In its initial stages, nonalcoholic fatty liver disease presents hypertriglyceridemia and accumulation of lipids in the liver (hepatic steatosis). Bempedoic acid is an ATP:citrate lyase inhibitor that promotes a dual inhibition of the synthesis of cholesterol and fatty acids. However, its effect in the prevention/treatment of hepatic steatosis and hypertriglyceridemia has not been investigated. The aim of our work has been to elucidate whether bempedoic acid, through a mechanism other than ATP:citrate lyase inhibition, reverses these metabolic alterations.
Experimental designThe study was carried out in female Sprague-Dawley rats fed, for three months, with a high fat diet supplemented with fructose (10% w/v) in drinking water. During the last month, bempedoic acid (30 mg/kg/day) was administered to a group of animals. Zoometric and plasmatic parameters were analyzed, gene and protein expression analysis were performed in liver samples and PPAR-PPRE binding activity was determined.
ResultsOur interventional model developed hepatic steatosis and hypertriglyceridemia. Despite an increase in total caloric intake, there was no increase in body weight of the animals. The administration of bempedoic acid significantly reduced hepatic steatosis and promoted a marked hepatocyte hypertrophy. There was a 66% increase in the liver weight of the animals treated with the drug that was not accompanied by modifications in the markers of inflammation, oxidative stress, or endoplasmic reticulum stress. Bempedoic acid activated the peroxisome proliferator activated nuclear receptor (PPARα) and its target genes.
ConclusionsBempedoic acid could be an effective therapy for the treatment of fatty liver and associated cardiovascular risk. Bempedoic acid has other mechanisms of action besides the inhibition of ATP:citrate lyase, such as the activation of PPARα, which could explain the reduction in hepatic steatosis and the increase in liver weight observed in animals treated with the drug.
La enfermedad del hígado graso no alcohólico cursa, en sus fases iniciales, con hipertrigliceridemia y acúmulo de lípidos en el hígado (esteatosis hepática). El ácido bempedoico es un inhibidor de la ATP:citrato liasa que promueve una inhibición dual de la síntesis de colesterol y ácidos grasos. Sin embargo, no se ha investigado su efecto en la prevención/tratamiento de la esteatosis hepática y la hipertrigliceridemia. El objetivo de nuestro trabajo ha sido elucidar si el ácido bempedoico, mediante un mecanismo diferente/alternativo a la inhibición de la ATP:citrato liasa, revierte estas alteraciones metabólicas.
Diseño experimentalEl estudio se realizó con un modelo animal de rata Sprague-Dawley hembra alimentada, durante tres meses, con una dieta rica en grasa saturada suplementada con fructosa al 10% (p/v) en el agua de bebida. Se administró, durante el último mes, ácido bempedoico (30 mg/kg/día) a un grupo de animales. Se analizaron parámetros zoométricos, se realizaron valoraciones plasmáticas, de expresión génica y proteica en muestras de hígado y se determinó la actividad de unión PPAR-PPRE.
ResultadosNuestro modelo de intervención dietética desarrolló esteatosis hepática e hipertrigliceridemia. A pesar de un aumento en la ingesta calórica total, no se observó un incremento de peso corporal de los animales. La administración de ácido bempedoico redujo significativamente la esteatosis hepática y promovió una marcada hipertrofia de los hepatocitos. Se observó un incremento del 66% en el peso del hígado de los animales tratados con el fármaco, que no se acompañó de modificaciones en los marcadores de inflamación, estrés oxidativo o estrés de retículo endoplasmático. El ácido bempedoico activó el receptor nuclear activado por proliferadores peroxisómicos (PPARα) y a sus genes diana.
ConclusionesEl ácido bempedoico podría ser una terapia efectiva para el tratamiento del hígado graso y el riesgo cardiovascular asociado. El ácido bempedoico presenta otros mecanismos de acción diferentes a la inhibición de la ATP:citrato liasa, como sería la activación de PPARα, lo que podría explicar la reducción de la esteatosis hepática y el incremento del peso del hígado observado en los animales tratados con el fármaco.
The incidence of non-alcoholic fatty liver disease (NAFLD) has increased over recent decades, and its prevalence is now higher than 25%.1 NAFLD is characterized by alterations which chiefly derive from hepatic insulin resistance, and they are included in the manifestations of metabolic syndrome, and they are also associated with increased cardiovascular risk.2 Although NAFLD has a multiple causes, the consumption of a “Western” diet with a high calorie intake in the form of simple sugars and saturated fats plays a decisive role in its appearance.3 This type of diet in combination with a sedentary lifestyle leads to obesity and insulin resistance (IR), which are two key etiopathogenic factors in the development of NAFLD. IR induces a higher influx of fatty acids (FA) towards the liver from adipose tissue4 and hepatic activation of de novo lipogenesis (DNL).5,6 This in turn leads to an increase in the synthesis of hepatic triglycerides which in principle are exported to the plasma in very low density lipoproteins (VLDL). The overproduction of VLDL in individuals with NAFLD leads to dyslipidaemia characterized by high levels of triglycerides and low levels of HDL-cholesterol, as well as an increase in small dense LDL particles, the characteristic triad of what is known as atherogenic dyslipidaemia.7,8
Approximately 25% of the patients who have NAFLD eventually develop non-alcoholic steatohepatitis or NASH, which may leads to the appearance of hepatic fibrosis and cirrhosis.9 Selective PPARα receptor modulating drugs are currently under development, such as pemafibrate10, PPARα/δ agonists such as elafibranor11 or acetyl-CoA carboxylase (ACC) inhibitors such as GS-0976 or firsocostat12 for the treatment of NASH. Nevertheless, the accumulation of triglycerides in the liver (steatohepatitis), which is considered to be an alteration which appears in the early phases of NAFLD, currently lacks a fully effective pharmacological treatment. In the search for new pharmacological targets, ATP- citrate lyase (ACL) is highly interesting, as this enzyme has key functions in the intersection between nutrient catabolism and the biosynthesis of cholesterol and fatty acids.13 Bempedoic acid (Bempedoic acid; ETC-1002, 8-hydroxy-2,2,14,14-tetramethylpentadecanodioic acid) is an activator of AMP-protein kinase (AMPK) and an inhibitor of ACL. Its effects have been studied in in vivo models as a dual inhibitor of cholesterol and fatty acid synthesis,14,15 and its commercialization was recently approved by the European Medicines Agency (EMA). Clinical studies undertaken in patients with hypercholesterolaemia by Ballantyne et al.16 showed that bempedoic acid only reduced levels of cholesterol in plasma, without affecting triglyceride levels. This result differs from findings in rodents,17 and this is probably due to the low relevance of DNL in the absence of insulin resistance. Nevertheless, no research has taken place into the effect of bempedoic acid in the prevention or treatment of steatohepatitis and the hypertriglyceridaemia associated with NAFLD.
Based on our experience in the study of the effects of consuming 10% (w/v) on lipid metabolism,18,19 we designed a dietary model of a fatty liver without inflammation in female Sprague-Dawley rats fed with a diet rich in saturated fatty acids (palmitic and stearic), with no cholesterol and supplemented with liquid fructose. This work studies whether bempedoic a acid may be a therapeutic alternative for the treatment of steatohepatitis and/or hypertriglyceridaemia observed in the early phases of NAFLD, elucidating the mechanism that is involved.
Material and methodsExperimental designFor this study 24 female Sprague-Dawley rats were used, supplied by Charles River (Barcelona, Spain). The animals were kept with water and food ad libitum, at a constant temperature and humidity with a light/dark cycle of 12 h during one week. After acclimatisation the rats were distributed at random into three study groups (n = 8): one control group (CT) fed with a standard diet and drinking water, and one group fed with a high fat diet (Teklad Custom Diet TD.180456, by Envigo, Spain). The content of this diet in %kcal is: proteins 15.5%, carbohydrates 37.5% and fats 47%, of which 210 g/kg are cocoa butter which contains approximately 25% of palmitic acid and 35% of stearic acid.20 This diet was supplemented with a 10% solution (weight/volume) of fructose in the drinking water (HF-FR). A third group of rats were fed with HF-FR and received, in the last month, an oral daily dose of bempedoic acid of 30 mg/kg, diluted to 0.5% of carboximethylcelulose and 0.025% of tween-20 (Bempedoic acid). The other groups were administered the corresponding volume of the drug excipient. The intake of food and drink was monitored every 2 days, and the animals were weighed once a week. The rats were sacrificed after 3 months of the study by blood loss under anaesthesia with ketamine:xylazine (proportion 9:1) after fasting prior to sacrifice for 2 h.
The whole process was implemented according to the Guidelines set by the Bioethics Committee of Barcelona University, as stipulated in Law (5/1995) (21 July) of the Generalitat de Catalunya (file number: 10106).
Sample takingSamples of blood were taken by puncture of the saphenous vein to determine the levels of triglycerides and cholesterol in plasma. For the levels in serum of free fatty acids (FFA), AST and ALT, blood was collected by cardiac puncture at the moment the animals were sacrificed, in tubes by Sarstedt FA & Co. (Nümbrecht, Germany), and the serum was obtained by centrifuging at 1000×g during 10 min at ambient temperature. The livers of the rats were extracted, weighed and immediately frozen in liquid N2 at −80 °C until they were used. 10 mg–100 mg of hepatic tissue was used for the extraction of total RNA. Two additional samples of approximately 250 mg were used to quantify hepatic lipids, to obtain the total protein and nuclear extracts. One fragment of hepatic tissue was kept in formaldehyde for histological studies before it was frozen.
Determination of plasmatic and hepatic lipidsThe levels in plasma of triglycerides and cholesterol were determined using the Accutrend® Plus System glucometer (Cobas, Roche Farma, Barcelona) with specific reactive strips. Free fatty acids (FFA) were measured using the colorimetric test by Bioo Scientific (Austin, TX, U.S.A.), and AST and ALT levels were measured using enzymatic kits by Spinreact (Girona, Spain).
Hepatic lipids were extracted by basically following the protocol described by Qu et al.21 40 mg of liver was homogenized in 800 μl acetone using a mechanical homogenizer (Polytron® PT 1200E, Selecta). After 12 h’ incubation under constant agitation and at ambient temperature, the samples were allowed to rest for 15 min. Triglyceride and cholesterol levels were then evaluated using colorimetric kits: 41030 Triglycerides and MD41021 Cholesterol, by Spinreact (Girona, Spain).
Histological studiesThe hepatic tissue samples were dehydrated and embedded in paraffin using a Leica TP1020 automatic tissue processor. The block was formed in the centre of an EG1140H tissue embedding centre and the samples were cut at 5 μm in the PFM Rotary 3004M. Staining with haematoxylin-eosin was carried out automatically in a Thermo Shandon Varistain 24−4. Images were acquired using a Leica DMSL microscope equipped with a DP72 camera. Hypertrophy was quantified using the ImageJ computer programme, and it was expressed in % of the surface occupied by hepatic nuclei per area of image. All of the procedures were carried out in the Animal Histopathology laboratory of Barcelona University.
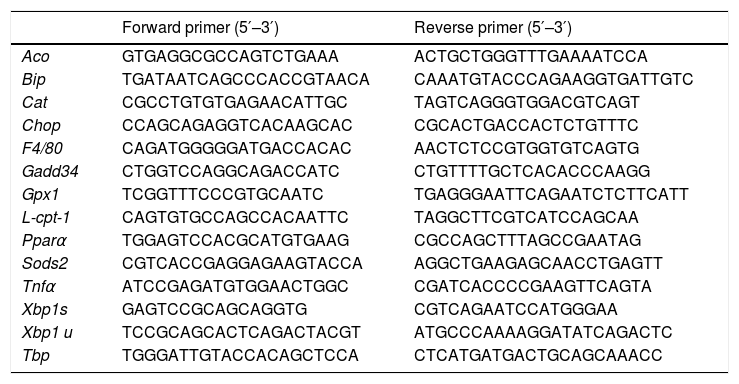
RNA analysis: RT-qPCRThe total RNA was isolated from 100 mg of hepatic tissue by TRIsure™ reagent (Meridian Biosciences, Memphis, TN, U.S.A.), according to the manufacturer’s instructions. The concentration and quality of the RNA were determined by spectrophotometry in a NanoDrop ND-1000 (Thermo Fischer Scientific, Madrid, Spain) at 260−230 nm. Relative levels of each specific RNA were determined using the reverse transcriptase (RT) technique associated with PCR in real time (qPCR).22 Complementary DNA was obtained from 1 μg of total RNA and it was amplified by polymerase chain reaction using 5−10 ng DNAc, SYBR green PCR Master Mix, specific primers (shown in Table 1) and the StepOne-Plus sequence detection system, all by Applied Biosystems (Foster City, CA, U.S.A.). TBP (TATA-box-binding protein) was used as the internal control. The results were calculated using the 2−ΔCt method.
Specific oligonucleotides for qPCR.
| Forward primer (5′–3′) | Reverse primer (5′–3′) | |
|---|---|---|
| Aco | GTGAGGCGCCAGTCTGAAA | ACTGCTGGGTTTGAAAATCCA |
| Bip | TGATAATCAGCCCACCGTAACA | CAAATGTACCCAGAAGGTGATTGTC |
| Cat | CGCCTGTGTGAGAACATTGC | TAGTCAGGGTGGACGTCAGT |
| Chop | CCAGCAGAGGTCACAAGCAC | CGCACTGACCACTCTGTTTC |
| F4/80 | CAGATGGGGGATGACCACAC | AACTCTCCGTGGTGTCAGTG |
| Gadd34 | CTGGTCCAGGCAGACCATC | CTGTTTTGCTCACACCCAAGG |
| Gpx1 | TCGGTTTCCCGTGCAATC | TGAGGGAATTCAGAATCTCTTCATT |
| L-cpt-1 | CAGTGTGCCAGCCACAATTC | TAGGCTTCGTCATCCAGCAA |
| Pparα | TGGAGTCCACGCATGTGAAG | CGCCAGCTTTAGCCGAATAG |
| Sods2 | CGTCACCGAGGAGAAGTACCA | AGGCTGAAGAGCAACCTGAGTT |
| Tnfα | ATCCGAGATGTGGAACTGGC | CGATCACCCCGAAGTTCAGTA |
| Xbp1s | GAGTCCGCAGCAGGTG | CGTCAGAATCCATGGGAA |
| Xbp1 u | TCCGCAGCACTCAGACTACGT | ATGCCCAAAAGGATATCAGACTC |
| Tbp | TGGGATTGTACCACAGCTCCA | CTCATGATGACTGCAGCAAACC |
Total liver protein and nuclear protein extracts were obtained according to the method described above.23 Quantification of the protein concentration in each fraction was undertaken using the Bradford method.24
30 μg of protein was subjected to electrophoresis in 10% polyachrylamide-SDS gel. After transferring the proteins to PVDF membranes (Immobilon-P polyvinylidene difluoride; Millipore Iberica, Bedford, MA, U.S.A.) they were buffered during 1 h at ambient temperature in a solution of TBS-0.1% Tween-20 (TBS-Tween) with 5% skimmed powdered milk. The membranes were incubated for the entire night at 4 °C with the primary antibody (PPARα by Abcam, dilution 1:500; p-IRE-1, IRE, p-PERK and PERK by Cell Signalling, dilution 1:1000 in TBS-Tween and 5% BSA) and 1 h. at ambient temperature with the secondary antibody (antirabbit, dilution 1:3000). Detection was performed using the ECL chemiluminescence reagent kit HRP (Thermo Fisher Scientific) and a ChemidocTM XRS by Bio-Rad (Hercules, CA, U.S.A.). As load control the membranes were incubated with the TATA-box-binding protein (TBP) (Abcam, Cambridge, UK, dilution 1:2500) or β-actin (Sigma-Aldrich, San Louis, MO, U.S.A., dilution 1:3000). The size of the proteins was verified using Bio-Rad molecular weight markers. ImageLab (BioRad) software was used for relative quantification of the proteins.
Binding assay or PPARα binding activityThe ab133107 PPAR alpha Transcription Factor assay kit by Abcam (Cambridge, UK) was used to determine the binding activity of PPARα to DNA in samples of rat liver nuclear extract. This technique is an ELISA where, in each well on a plate of 96 wells, there is a specific DNA containing the response element to PPAR (PPRE). The PPAR present in the nuclear extracts (extracts obtained using the Abcam ab113474 kit) bind specifically to the PPRE and are quantified by colorimetry at 450 nm. A positive control was added to the experiment, with a binding competitor and targets to confirm that the procedures were correct. The results are expressed as binding % of the PPARα to the DNA or PPRE.
StatisticsResults are shown as averages ± standard deviation. The plasma samples were studied in duplicate. Statistical differences were evaluated by variance analysis (ANOVA) for unpaired samples and Sidák post hoc analysis, using the GraphPad Prism computer programme (GraphPad Software V8.3). When deviations between groups were not homogeneous, a non-parametric ANOVA was applied with a Kruskal–Wallis post-test. The limit for statistical significance was set at P < .05.
Results and discussionThe prevalence of NAFLD has increased over recent years, accompanied by a higher incidence of energy metabolism pathologies such as diabetes and metabolic syndrome.1,25 NAFLD is a spectrum of disorders which in their initial phases are characterized by hypertriglyceridaemia and the accumulation of triglycerides in the liver (steatohepatitis).2 To date, and in spite of the almost epidemic proportions attained by the said alterations, no drug has been approved for their treatment, which clearly constitutes an unmet need within the field of medicine.
To seek new therapeutic targets for the treatment and prevention of NAFLD and, based on previous studies by our research group,19,22,26 for this work we designed an experimental model consisting of dietary and pharmacological intervention. Female Sprague-Dawley rats were fed during 3 months with a diet rich in saturated fat and 10% (w/v) liquid fructose, to induce steatohepatitis and hypertriglyceridaemia. Bempedoic acid was administered orally during the last month, to study whether these effects could be reversed by the drug, and to elucidate other involved molecular mechanisms, as well as the inhibition of ATP- citrate lyase (ACL) which is the intrinsic mechanism of action of bempedoic acid.
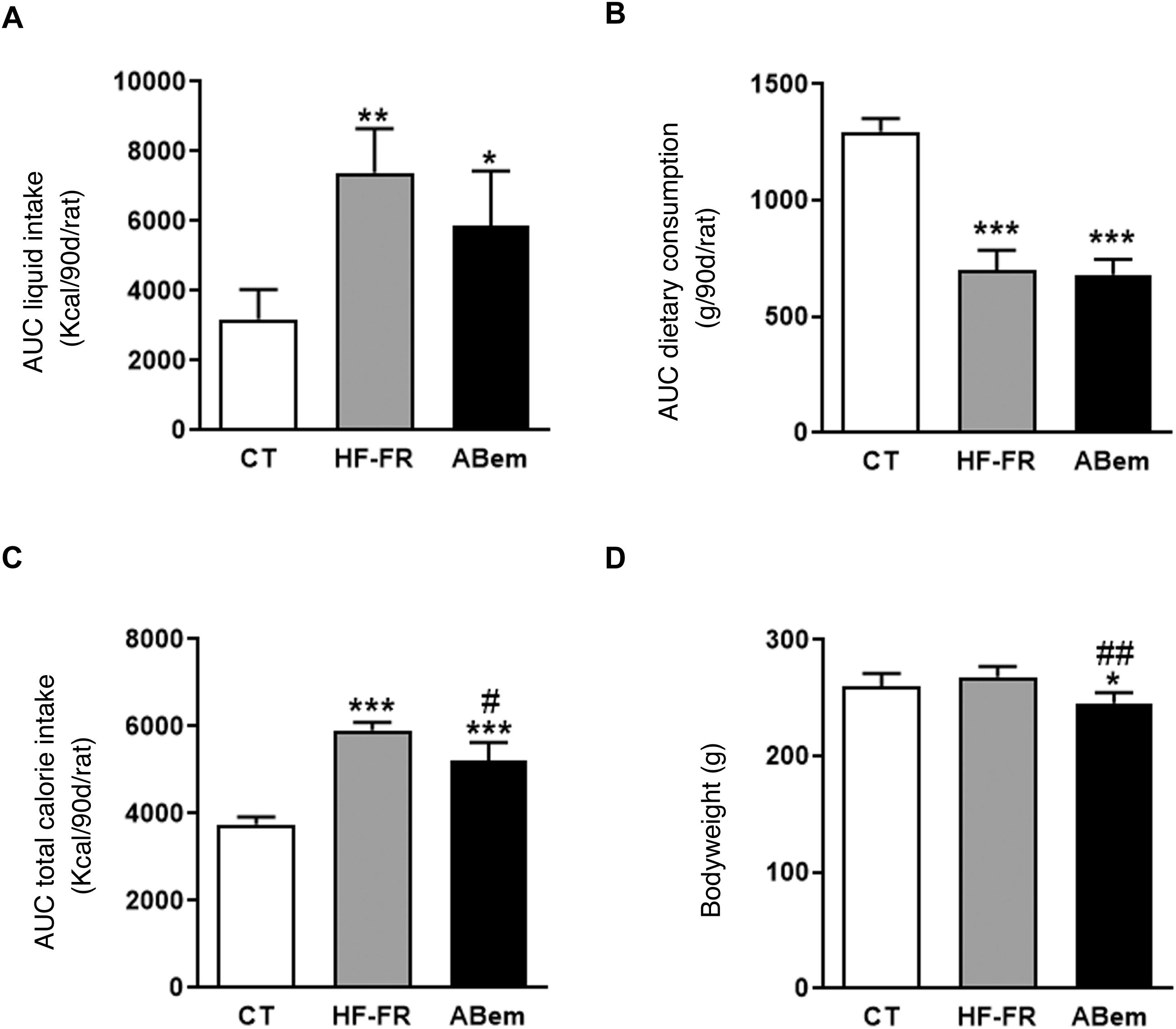
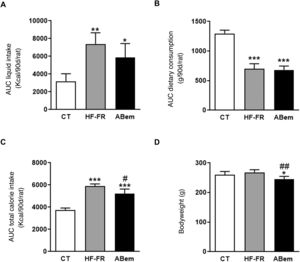
As Fig. 1 shows, the area under the drink consumption curve showed that both groups of animals which received a supplement of 10% (w/v) of fructose, HF-FR and bempedoic acid, drank 2.3 and 1.8 times more than the animals in the control group, respectively (Fig. 1A). This increase was accompanied by a lower intake of solid food, and this was found to be statistically significant in both groups (Fig. 1B). The changes that were observed in consumption of the diet and fructose led to an increase in the total calorie intake by the HF-FR animals. This increase amounted to 60% in the HF-FR group and 38% in the bempedoic acid group, in comparison with the CT group (Fig. 1C). Nevertheless, the HF-FR diet did not modify the bodyweight of the animals throughout the study in comparison with those in the CT group (Fig. 1D). In previous papers we have described how mice fed a Western diet supplemented with liquid fructose showed an increase in bodyweight that was chiefly due to an increase in total calorie intake.22 However, this effect was not observed in the current model. This fact may be due to the composition of the HF diet which, unlike the Western diet, does not contain cholesterol and has a lower proportion of palmitic acid, counterbalanced by the included of stearic acid (saturated fatty acid that is metabolized to oleic acid27). This peculiar composition, as we described recently,28 may explain why the bodyweight of the animals did not increase, even though they receive approximately 1.6 more calories than the control rats.
Drink and food consumption, total calorie intake and animal weights. (A) Area under the curve (AUC) of drink consumption per rat during 90 days, expressed in ml (ml/90d/rat). (B) Area under the curve (AUC) of solid food consumption per rat during 90 days, expressed in g (g/90d/rat). (C) Area under the curve (AUC) of total calorie intake per rat during 90 days, expressed in kcal (kcal/90d/rat). (D) Bodyweight of the animals at the end of treatment, expressed in g. Results expressed as an average ± SD for n = 8 animals/group. Bpda: group fed with HF-FR and treated with bempedoic acid; CT: control group; HF-FR: group fed with a high-fat diet and supplemented with fructose in the drinking water. *P < .05, **P < .01, ***P < .001 vs. the CT group. #P < .05, ##P < .01 vs. HF-FR group.
Although treatment with bempedoic acid did not change the area under the curve for drink consumption or diet, it did reduce total calorie intake by 12% in comparison with the HF-FR group (Fig. 1). Likewise, the drug reduced the bodyweight of the bempedoic acid animals by 9% and 10% vs. the CT and HF-FR groups, respectively, in a statistically significant way (Fig. 1D). The fall in bodyweight observed in the bempedoic acid group may be an effect of the drug that it unconnected with the lower calorie intake of these animals, as the bempedoic acid group had a total calorie intake 1.38 time those of the control group and a 9% fall in bodyweight vs. the CT. Our results agree with those of other published studies, which show that administering bempedoic acid at a dose of 30 mg/kg per day during 3 weeks to rodents fed with a high-fat diet and cholesterol reduces their bodyweight in comparison with animals fed only with the diet.14
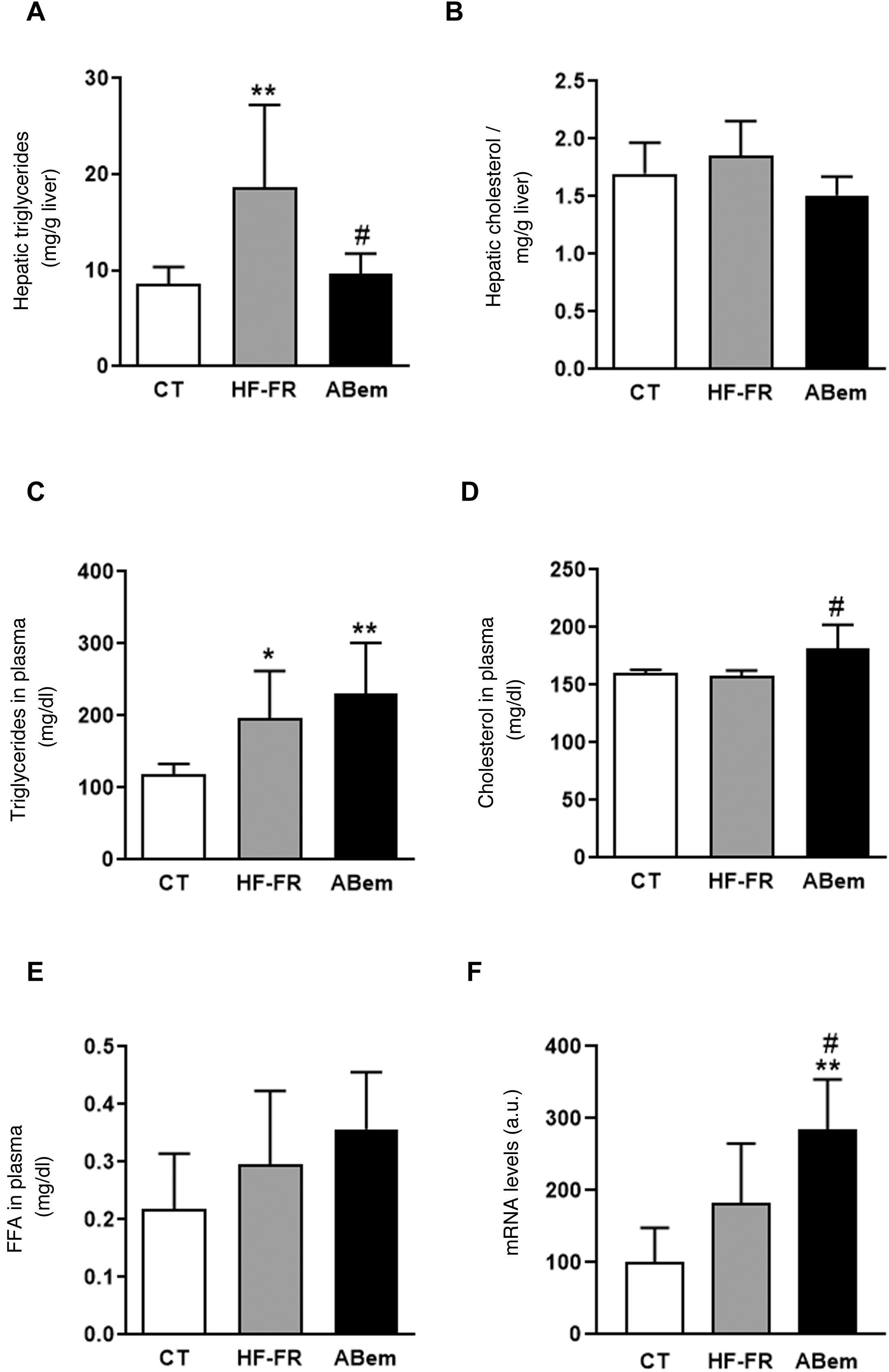
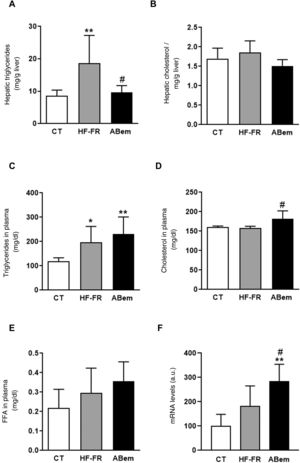
In our study, the administration of a fatty diet and liquid fructose during 3 months induced the appearance of steatohepatitis. Hepatic triglyceride content increased in the HF-FR group, where this increase was 2.2 times the increase in the control animals (Fig. 2A). Likewise, histopathological analysis of liver sections using haematoxylin and eosin staining showed a higher number of vesicles corresponding to the accumulation of triglycerides (Fig. 3A). On the other hand, no differences were found in intrahepatic cholesterol levels in HF-FR respecting the CT group (Fig. 2B). In plasma, only triglyceride levels were found to have increased markedly by 66% in the HF-FR group vs. the CT group (Fig. 2C), while cholesterol and FFA levels did not change with the diet (Fig. 2D and E).
Plasmatic and hepatic lipids. (A) Hepatic triglyceride content expresses as mg triglycerides/gram of liver. (B) Hepatic cholesterol content expresses as mg cholesterol/gram of liver. (C) Levels of triglycerides in plasma. (D) Levels of cholesterol in plasma. (E) Levels of free fatty acids (FFA) in plasma, expressed as mg lipid/dl plasma. (F) mRNA levels of the Acl gene expressed in arbitrary units (a.u.). Results expressed as an average ± SD for n = 8 animals/group. Bpda: group fed with HF-FR and treated with bempedoic acid; CT: control group; HF-FR: group fed a high-fat diet supplemented with fructose in drinking water. *P < .05, **P < .01 vs. CT group. #P < .05 vs. HF-FR group.
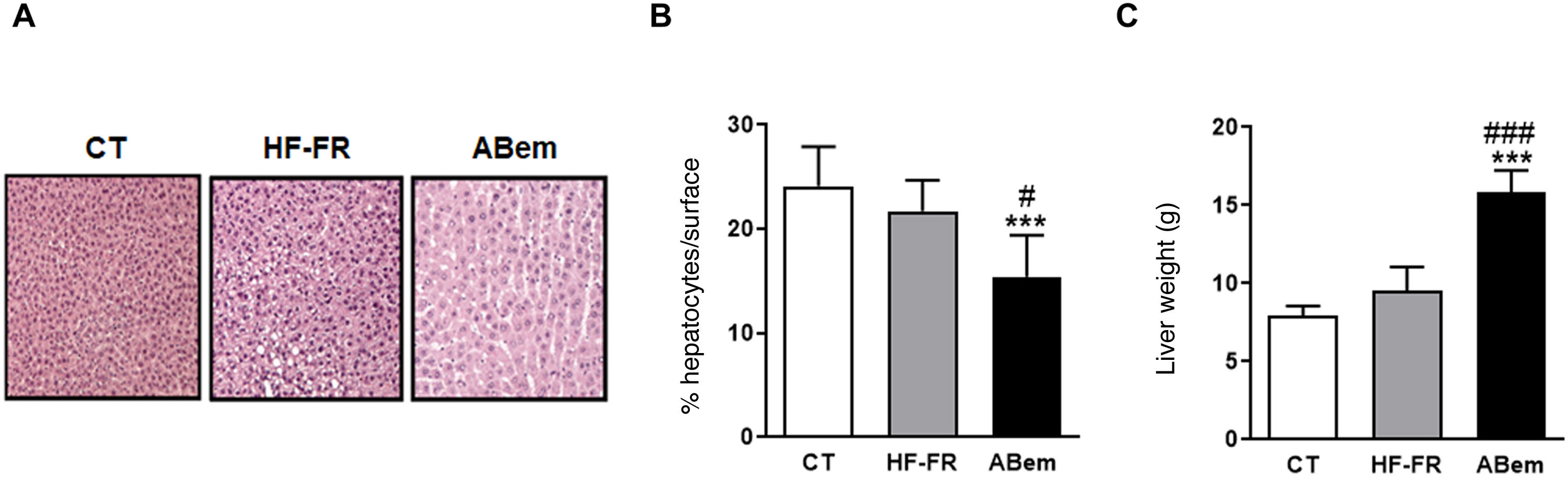
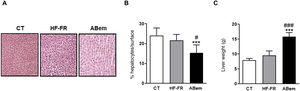
Hepatic hypertrophy and liver weight. (A) Image showing staining of liver slices by haematoxylin and eosin for the three study groups. (B) Percentage (%) of hepatocyte nuclei per quantified surface for evaluation of hepatic hypertrophy. (C) Liver weight at the end of treatment expressed in g. The results are the average ± SD para n = 8 animals/group. Bpda: group fed with HF-FR and treated with bempedoic acid; CT: group control; HF-FR: group fed with a high-fat diet supplemented with fructose in their drinking water. ***P < .001 vs. CT group. #P < .05, ###P < .001 vs. HF-FR group.
Several authors describe bempedoic acid as, able to reduce cholesterol levels in plasma in patients with hypercholesterolaemia without modifying triglyceride levels, unlike what has been observed in rodent models.16,17 Nevertheless, in our experimental model the drug did not change the hypertriglyceridaemia induced by the diet, (Fig. 2C), it increased the levels of cholesterol by 15% in comparison with the HF-FR group. Our hypothesis is that bempedoic acid may induce a higher rate of lipid transport from the liver to the plasma through the VLDL (Fig. 2D), which would justify the increase in plasmatic levels of cholesterol, triglycerides, although this is not significant in the latter case.
Treatment with bempedoic acid reversed the steatohepatitis induced by theHF-FR diet, reducing the content of triglycerides in the liver by 51% vs. the HF-FR group, without modifying intrahepatic levels of cholesterol (Fig. 2A and B). Bempedoic acid reduces the synthesis of cholesterol and fatty acids in the liver, by a mechanism which involves the inhibition of ATP-citrate lyase (ACL).15,29 In our animals, the levels of ACL mRNA increased strongly in the bempedoic acid group (1.56X vs. HF-FR and 2.85X vs. CT) (Fig. 2F). This induction in the expression of ACL may be a mechanism to counterbalance the inhibition of the enzymatic activity triggered by the drug. ACL inhibition involves less hepatic synthesis of fatty acids, and therefore of triglycerides, too. This would accord with the fall in hepatic triglyceride content observed in the bempedoic acid group vs. the HF-FR group.
In spite of the improvement observed in steatohepatitis following treatment with bempedoic acid, livers in the bempedoic acid group showed marked hepatocyte hypertrophy (Fig. 3B), with a 30% reduction in cellular nuclei per quantified area in comparison with the CT group, and 36% in comparison with the HF-FR group. Parallel with this, liver weight in the bempedoic acid group underwent a major increase, to 1.7X that of the HF-FR animals (Fig. 3C). Both effects could not be caused by the accumulation of hepatic lipids, as triglyceride levels were reduced in the bempedoic acid group livers, and cholesterol levels did not change (Fig. 2A and B).
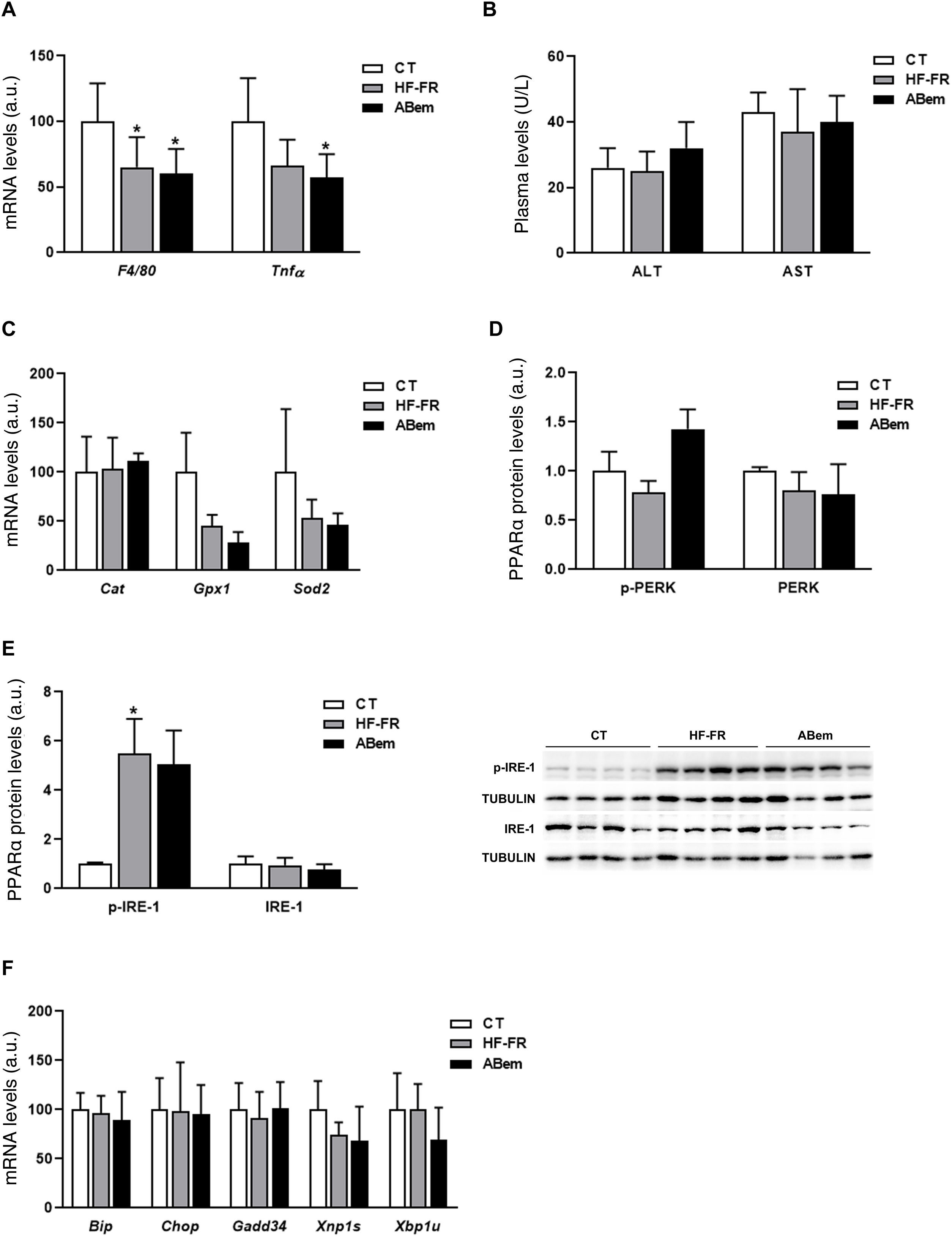
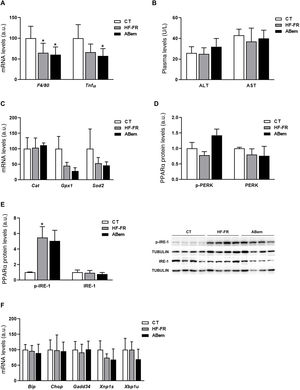
Hepatic inflammation, rising oxidative stress markers and endoplasmic reticulum stress are all intrinsic complications of NAFLD.30,31 We therefore analysed whether the hepatic hypertrophy and increased liver weight found in the bempedoic acid were due to an alteration in one of these three pathways. As Fig. 4 and A shows, the mRNA levels of inflammation biomarkers such as Tnf-α and F4/80 were found to be reduced in both groups of animals which received the HF-FR diet (35% in the HF-FR group and 40% in the bempedoic acid group vs. CT). Administration of the drug did not modify the expression of any of these genes when the group was compared with the HF-FR group. Nor were any differences found between the groups studied in terms of plasma levels of AST and ALT, which are biochemical markers of hepatic lesion and inflammation (Fig. 4B). Likewise, the levels of expression of Cat, Gpx1 and Sods2, which are oxidative stress markers, did not change in either of the HF-FR and bempedoic acid groups (Fig. 4C). When the reticulum stress signalling pathways IRE-1 and PERK are analysed, we find that only levels of p-IRE-1 protein increased in liver samples from HF-FR animals (Fig. 4D), while the levels of p-PERK did not change (Fig. 4E). In spite of activation due to IRE-1 phosphorylation, no significant change occurred in the gene expression of the Bip, Chop, Gadd34, Xbp1s and Xbp1u markers, which are intrinsic to both of these pathways, in the HF-FR rat livers vs. CT (Fig. 4F). Administration of bempedoic acid did not modify the p-IRE-1 protein levels of any of the genes studied in a statistically significant way, if they are compared with those in the HF-FR group (Fig. 4D and F). These results allow us to confirm that our dietary model induces fatty liver, as in the first stages of NAFLD, while avoiding the proinflammatory effect characteristic of diets rich in fat,32 as it does not induce inflammation, oxidative stress or endoplasmic reticulum stress. Nor does treatment with bempedoic acid modify any of these parameters when compared with the CT animals.
Inflammation markers, oxidative stress and reticulum stress. (A) Inflammation marker gene mRNA levels expressed in arbitrary units (a.u.). (B) Levels in plasma of ALT and AST expressed in units/litre of plasma (U/L). (C) Oxidative stress marker gene mRNA levels expressed as arbitrary units (a.u.). (D) Levels of PERK protein (phosphorylated and total) expressed in arbitrary units (a.u.) (E) Levels of IRE-1 protein (phosphorylated and total) expressed in arbitrary units (a.u.). At the right of the graph an image shows the western blot experiment performed for p-IRE1 and IRE-1 for n = 3 samples per study group. (F) mRNA levels of key genes of the different reticulum stress pathways, expressed in arbitrary units (a.u.). The results are the average ± SD for n = 8 animals/group. Bpda: group fed withHF-FR and treated with bempedoic acid; CT: control group; HF-FR: group fed with a high-fat diet supplemented with fructose in their drinking water. *P < .05 vs. CT group.
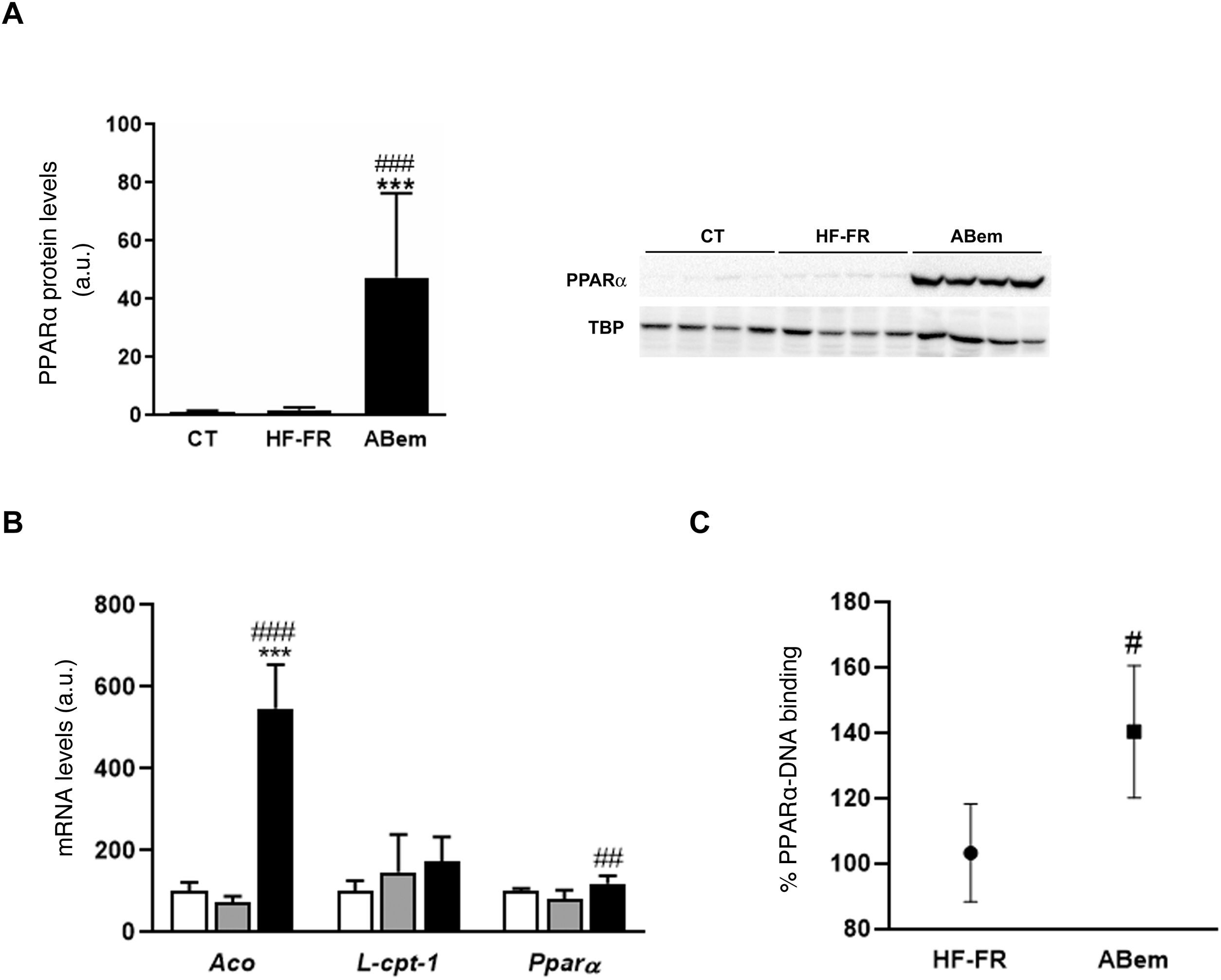
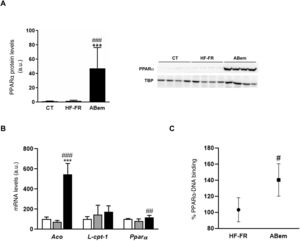
The phenomenon of peroxisome proliferation is another cause of hepatocellular hypertrophy that involves an increase in the size and number of peroxisomes and an increase in fatty acids β-oxidation activity and gene expressions associated with this pathway and regulated by PPARα.33,34 The fibrates, hypolipimiant drugs that are agonists of the PPARα nuclear receptor, are known to be inducers of this phenomenon of peroxisome proliferation in rodents.35 Studies by Samsoondar et al.,17 in a murine model, suggested that bempedoic acid may act in a similar way to a peroxisome proliferator; the increase observed in the liver weight of the animals treated with bempedoic acid was correlated with a slight effect of peroxisome proliferation, an increase in fatty acid β-oxidation and an increase in the expression of Aco (acyl-CoA oxidase, an enzyme which limits the velocity of the peroxisome β-oxidation reaction and the target of PPARα). Our results are in accordance with the said hypothesis. Consumption of the HF-FR diet did not modify the protein expression of PPARα or that of its target genes (Figs. 5A and B). However, treatment with bempedoic acid increased the expression of PPARα, in hepatic protein levels (25X vs. HF-FR) (Fig. 5A) and mRNA levels (1.46X vs. HF-FR). The activation of PPARα by the drug increased the mRNA levels of its target genes, and the gene expression of Aco was 7.4X in the bempedoic acid group vs. the HF-FR group (Fig. 5B). To elucidate whether in our model bempedoic acid behaves as a direct activator of PPARα, we performed binding studies or studies of binding activity of PPARα to its response element (PPRE), in hepatic samples from HF-FR and bempedoic acid animals, using the commercial PPAR alpha Transcription Factor assay kit by Abcam. The animals treated with bempedoic acid had 40% more PPARα - PPRE binding capacity than the animals in the HF-FR group, as is shown in Fig. 5C. Taken together, all of these results suggest that the bempedoic acid behaves as a direct activator of the PPARα receptor. PPARα activation would significantly contribute to the effect of bempedoic acid on diet-induced steatohepatitis, and at the same time, it may cause hepatocyte hypertrophy and the increase in liver weight observed in the treated animals.
Determination of PPARα expression and activity (A) PPAR protein levels expressed in arbitrary units (a.u.). An image shows the western blot assay for n = 4 samples per study group. The results were normalized using the TATA-box-binding protein (TBP) as control. (B) mRNA levels of PPAR target genes expressed in arbitrary units (a.u.). (C) PPARα to PPRE binding activity expressed as a percentage (%) of binding in the HF-FR and bempedoic acid groups. Results expressed as average ± SD for n = 8 animals/group. Bpda: group fed with HF-FR and treated with bempedoic acid; CT: control group; HF-FR: group fed with a high-fat diet supplemented with fructose in their drinking water. ***P < .001 vs. CT group. #P < .05, ##P < .01, ###P < .001 vs. HF-FR group.
Our results allow us to conclude that the administration of a fatty acid rich and cholesterol-free diet to female Sprague-Dawley rats, supplemented with 10% (w/v) fructose in their drinking water during 3 months, induces steatohepatitis and hypertriglyceridaemia. It does so without altering proinflammatory markers, inducing oxidative stress or altering endoplasmic reticulum stress pathways, indicating that we are in the initial phases of NAFLD. Bempedoic acid reverses steatohepatitis through a mechanism that would involve, at least partially, the direct activation of the nuclear receptor PPARα. This mechanism differs from, although it may not exclude, inhibition of the ACL, the chief mechanism of action of bempedoic acid.
FinancingThis work was financed by the Spanish Society of Arteriosclerosis by a FEA/SEA 2020 Basic Arteriosclerosis Research Grant, and a Ministry of Science and Innovation project, reference SAF2017-82369.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Bentanachs R, Velázquez AM, Sánchez RM, Alegret M, Laguna JC, Roglans N. El ácido bempedoico como activador PPARα: Nuevas perspectivas para el tratamiento de la esteatosis hepática en un modelo experimental de rata hembra. Clin Investig Arterioscl. 2022;34:57–67.