Treatment of lung carcinoma is multidisciplinary. There are different therapeutic strategies available, although surgery shows the best results in those patients with lung carcinoma in early stages. Other options such as stereotactic radiation therapy are relegated to patients with small tumours and poor cardiopulmonary reserve or to those who reject surgery. Adjuvant chemotherapy is not justified in patients with stage I of the disease and so double adjuvant chemotherapy should be considered. This adjuvant chemotherapy should be based on cisplatin after surgery in those patients with stages II and IIIA.
El tratamiento del carcinoma brongénico es multidisciplinar. Se dispone de diferentes estrategias terapéuticas, siendo la cirugía la que presenta mejores resultados en aquellos pacientes con carcinoma broncogénico en estadios precoces. Otras opciones como la radioterapia estereotáctica quedan relegadas a pacientes con pequeños tumores y mala reserva cardiopulmonar, o a aquellos que rechacen la cirugía. La quimioterapia adyuvante no está justificada en pacientes con enfermedad en estadio I, planteándose doble quimioterapia adyuvante basada en cisplatino tras la cirugía en aquellos con estadios II y IIIA.
Non-small cell lung cancer (NSCLC) accounts for 85% of lung carcinomas. The therapeutic goals depend on the disease stage at the time of diagnosis. For patients in stages I, II, and III, the goal is cure. For patients in stage IV, the goal is to alleviate symptoms and prolong survival.
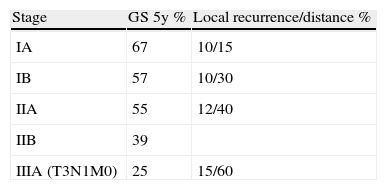
Bronchogenic carcinomas are considered to be in the initial stages when the tumour is localised within the lung, with or without affecting the hilar lymph nodes (stages I and II and some cases of IIIA). The 5-year survival rate of patients with pathological stage IA is 67%; stage IB, 57%; and stage IIIA, 25%1 (Table 1).
The therapeutic options for NSCLC in stages I and II are mainly local (surgery, conventional radiotherapy [RT], stereotactic body radiotherapy [SBRT], radiofrequency ablation [RFA], cryosurgery, and brachytherapy). Few cases are treated with adjuvant chemotherapy.
Surgical TreatmentIt is estimated that approximately 20% of bronchogenic carcinoma patients are diagnosed at an early stage. The therapeutic objective in these patients is to cure the disease, which is achieved in 60%–80% of patients in stage I and in 40%–50% of patients in stage II.
LobectomyThe most important curative therapy for these cases is surgery, which can encompass lobectomy or tumorectomy, depending on the T and N extension grades of the tumour.
Since 1995, it has been assumed that sub-lobular resections have worse results in terms of local recurrence (three-fold) and 5-year survival rate (30% less) compared with lobectomies. These data were obtained from a randomised clinical study conducted by Ginsberg in 247 patients with stage IA NSCLC who randomly underwent either a lobectomy or a sub-lobular resection (segmentectomy or wedge resection).2 A 4–5-year follow-up was conducted. However, there was no further discussion on which patients underwent segmentectomy and which patients underwent wedge resection (with worse results). Progression control was conducted using chest Rx, which did not diagnose early recurrence in the lobectomy cases. The statistical analysis has also been criticised. After a statistical revision in 2003 by Patel, no significant differences were found in the 5-year survival rates.3
In 1995, Martini published the results from 598 stage I NSCLC patients who underwent surgery at Memorial Sloan Kettering Hospital over a 15-year period. Of these patients, 4% underwent pneumonectomy, 85% underwent lobectomy, and 11% underwent sub-lobular resection. Only patients who could not tolerate a more extensive surgery underwent sub-lobular resections, which had a 50% recurrence index. However, in the cases where appropriate staging was conducted via lymphadenectomy, the recurrence rate was found to be only 5%. Furthermore, tumour size was also identified as a risk factor for recurrence. The 5-year survival rate for sub-lobular resections was 59%, whereas the rate for lobectomies/pneumonectomies reached 77%.4
Similar results were published in a study conducted at the Mayo Clinic in 2002. This study compared results from 100 patients with stage I NSCLC and T≤1cm who underwent either a lobectomy, bi-lobectomy, segmentectomy, or wedge resection. The 5-year survival rate was 92% for lobectomies and 47% for sub-lobular resections. Interestingly, when the sub-lobular resections were examined, the anatomical segmentectomies had a 75% survival rate compared with only 42% for wedge resections. In fact, no significant differences were found between anatomical segmentectomies and lobectomies.5
More recently, in December of 2011, Whitson et al. at the University of Minnesota conducted a retrospective study assessing 6810 bronchoalveolar adenocarcinoma patients who had undergone lung resection (anatomical segmentectomy, wedge resection, or lobectomy). The authors concluded that lobectomies had the best outcomes in all cases. After the data were adjusted for confounding factors and tumour characteristics for each patient, it was found that both anatomical segmentectomy and lobectomy had similar results. This was not the case for wedge resections. The results were also independent of age, gender, tumour size, and degree of differentiation.6
Sub-lobular ResectionsDue to an improvement in diagnostic techniques, small and peripheral lesions are more frequently diagnosed than more advanced tumours. These lesions are easier to excise by segmentectomy. For this reason, other analyses have been conducted to compare these therapeutic options.
In 1995, Landrenau published an interesting multicentre analysis using a non-random and prospective comparison of stage I NSCLC cases treated with lobectomies with cases that underwent open wedge resection or videothoracoscopy. Of the 219 patients studied, 117 underwent lobectomies, and 102 underwent wedge resection. The local recurrence rate was 19% for sub-lobular resections and 9% for lobectomies. However, no statistically significant differences were found in terms of 5-year survival rates.
Moreover, when cancer-specific survival was taken into account, segmental resections were favoured, given the decrease in perioperative morbidity and mortality, especially in patients with insufficient cardio-respiratory reserve. Finally, the possibility of intraoperative RT for sub-lobular resection cases was discussed and could achieve better resection margins and decrease local recurrence.7
El-Sherif published a retrospective analysis in 2006 comparing 784 stage IA NSCLC patients who underwent lobectomies or segmentectomies and did not find any significant differences between the two treatments. Other retrospective analyses have also shown similar results,8 but only when lesions were <3cm and, especially, <2cm.9–11
Nodular lesions with ground glass-like radiological patterns deserve special mention. Their incidence has increased due to advances in tomography techniques and the implementation of screening programmes using computerised tomography (CT), which is currently the case in Japan. Many authors, especially those from Asian countries, have suggested that sub-lobular resection without lymphadenectomy is sufficient treatment for these lesions.12,13 According to the analysis by Nogushi, pure nodular lesions with ground glass patterns <2cm usually correspond to adenocarcinomas without fibroblast proliferation, exhibiting lipid growth and basal membrane invasion and with a very low probability of lymphatic metastasis. In these particular cases, 5-year survival rates of 100% after wedge resection have been reported.12
Since this study was published, many others, especially those from Japan, have obtained similar results. Based on the collection of observations from retrospective studies where the oncological results from sub-lobular resections are known, a randomised phase III clinical trial in 908 patients was initiated by the Cancer and Leukaemia Group B. This study is based on the comparison between patients with tumours ≤2cm who underwent lobectomy versus segmentectomy and were subsequently monitored for 3 years.14 Regardless, segmental pulmonary resection is the only option in patients of advanced age15 or with insufficient cardio-pulmonary reserve.
Okada et al. described a study conducted in 1272 stage I NSCLC patients that used tumour diameter ≤2cm and peripheral localisation as cut-off points. They found that 5-year survival rates after segmentectomy reached 90%.16 Furthermore, in the case of solid tumours, lymphadenectomy needs to be conducted for lobular resections due to the increased compromise of lymph nodes and should be performed to avoid infra-staging with corresponding sub-optimal surgery.17
To minimise local recurrence in patients who have undergone sub-lobular resections, several studies have studied the most effective resection margins. A prospective multicentre analysis conducted by Sawabata et al. determined that in NSCLC patients who underwent lobular resection, no malignant cells were found along the resection margins as long as the margins were larger than the tumour diameter.18 Passlick et al. recommend margins ≥1cm and intraoperative marginal analysis. If tumours are larger, they recommend expanding to bi-segmental surgery or lobectomy.19
Other thoracic surgeons recommend the use of intraoperative brachytherapy with 125I as adjuvant therapy. This would increase the negative resection margins and displays low lung toxicity.20
However, due to the refinement of minimally invasive surgical techniques, patients who were previously considered inoperable due to their insufficient ventilatory reserves are now considered operable. Improved post-operative pain management and decreased morbidity associated with the techniques are responsible for this increased operability.21
Furthermore, no differences in mortality, post-operative stay, surgical wound infection, or cardiopulmonary alterations have been found when open-chest surgery was compared to videothoracoscopy.22
A prospective study by Whitson et al. conducted in 147 stage I NSCLC patients who had undergone surgery between 1998 and 2005 compared the results obtained after open lobectomy and video-assisted thoracic surgery (VATS). Although it was possible to obtain more samples from lymph node regions using open lobectomies, there was a decrease in postoperative hospital stay durations and the frequency of pneumonia cases in patients who underwent VATS. Furthermore, the 5-year survival rates for both types of surgery were similar.23 These results further support the use of lobectomy-VATS in patients with co-morbidities.
Cryosurgery24Freezing-induced tumour necrosis has been previously used in hepatic, breast, renal, and prostate tumours. This technique was initially used in tumours of respiratory origin to treat unresectable endobronchial tumours. Due to technological advances, cryotherapy can now also be used directly during surgery or in a percutaneous fashion. Cryosurgery is considered to be a safe technique, with infrequent hemoptysis and pneumothorax.
Although its indications are more extensive and geared towards unresectable disease, cryosurgery can be a therapeutic option for early-stage NSCLC cases with small endobronchial lesions as well as for patients with peripheral lesions who do not tolerate pulmonary resection.
A cryoprobe is required to use this treatment, achieving temperatures of −160°C at the tumour site after 2 or 3 sets of 5–10min. In cases of direct application during surgery and percutaneous application, the aim is to achieve an increasing freezing zone. Treatment must be maintained until the freezing zone achieves margins of 1cm. In cases of endoluminal treatment or direct intraoperative treatment, the necrotic zone is directly removed; in cases of percutaneous application, it is left to be resorbed.
The results of direct cryosurgery are similar to those of percutaneous use. Patients have a mean survival time of 5–61 months (mean 23-months) and 1-, 2-, 3-, 4-, and 5-year global survival rates of 68, 52, 34, 26, and 21%, respectively.
Non-surgical TreatmentsRadiotherapyRT is especially necessary in stages I and II NSCLC patients who are inoperable due to either advanced age or because of inadequate respiratory reserves. The results from inoperable NSCLC patients who randomly received different RT doses were compared in the clinical trial RTOG 73-01. It was concluded that better local control and 2-year survival rates were obtained with total doses of 60Gy, which were administered in daily fractions of 2YGy.25
When sub-lobular resections were compared with other therapeutic options, such as traditional RT, in patients with inadequate functional lung reserves presenting with stage I NSCLC, it was found that surgery was superior in terms of survival.26 In this sense, the mean survival rate of patients with stage I NSCLC treated with conventional RT fluctuates between 15 and 48%, with a local recurrence index of 50%.27
The main limitation of RT is damage to healthy lung tissue as a result of high radiation doses. This is most likely responsible for the worse results compared with surgery. Due to advances in helical CT and software, three-dimensional localisation of the tumour and adjacent healthy tissue is possible and has greatly improved the safety profile of this technique. Other advances contributing to improved RT, maximising the radiation dose issued directly to the tumour and minimising damage to adjoining healthy tissue, include respiratory movement coordination (gating), intensity-modulated RT, and the use of PET to guide the RT towards its target.
Stereotactic Body RadiotherapyThis technique utilises multiple photon beams, which emit a high dose of radiation to a tumour of defined volume, achieving a high level of precision and a small number of fractions. As well as better induction of cell death by radiation (DNA alterations), it allows a decrease in the dose received by healthy surrounding tissues.
SBRT is recommended for tumours <5cm, which require the coordination of radiation emission with the respiratory cycle. The total SBRT dose varies between a 30-Gy single dose and 60Gy split into 3 or 5 fractions (extreme hypofractionation). This differs from the 30 fractions of conventional RT spread over 6 weeks. The effective biological dose of these treatments is greater than the absolute value of the dose. Thus, 60Gy administered in 3 fractions corresponds to an effective biological dose of 150Gy of conventional RT emitted at daily 2-Gy doses.
In patients with inoperable NSCLC, SBRT yields excellent results, with local control percentages between 85 and 96% and 5-year survival rates of 50%.28 Furthermore, patients are not required to have a minimal lung function to be eligible for SBRT.
SBRT-associated toxicity is generally low, with serious adverse events described in less than 5% of patients.29 These events include lung damage, thoracic pain, and rib fractures. These complications are more severe when central tumours are treated with SBRT due to their proximity to bronchi and large vessels.29
SBRT is mainly indicated for treatment of inoperable stage I NSCLC patients.30 Due to its low toxicity and high effectiveness, which achieve excellent local control rates, several investigators have suggested that SBRT could be effective in high-risk patients (who are generally treated with sub-lobular resections) and even in patients with standard surgical risk (who are usually treated with lobectomies). However, this technique is less widely accepted, meriting a study. Investigators at the American College of Surgeons Oncology Group and the Radiation Oncology Group have collaborated in the development of a randomised phase III study comparing SBRT to sub-lobular resections (with or without brachytherapy) in high-risk, operable NSCLC patients. This study (American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021) recently opened for enrollment.31
Unlike surgery, different studies regarding quality of life after SBRT have shown that there is no decrease in quality of life, given that SBRT minimises healthy lung damage and does not affect respiratory function.32,33
Due to the excellent local control and low toxicity associated with SBRT in patients with early-stage NSCLC, some high-risk patients without absolute surgical contraindications are being proposed as candidates for this treatment. These are “borderline operable patients”. In these cases, survival rates are similar to the ones obtained after surgery.34,35 Currently, randomised clinical trials comparing surgical resection with SBRT are being conducted in operable and borderline stage I NSCLC patients (NCT00840749 and NCT01336894).
Along these lines, more cases of borderline patients refusing surgery and opting for treatment with SBRT instead are being reported. However, these patients often develop local recurrence afterwards, and they then undergo successful surgical resection with excellent results.36
Radiofrequency AblationIn RFA, a needle-electrode is introduced into the tumour using imaging techniques such as ultrasound, CT, or nuclear magnetic resonance (NMR). Electromagnetic energy is emitted through this needle using a high-frequency alternate current. In this way, very high temperatures are achieved within the tumour, resulting in tissue necrosis.
Pathological studies have shown that RFA produces an area of coagulative necrosis around the electrode. One of the main problems of RFA is heat loss through convection due to blood circulation within this zone, which is known as a “heat sink” effect. This effect is especially problematic when the tumour is localised close to blood vessels and >3mm in diameter.
The most frequent complications due to RFA are pneumothorax requiring drainage (11%), pleural effusion, and intrapulmonary haemorrhage. However, no significant deterioration of lung function as a consequence of RFA has been described.
RFA is indicated as a treatment for inoperable stage I NSCLC patients or as treatment for recurrent disease.
Recently, a prospective analysis conducted in patients with primary lung tumours or lung metastases from other origins showed that RFA causes total destruction in 80% of cases with lesions ≤3.5cm in size (imaging control).37 Furthermore, these cases achieve 1-, 2-, and 5-year survival rates of 70, 48, and 27%, respectively. RFA results are modified by the tumour size, with lower efficacy for lesions >3cm in diameter.
The experience of 64 patients who were lobectomy-intolerant and non-randomly subjected to sub-lobular resection, cryotherapy, or RFA has also been described. The general survival and cancer-specific results were similar in all three groups.38
Some authors defend the use of RFA over SBRT, given that the former can be conducted in an outpatient fashion and requires only one session. To determine which technique is most appropriate, Renaud recently conducted a meta-analysis comprising 90 RFA articles and 112 SBRT articles and determined that SBRT, if available, should be the first choice for treating inoperable NSCLC patients. Nonetheless, no randomised prospective study comparing both techniques exists to date.39
Intraoperative Brachytherapy With 125IThe benefit of intraoperative brachytherapy as an adjuvant to sub-lobular surgery20 or cryosurgery40 has been previously reported. This is due not only to its capacity of widening resection margins but also because it eliminates the problem of coordinating respiratory movements with other types of RT and minimises lung toxicity.
The American College of Surgeons Oncology Group (ACOSOG Z4032) is currently conducting a large randomised clinical trial comparing segmentectomy or wedge resection alone or in combination with brachytherapy with 125I, which is applied through a vicryl mesh implant, in stage I NSCLC patients (tumour size ≤3cm) with inadequate cardiorespiratory reserves.41 During this study, it was found that exposure of physicians and personnel to radiation during segmentectomy and implantation of the 125I-vicryl mesh is very low; this method is thus safe for healthcare professionals.42 Additionally, this study showed that intraoperative brachytherapy does not pose a greater risk for worsening respiratory function nor does it increase hospital stays compared with surgery alone.43 The medium- and long-term oncological results of this study remain to be published.
ChemotherapyThe main cause of death for NSCLC patients who undergo lung resection is relapse due to micro-metastases that were previously undetected.
Different clinical trials have determined the suitability of adjuvant chemotherapy treatment after surgery:
Stage IAA relatively small number of patients with stage IA NSCLC have been included in randomised clinical trials for adjuvant chemotherapy. The LACE meta-analysis has shown that the benefit of adjuvant chemotherapy varies significantly with stage and is potentially detrimental for stage IA patients.44
Currently, adjuvant chemotherapy is not recommended for patients with stage IA NSCLC.
Stage IBThe benefit of adjuvant chemotherapy in patients with stage IB NSCLC is less evident.
There is no solid proof in any of the randomised clinical trials that supports adjuvant chemotherapy for these patients.
Controversy exists for the subgroup of patients with stage IB defined by tumour size. An unplanned subgroup analysis of the CALGB 9633 data revealed that adjuvant chemotherapy was beneficial for patients with tumour diameter >4Ycm.45 At the 2009 American Society of Clinical Oncology (ASCO) meeting, the results of the JBR.10 study were presented, and the data of the same subgroup were reviewed. It was also concluded that patients with tumours >4cm in diameter showed a trend, albeit not significant, in favour of adjuvant chemotherapy.46 However, caution should be used when emphasising these results, given that the subgroup analysis was not planned. It has been recommended that patients in this subgroup be considered in an individualised manner for adjuvant chemotherapy after extensive discussion of risks and benefits.
Stage IIThe beneficial effect of chemotherapy has been consistently established for patients with stage II NSCLC. To date, the JBR.10 study has shown the largest benefit in survival in these patients, with a 20% absolute increase in 5-year survival rates.47 Similarly, the ANITA study showed a 13% increase, whereas the LACE study showed a 10% increase in 5-year survival rates.44,48
Stage IIIAAdjuvant chemotherapy is clearly beneficial for patients with stage IIIA NSCLC who have undergone complete resection. The ANITA study found a 16% absolute increase in the 5-year survival rate.48 The IALT study found the greatest beneficial effect in stage IIIA patients.49 Lastly, the LACE study showed a 13% increase in 5-year survival rates.44
DiscussionThe treatment of NSCLC is multidisciplinary and varies according to disease stage at the time of diagnosis and the condition of the patient.
There is no doubt that the treatment of choice for patients with stages I and II NSCLC is lobectomy with hilar and mediastinic lymphadenectomy. VATS surgery can increase tolerance of lobectomy in patients with advanced age or with other co-morbidities.
In patients who do not tolerate lobectomies, sub-lobular resections must be conducted with wide margins of at least half the size of the maximal tumour diameter. Patients with pure ground-glass nodules <2cm can undergo wedge resection surgery.
Anatomical segmentectomy can be used in patients with stage I NSCLC and T≤2cm together with hilar and mediastinic lymphadenectomy. In these patients, it is crucial to achieve good resection margins to avoid local recurrence. Furthermore, bi-segmentectomy or lobectomy is recommended for cases with margins <1cm. However, no randomised clinical trials are currently being conducted to support the decision of whether to offer sub-lobular resection to patients who tolerate surgery. Thus, we must wait for the results of a randomised phase III clinical trial conducted by the Cancer and Leukaemia Group B.
SBRT is the best option for patients with NSCLC in the initial stages but with high risk (excess post-exercise oxygen consumption (COPD), advanced age, etc.). SBRT is an even better option for borderline operable patients who do not want to accept the morbidity and mortality risks associated with surgery. Recurrent post-treatment cases can be operated on using surgery with curative intent.
However, chemotherapy is a fundamental complementary treatment to surgery in patients with stage II and IIIA NSCLC. Standard treatment for patients with stages II and IIIA NSCLC consists of double cisplatin adjuvant chemotherapy after surgery, which improves survival. This treatment must be initiated within the first 2–3 months after surgery, which makes it suitable only for patients with good overall health and without any post-surgery complications. The benefit of chemotherapy for patients with stage IB NSCLC is less evident; this is likely due to the heterogeneity of this population. The latest review of the TNM staging criteria should help in stratifying risk. Whereas standard chemotherapy consists of four cycles, toxicity is increased after lung resection; therefore, only 60%–70% of patients are able to complete treatment. The most common adverse effects are neutropenia, anaemia, nausea, vomiting, fatigue, and neuropathy.40
Although the introduction of adjuvant therapy represents one of the most significant advances for the management of NSCLC in the last decade, the greatest benefits are still to be seen. The future of the treatment of NSCLC patients is changing alongside new strategies employing gene expression profiling and pharmacogenomics, allowing for personalised treatment and maximal therapeutic benefit. Furthermore, research efforts continue to unravel the molecular mechanisms behind lung tumorgenesis, revealing new objectives and forming the foundation for modern adjuvant therapy clinical trials. These therapies include bevacizumab and erlotinib as well as the MAGE-A3 vaccine. It remains to be seen whether these approaches will improve the current standard of double cisplatin-based chemotherapy.
DisclaimerModifications of the following publications have been obtained for this document:
- 1.
Gadgeel SM, Ramalingam SS, Kalemkerian GP. Treatment of lung cancer. Radiol Clin N Am. 2012;50:961–974.
- 2.
Yagui-Beltrán A, Jablons DM. Optimal surgical management of stage I non-small cell lung cancer in an increasingly aging population: Challenges and recent progress. Expert Rev Respir Med. 2007;1(3):343–353.
The authors declare no conflicts of interest.
Please cite this article as: Meneses JC, Ávila Martínez RJ, Ponce S, Zuluaga M, Bartolomé A, Gámez P. Tratamiento del carcinoma broncogénico de célula no pequeña en estadios precoces. Cir Esp. 2013;91:625–632.