The aim of our study was to identify those patients with preoperative diagnosis of ductal carcinoma in situ (DCIS) and high risk of upstaging to invasive breast carcinoma (IBC), in whom sentinel lymph node biopsy (SLNB) should be considered.
Materials and methodsOne-hundred and five DCIS patients treated with breast-conserving surgery (BCS) or mastectomy were studied. Preoperative features of the tumours were analyzed to investigate its association with underestimation of IBC on final pathology.
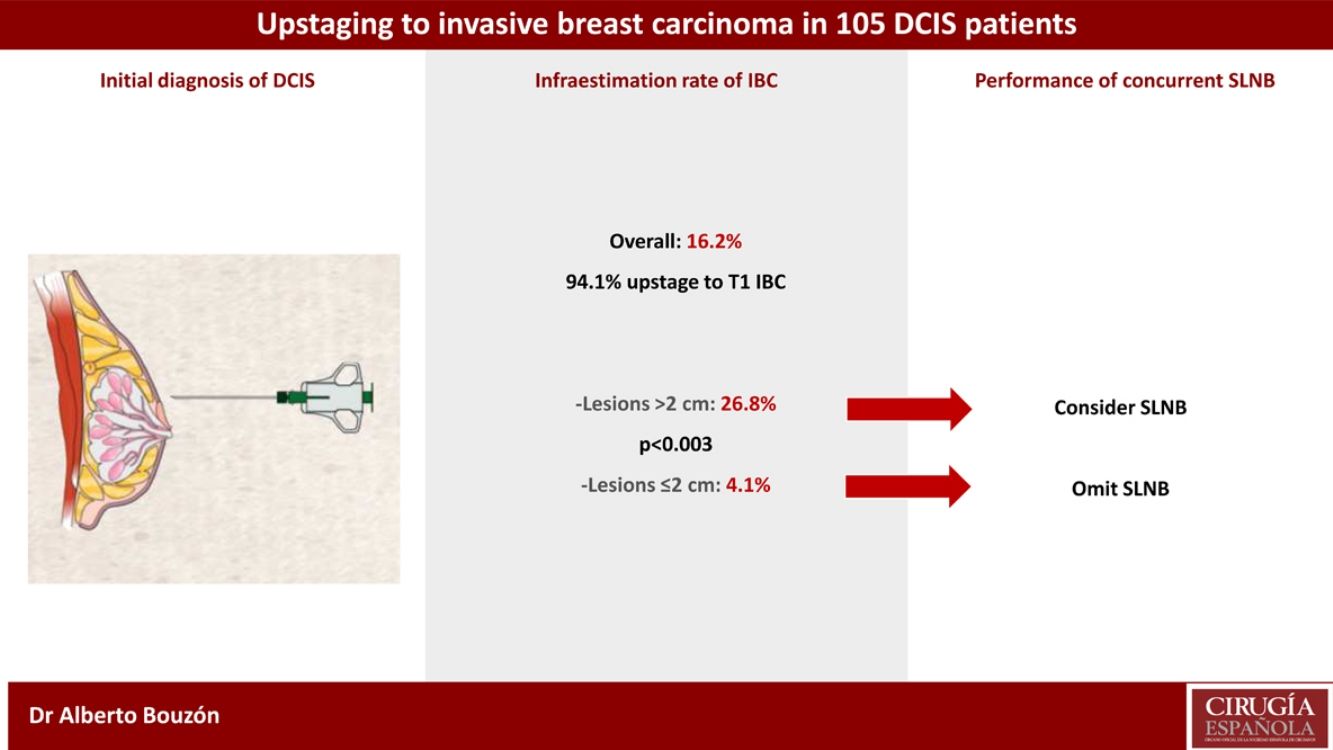
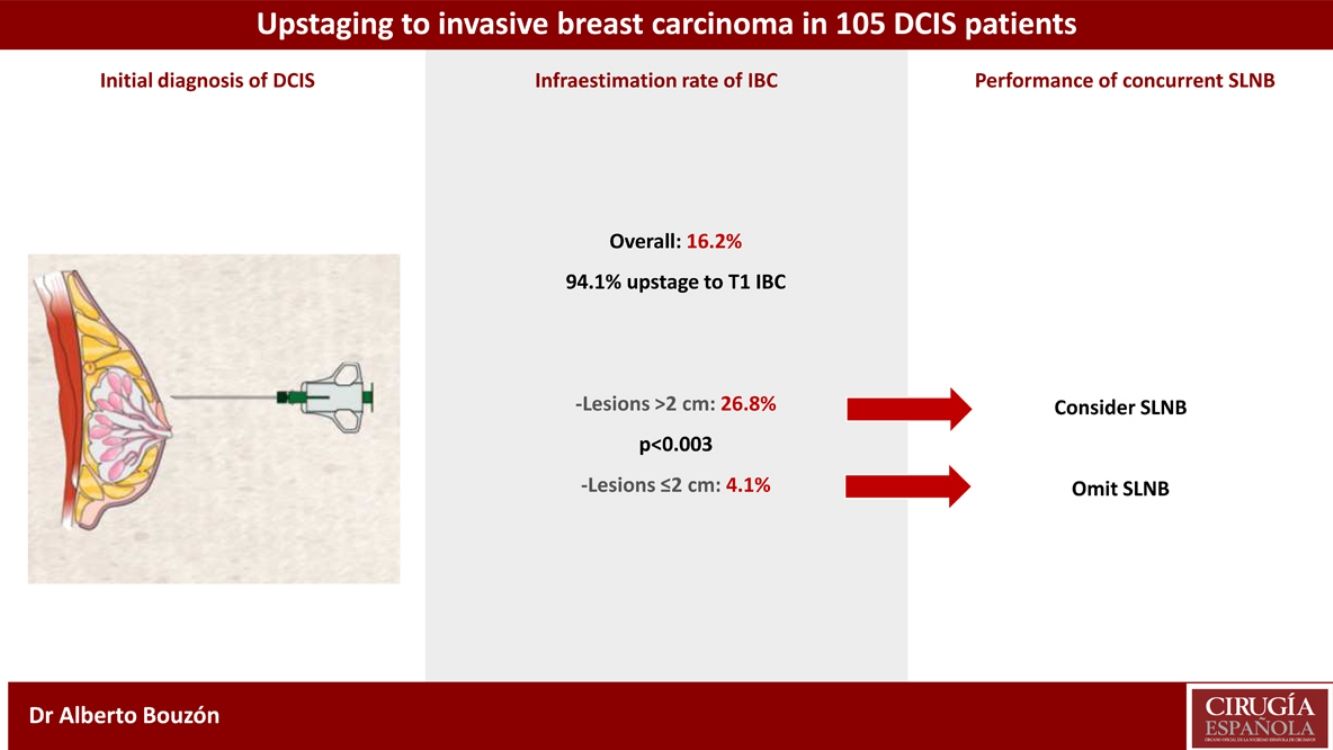
ResultsOverall, the underestimation rate of IBC was 16.2%. The underestimation rate was highest in lesions with initial size >2 cm compared with those with size ≤2 cm (26.8% vs. 4.1%, respectively; p < 0.003). Eighty-eight patients (83.8%) underwent concurrent SLNB and only one case had lymph node involvement (1.1%).
ConclusionsSLNB should be considered in DCIS patients receiving BCS with lesions greater than 2 cm since approximately one in four will harbour an IBC.
El objetivo de nuestro estudio consistió en identificar aquellas pacientes con diagnóstico preoperatorio de carcinoma ductal in situ (CDIS) y alto riesgo de presentar un carcinoma infiltrante en la lesión, en las que se debería considerar realizar una biopsia selectiva de ganglio centinela (BSGC).
MétodosSe estudiaron 105 pacientes con CDIS tratadas mediante cirugía conservadora o mastectomía. Se analizaron las características preoperatorias de los tumores para investigar su asociación con la infraestimación de carcinoma infiltrante.
ResultadosEl porcentaje global de infraestimación de carcinoma infiltrante fue del 16,2%. El porcentaje de infraestimación fue mayor en las lesiones con un tamaño inicial superior a 2 cm en comparación con las lesiones con un tamaño igual o menor a 2 cm (26,8% vs. 4,1%, respectivamente; p < 0,003). Se realizó la BSGC en ochenta y ocho pacientes (83,8%), encontrándose afectación ganglionar en un solo caso (1,1%).
ConclusionesEn pacientes con diagnóstico inicial de CDIS tratadas mediante cirugía conservadora, se debería considerar realizar una BSGC cuando el tamaño de la lesión es superior a 2 cm, ya que uno de cada cuatro casos albergará la presencia de un carcinoma infiltrante.
Ductal carcinoma in situ (DCIS) is considered a non-obligatory precursor lesion to invasive breast cancer. The proportion of DCIS diagnoses has increased since the implementation of the breast screening program, accounting for about 20% of all new female breast carcinomas.1 Up to 90% of cases are presented mammographically as suspicious microcalcifications.2
DCIS local treatment includes breast-conserving surgery (BCS), usually followed by radiotherapy, and mastectomy, depending on the breast-tumor size index and patient preference. Most of screen-detected DCIS lesions could be treated by BCS. Radiotherapy following BCS in DCIS patients decrease the risk of any ipsilateral breast recurrence by 15.2%.3
Sentinel lymph node biopsy (SLNB) is the standard of care for axillary staging in patients with early-stage invasive breast carcinoma (IBC), but is not routinely recommended in patients with preoperative diagnosis of DCIS undergoing BCS. However, it has been suggested to perform concurrent SLNB for patients with DCIS and high risk of upstaging to IBC.4
The aim of the study was to investigate the incidence of upstaging to IBC in our series of patients with DCIS diagnosed by image-guided needle biopsy and identify those cases in whom the presence of IBC is most likely to be underestimated and, therefore, in whom concurrent SLNB should be considered.
MethodsOne hundred and five patients with an initial diagnosis of breast DCIS were treated from January 2014 through October 2019. Patients with DCIS associated with Paget disease, DCIS with microinvasion, history of prior breast cancer, previous breast surgical biopsy or contraindication for radiotherapy were excluded. This retrospective study was approved by the Institutional Research Ethics Committee (No.2019/446).
Patients were diagnosed by core-needle biopsy (11-gauge needle) in case of a mass, nodule or architectural distortion or by vacuum-assisted biopsy (10-gauge needle) in case of calcifications. The specimens obtained with vacuum-assisted biopsy were subjected to x-ray examination to confirm the presence of calcificactions. In small lesions a clip was placed after biopsy.
Biopsy samples were fixed in 10% buffered formalin, embedded in paraffin and stained with standard hematoxylin and eosin for histologic examination. Nuclear grade, arquitectural pattern, presence of necrosis and status of estrogen receptor (ER) were documented in most cases.
All patients underwent breast surgery. Surgical specimens were sectioned in 5 mm slices and routinely processed. The presence of IBC and the extension of the disease were noted in the final pathology report. Microinvasive disease was defined as tumor focus ≤ 1 mm. A margin of 2 mm was accepted as negative for DCIS patients.
The sentinel lymph node (SLN) was identified using technetium 99m-labeled sulfur colloid or isosulfan blue dye. SLNs were sectioned at 2 mm intervals and submitted for routine processing. Lymph node involvement was categorized as macrometastases (>2 mm), micrometastases (>0.2 and ≤2 mm) or isolated tumor cells (≤0.2 mm).
Statistical analysisThe incidence of upstaging to IBC was calculated. Univariate analysis was performed to examine the association between initial tumor features and presence of invasive carcinoma on final pathology using the chi-squared test or Fisher´s exact test for categorical variables. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 23).
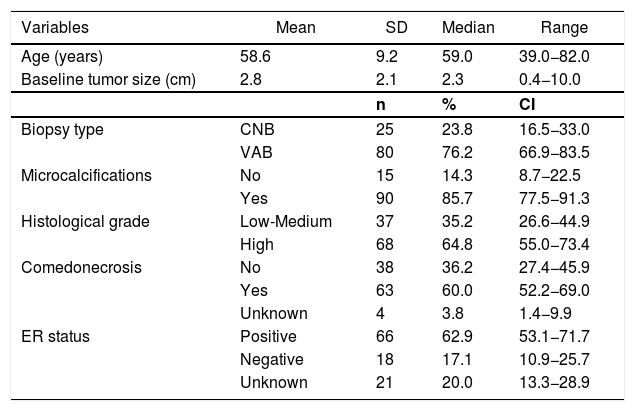
ResultsMost DCIS lesions were screen-detected (74.3%). Clinicopathologic features of the patients are summarized in Table 1. The mean age of the patients was 58.6 years and mean preoperative tumor size was 2.8 cm. Mammographic microcalcificactions at presentation were found in 90 patients (85.7%). Preoperative diagnosis of DCIS was performed by stereotactic vacuum-assisted biopsy in 80 patients (76.2%).
Clinical and tumoral characteristics (n = 105).
| Variables | Mean | SD | Median | Range |
|---|---|---|---|---|
| Age (years) | 58.6 | 9.2 | 59.0 | 39.0−82.0 |
| Baseline tumor size (cm) | 2.8 | 2.1 | 2.3 | 0.4−10.0 |
| n | % | CI | ||
| Biopsy type | CNB | 25 | 23.8 | 16.5−33.0 |
| VAB | 80 | 76.2 | 66.9−83.5 | |
| Microcalcifications | No | 15 | 14.3 | 8.7−22.5 |
| Yes | 90 | 85.7 | 77.5−91.3 | |
| Histological grade | Low-Medium | 37 | 35.2 | 26.6−44.9 |
| High | 68 | 64.8 | 55.0−73.4 | |
| Comedonecrosis | No | 38 | 36.2 | 27.4−45.9 |
| Yes | 63 | 60.0 | 52.2−69.0 | |
| Unknown | 4 | 3.8 | 1.4−9.9 | |
| ER status | Positive | 66 | 62.9 | 53.1−71.7 |
| Negative | 18 | 17.1 | 10.9−25.7 | |
| Unknown | 21 | 20.0 | 13.3−28.9 |
SD: standard deviation; CI: confidence interval; CNB: core needle biopsy; VAB: vaccum-assisted biopsy; ER: estrogen receptor.
Overall, the upstaging rate from DCIS to invasive carcinoma was 16.2% (17/105). Most of these patients upstage to T1 disease (94.1%). The median size of the invasive carcinoma was 0.7 cm (range 0.1–3.6 cm). Nine patients (8.6%) had no residual DCIS or IBC in the surgical specimen.
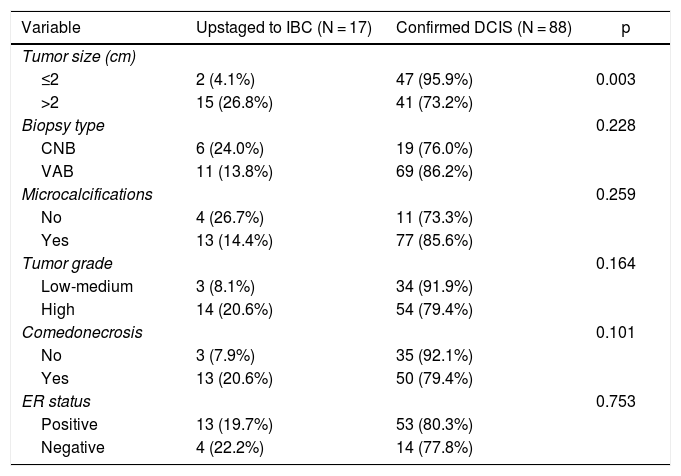
Univariate analysis of clinicopathologic predictors of underestimation of IBC in preoperative DCIS patients is summarized in Table 2. Among all tumor features examined, the only factor associated with upstaging to IBC was initial tumor size. The underestimation rate of IBC differed significantly between patients with initial lesion extent >2 cm or ≤2 cm (26.8% vs. 4.1%; p < 0.003). Type of biopsy, presence of microcalcifications, nuclear grade, presence of comedonecrosis and ER status were not associated with upstaging to invasive disease.
Predictors of upstaging to invasive disease. Univariate analysis.
| Variable | Upstaged to IBC (N = 17) | Confirmed DCIS (N = 88) | p |
|---|---|---|---|
| Tumor size (cm) | |||
| ≤2 | 2 (4.1%) | 47 (95.9%) | 0.003 |
| >2 | 15 (26.8%) | 41 (73.2%) | |
| Biopsy type | 0.228 | ||
| CNB | 6 (24.0%) | 19 (76.0%) | |
| VAB | 11 (13.8%) | 69 (86.2%) | |
| Microcalcifications | 0.259 | ||
| No | 4 (26.7%) | 11 (73.3%) | |
| Yes | 13 (14.4%) | 77 (85.6%) | |
| Tumor grade | 0.164 | ||
| Low-medium | 3 (8.1%) | 34 (91.9%) | |
| High | 14 (20.6%) | 54 (79.4%) | |
| Comedonecrosis | 0.101 | ||
| No | 3 (7.9%) | 35 (92.1%) | |
| Yes | 13 (20.6%) | 50 (79.4%) | |
| ER status | 0.753 | ||
| Positive | 13 (19.7%) | 53 (80.3%) | |
| Negative | 4 (22.2%) | 14 (77.8%) |
IBC: invasive breast carcinoma; DCIS: ductal carcinoma in situ; CNB: core needle biopsy; VAB: vaccum-assisted biopsy; ER: estrogen receptor.
Eighty-three patients underwent BCS (79%) and 22 had mastectomy (21%) as initial treatment. Fourteen patients needed an additional surgery due to positive margins after BCS (92.9% underwent re-excision). All patients except one (a small low grade DCIS) were treated with adjuvant radiation therapy after BCS.
Of the 105 cases of the study, 88 patients (83.8%) underwent concurrent SLNB. The median number of SLNs removed was 2. Among patients treated with BCS, 79.5% had axillary staging with SLNB. Overall, only one patient (1.1%) had a positive SLNB (one involved lymph node with micrometastasis). One patient without concurrent SLNB and postoperative upstaging to IBC underwent an axillary staging at a second operation.
DiscussionAmerican Society of Clinical Oncology (ASCO) recommends performing routine SLNB in DCIS patients undergoing mastectomy, but not in patients treated with BCS.5,6 However, approximately 25% of patients initially diagnosed with DCIS will upstage to IBC on final pathology.7 In the setting of BCS, SLNB should be considered in DCIS patients with high risk of underestimation of invasive disease after a careful interdisciplinary discussion in an attempt to avoid reoperation for axillary assessment.
We reported an upstaging rate to IBC of 16.2%, similar to other studies.8–10 Gumus et al.10 reported an underestimation rate of IBC of 17.8%, but their study only included microcalcification lesions diagnosed by stereotactic vacuum-assisted biopsy.
We identified initial tumor size as a predictive factor of upstaging to IBC. This is consistent with the study of Kurniawan et al.,11 which reported an underestimation rate of 12.5% in DCIS lesions ≤2 cm compared with 26.7% in lesions >2 cm (p = 0.001). In the study of Marques et al.,12 tumor size was not associated with underestimation, explained by the high rate of DCIS diagnosed by screening.
In our series, underestimation rate of IBC was higher after core-needle biopsy, in non-calcified lesions, in high grade DCIS and in the presence of comedonecrosis, but the differences did not reach statistical significance due to the small sample size. However, several studies have demonstrated that these factors are predictive of underestimation of IBC on final pathology. It has been shown that DCIS diagnosed by core-needle biopsy has a higher risk of underestimation compared with vacuum-assisted biopsy due to the smaller amount of tissue removed.12–14 Schulz et al.8 and Kurniawan et al.11 noted that suspicious non-calcified findings on mammography were significantly associated with upstaging to IBC. Lee et al.15 found that nuclear grade was a significant risk factor for upstaging. Son et al.16 and Marques et al.12 reported that the presence of comedonecrosis was an independent predictor of underestimation of IBC.
The rate of lymph node metastasis is low in patients with pure DCIS, usually less than 2%.9,16–19 Almost 99% of SLNBs were negative in the present study. Only one patient treated with BCS had lymph node micrometastasis, with no evidence of residual tumor at the breast resection specimen. This patient didn´t undergo further axillary surgery. An undetected small foci of invasion removed by vacuum-assisted biopsy was suspected in this case.
Our data supports the current recommendation of omitting axillary surgery in preoperative DCIS patients. SLNB is a minimally invasive procedure compared with conventionally axillary lymph node dissection. However, an unnecessary SLNB implies an increase in morbidity, economic costs and surgical time. A prospective study conducted by Goldberg et al.20 in clinically node-negative breast cancer patients reported an incidence of lymphedema of 5% at a median follow-up of 5 years. Other complications such as seromas, pain, paresthesias and reduce range of motion can decrease the quality of life of these patients.
According to Lara et al.,21 micrometastasis in lymph nodes has no apparent clinical significance in DCIS patients. Recently, it has been suggested not to offer concurrent SLNB for DCIS patients with suspected microinvasion.22 Furthermore, Magnoni et al.23 found a good disease-free and overall survival in women with positive SLNB and microinvasive DCIS, suggesting that SLNB could not be useful in these patients.
In our study, 94.1% of patients with upstaging had T1 invasive carcinoma, in which imaging could replace surgical axillary staging in the next years. Currently, there are three ongoing prospective trials designed to evaluate the safety of omitting SLNB in clinically node-negative early breast cancer patients treated with BCS.24–26 The SOUND trial (Sentinel node vs Observation after axillary Ultra-SouND)24 includes patients with T1 disease, while the BOOG 2013-0825 and INSEMA (Intergroup-Sentinel-Mamma)26 trials include patients with T1-2 disease. As long as the results of these trials are not available, SLNB continues to be the standard of care for nodal staging in patients with clinically node-negative early breast cancer, and therefore, this procedure may be contemplated in select patients with DCIS and high risk of upstaging to IBC.
The retrospective single-institution study design and the limited number of patients are the main limitations of this analysis.
In conclusion, the rate of upstaging in our series was 16.2%. In DCIS patients treated with BCS, concurrent SLNB may be considered if breast lesion measures more than 2 cm since one in four will harbour an IBC.
DisclosuresThere are no conflicts of interests by any of the authors.
The authors thank Jorge Suances, member of the Department of Biostatistics of Complejo Hospitalario A Coruña, for their contribution in the statistical analysis. The authors gratefully acknowledge also Carmen Cereijo and Manuel Juaneda, members of the Breast Unit of Complejo Hospitalario A Coruña, for their contribution.
Please cite this article as: Bouzón Alejandro A, Iglesias López Á, Acea Nebril B, García Jiménez ML, Díaz Carballada CC, Varela Romero JR. Infraestimación de carcinoma infiltrante de mama en pacientes con diagnóstico inicial de carcinoma ductal in situ: el tamaño importa. Cir Esp. 2021;99:655–659.