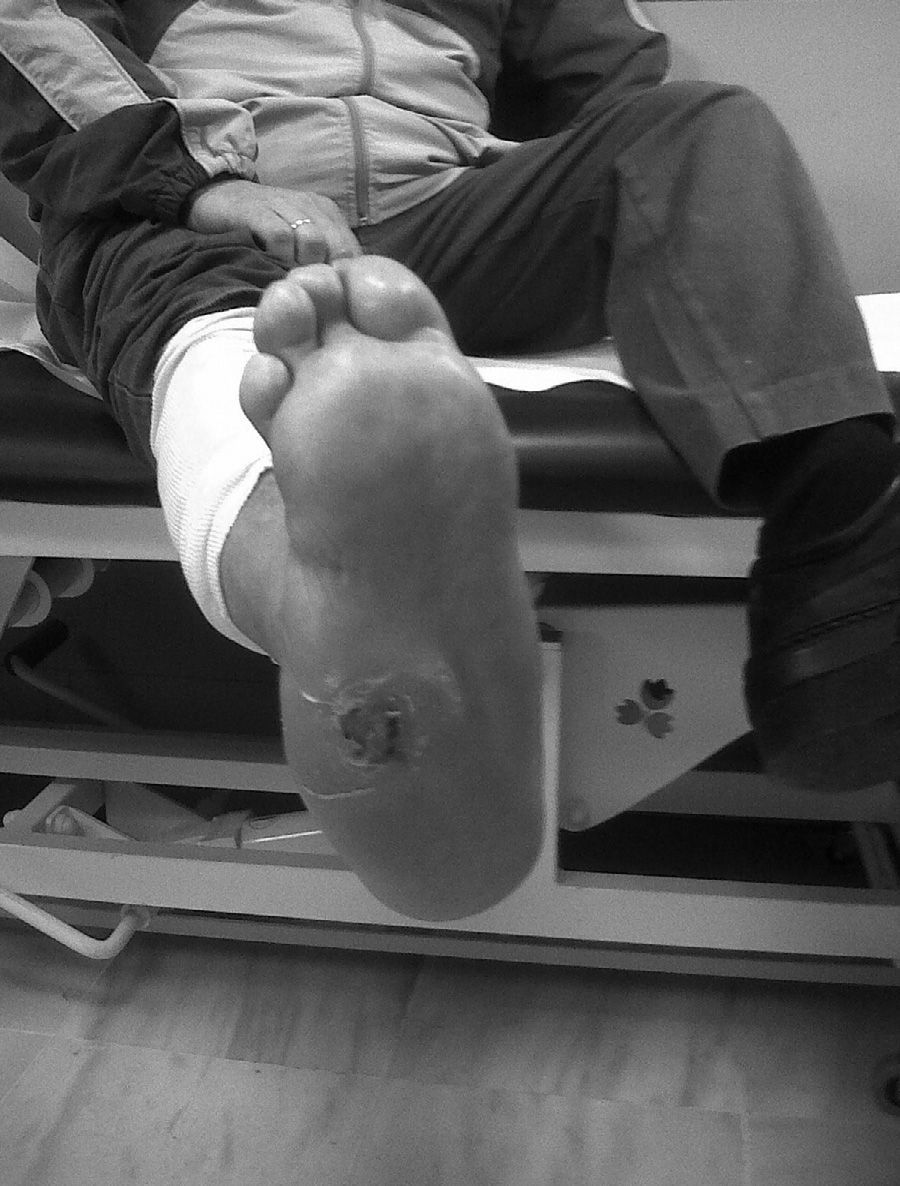
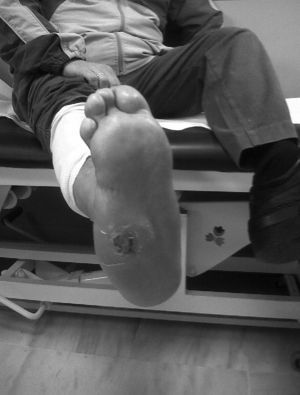
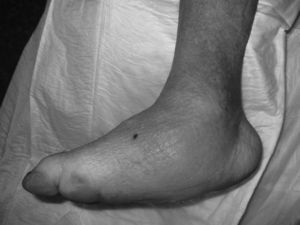
CF during the acute phase is characterised by “hot foot”, with erythema, oedema and mild pain on a neuropathic basis. The skin temperature raises 2–6 degrees compared to the skin in the other foot.4,6,7,16 In the chronic phase, the erythema and elevated temperature disappear and deformities develop.6 In our series, everyone was diagnosed in the acute phase. CF must be suspected for a diabetic patient with a long history of oedema, warm temperatures and unilateral flushing of the foot, absence or mild pain and deformity and instability of the joint (Fig. 1). Two of our patients were diagnosed erroneously with cellulitis. The average time of delay in diagnosis in our series was 10 weeks (minimum 1, maximum 24). In a study of Pakarinen,17 the diagnosis was delayed an average of 29 weeks.
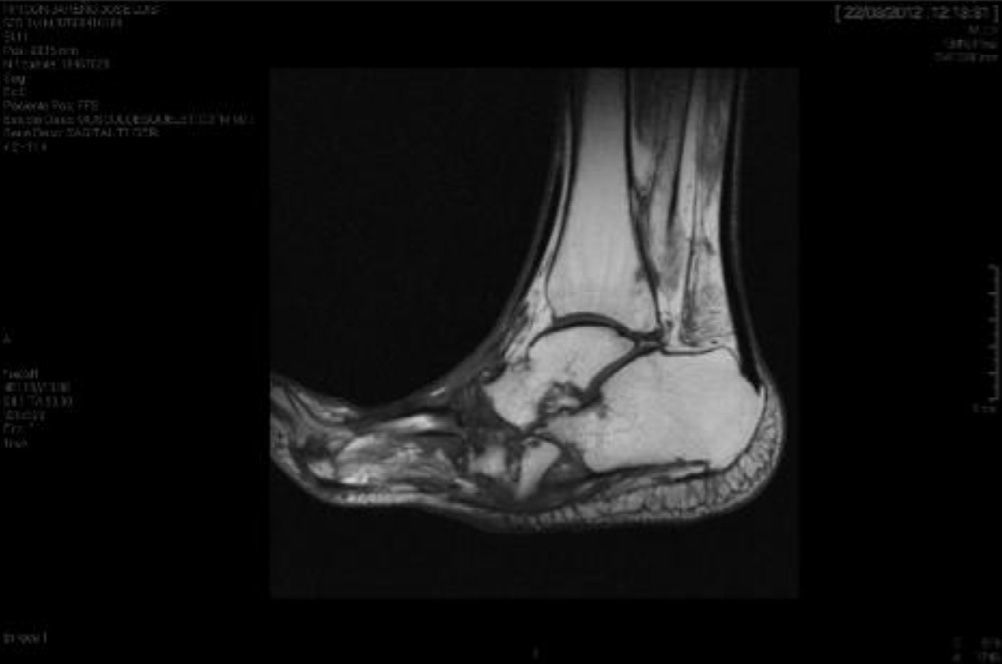
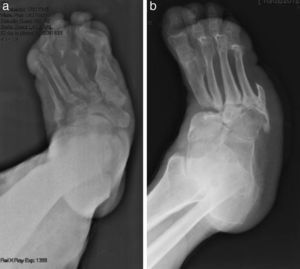
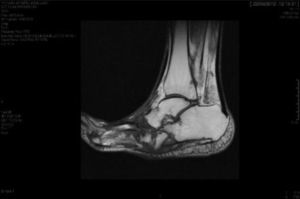
CF diagnosis is essentially clinical18; a differential diagnosis should be made, especially against cellulitis, osteomyelitis, DVT, gout and arthritis.4,6,7,11,16 Brodsky4,19,20 described a procedure that involves raising the affected limb for 10min with the patient in prone position; in the case of a CF, the oedema and erythema vanish, whereas the infection remains. Laboratory analyses are not diagnostic of CF but they can help to differentiate it from other infections.11.17 In our study, everyone had normal levels of white blood cells, C-reactive protein and the erythrocyte sedimentation rate. In early stages, the X-ray can be normal.6,11,21 As the disease progresses, radiographic findings appear. Bone changes associated with the neuroarthropathy are classified radiographically into atrophic and hypertrophic. The hypertrophic form is the most common: it occurs in the midfoot, hindfoot and ankle, and is characterised by fragmentation, destruction of joints, bone luxations, fractures and bone spurs. The atrophic form is not so common; it is located in the forefoot and is characterised by bone resorption and small fracture lines. The metatarsals have a radiographic image that resembles a “pencil point” or “skinny sugar cane”.6,7,14,20 In our patients, the atrophic form was the most frequent (5 cases) and the hypertrophic form occurred in 2 patients. MRI can detect changes in early stages and is the test most frequently used; it is also useful to differentiate it from osteomyelitis.6,11 In CF, the bone lesion is usually multiple, the cortex is not compromised and the lesion is self-limited. On the other hand, in osteomyelitis, it is usually unique, the cortex is compromised and the lesion is not self-limited.22 There is no definitive diagnostic test to distinguish CF from osteomyelitis; however, the 3-phase bone scan with Tc-99m, followed by In-111 with marked leukocytes, has shown a high sensitivity and specificity.6,8 The PET-CT scan is also very reliable to differentiate CF from osteomyelitis.1,8,11 The MRI scan is available at our centre; the rest of the tests have to be requested to the reference Hospital centre and are therefore, more difficult to obtain.
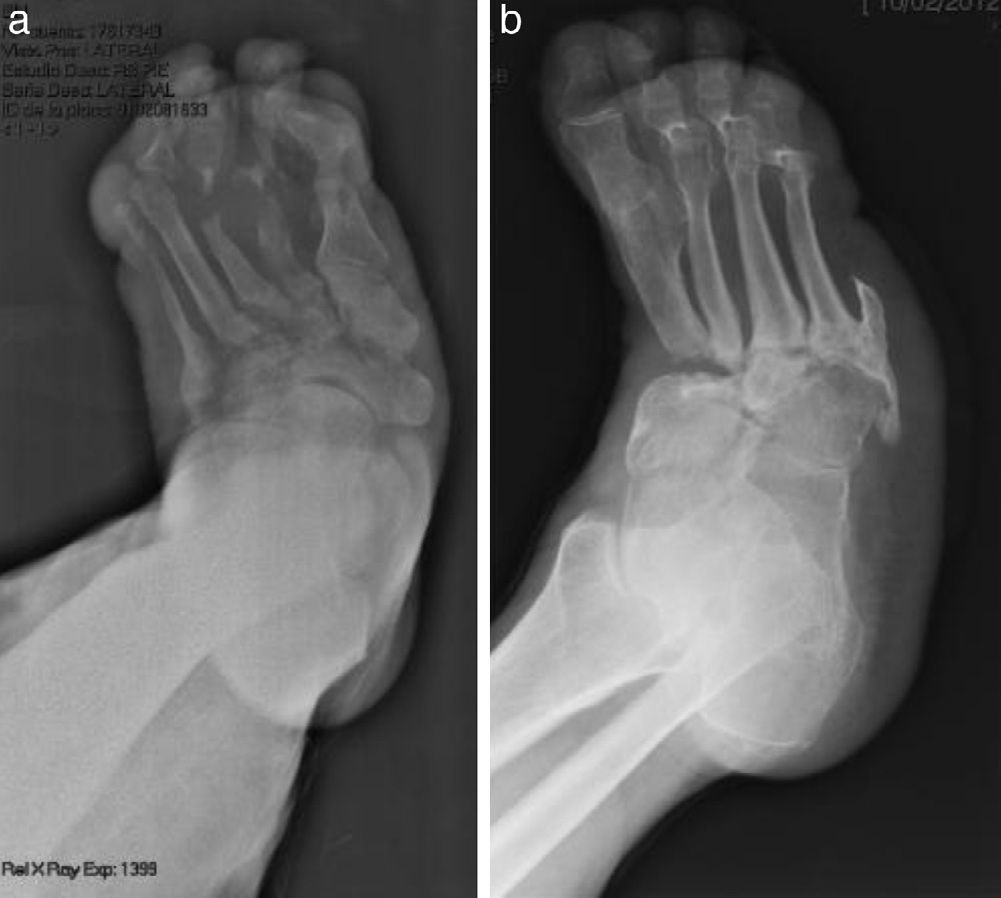
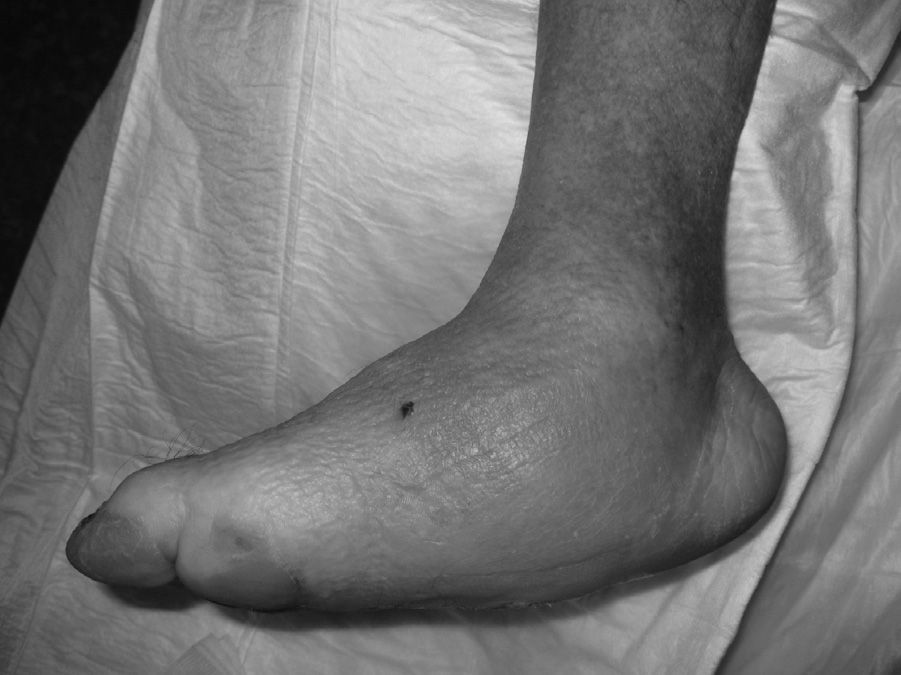
The evolution of CF usually follows a clinical–radiological pattern that was described by Eichenholtz in 3 stages: fragmentation, coalescence and reconstruction. There is a previous stage (stage 0) described by other authors,11,20,23 characterised by a “hot foot” with normal radiological findings, which may be confused with an infection. The “hot foot” also happens in stage I, but the X-ray shows osteopenia, subluxations, fractures, and peri-articular fragmentation. Stage II is characterised by decreased oedema and warm temperature; radiographically, it shows bone resorption, fusion of bone fragments and sclerosis. In stage III there is an absence of inflammation and a more stable foot, though often deformed; radiologically, it shows osteophytes, subchondral sclerosis and a decrease of space joint.6,10,11,16,17,20 All patients in our series were diagnosed in stage I. Sanders and Frikberg24 made a classification of CF depending on the anatomic area affected: type I affects the forefoot, namely the metatarsal-phalangeal and interphalangeal joints, and can be confused with osteomyelitis or osteoarthritis (Fig. 2A). Type II affects the Lisfranc joint, which is the joint most commonly affected in this disease (Fig. 2B). Type III affects the Chopart joint and the typical “rocking-chair foot” may occur, as well as type II, due to midfoot sinking, which causes a predisposition to ulcerations (Figs. 3 and 4). Type IV affects the ankle and type V affects the heel bone.6,10,17,24,25 As for our patients, the most frequent was type I (5 patients). A patient showed symptoms of type II and another patient had several areas affected at once (types II–IV).
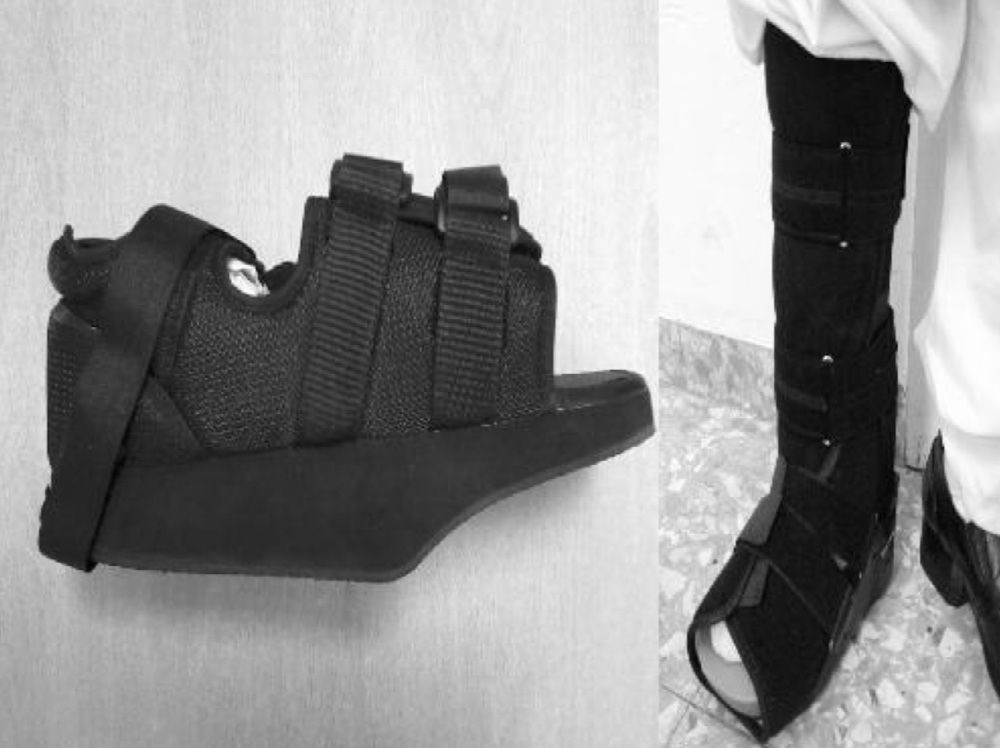
The initial treatment for CF in acute phases (stages 0 and I) is the immobilisation and offload of the affected limb, to prevent the disease from progressing and deformities from occurring. The total contact cast (TCC) is the gold standard for the initial treatment of the foot offload.1,4,6,7,10,11,16,20 This will change as oedema of the leg reduces until it disappears and radiological changes of coalescence are seen. A disadvantage is that it requires specialised personnel to make it and put it on, which is why alternatives are currently being sought out, such as pneumatic offload orthoses that allow for greater mobility, hygiene, and they can be pulled out at night.20 There is no experience with TCC at our centre, so physicians opted to indicate limb rest in bed with a Denis Browne splint and, once the oedema diminishes, an offload orthosis was placed according to the anatomical type. In CF type I (5 patients), an orthopaedic shoe was placed with offload in the forefoot (Fig. 5A), and for the rest of the patients with CF types II, III or IV (2 patients) a CAM (Controlled Ankle Movement) Walker® orthosis was placed (Fig. 5B). The other limb must be protected with a crutch or an adapted shoe, so that the healthy limb does not suffer.6 Tests were made with intravenous biophosphonates in the acute phase: although there are improvements in the clinical phase, there is no benefit in time to total immobilisation.18,26,27 Once the patient is in stage II, the TCC is replaced by a Charcot restraint orthotic walker (CROW) orthosis or similar for a month period, which will depend on the location and the extent of the destruction. At phase III, the patient will wear an orthopaedic shoe for mobility.4,6,10,11,16,20,28 Surgical treatment is indicated when soft tissue is affected, when the foot is unstable, or when it cannot be suited for footwear. There are various options, such as exostectomy, Arthrodesis and Achilles tendon section. When all treatment options have failed or if there are complications, the option should be below-knee amputation.6,10,28–30
ConclusionCF is a more common disease than we believe, and we should be aware in order to diagnose it in early stages and prevent irreversible stages. Within the multidisciplinary management of CF, the general surgeon is the anchor for diagnosis and initial treatment, since he/she is this person who initially “faces” the patient most of the time. Therefore, in the presence of inflammation and oedema of the foot in a patient with diabetes and severe neuropathy, once cellulitis, osteomyelitis, and DVT are discarded, a CN should be considered.
Conflict of InterestThe authors declare that there are no conflicts of interests.
Please cite this article as: Sellés Dechent R, Rueda Alcárcel C, Primo Romaguera V, Martínez Caamaño A, Asencio Arana F. Papel del cirujano general en el diagnóstico y tratamiento precoz del pie de Charcot. Cir Esp. 2015;93:320–325.