Bempedoic acid is a novel non-statin drug that was developed to treat hyperlipidemia in combination with other lipid-lowering drugs in those patients who need additional lipid lowering.
Objectives(1) To investigate the lipid efficacy of bempedoic acid; (2) to analyze the anti-inflammatory effects of bempedoic acid estimated through high sensitivity C-reactive protein (hsCRP).
MethodsWe performed a meta-analysis including randomized trials of bempedoic acid therapy, reporting low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), apolipoprotein B and hsCRP with a minimum of 4 weeks of follow-up. The primary endpoint was defined as the percentage change in lipids and hsCRP levels measured from baseline to follow-up, comparing groups of subjects on bempedoic acid versus placebo.
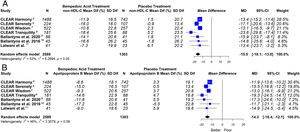
ResultsSeven eligible trials of bempedoic acid (3892 patients) were included. The bempedoic acid therapy was associated with a significant reduction in LDL-C levels [−20.3% (CI 95% −23.5 to −17.1)]; I2=43%]. Similarly, a significant percentage reduction in the apolipoprotein B levels [−14.3% (CI 95% −16.4 to −12.1)]; p<0.05; I2=46%], non-HDL-C levels [−15.5% (CI 95% −18.1 to −13.0)]; p<0.05; I2=53%] and hsCRP [−23.4% (CI 95% −32.6 to −14.2)]; p<0.05; I2=69%] was demonstrated with the bempedoic acid use. The sensitivity analysis showed that the results were robust.
ConclusionOur data suggests that the use of bempedoic acid significantly reduces the levels of all atherogenic lipid markers, including LDL-C, non-HDL-C and apolipoprotein B. Furthermore, considering hsCRP levels, the drug produces an anti-inflammatory effect.
El ácido bempedoico es un fármaco nuevo no perteneciente al grupo de las estatinas, que fue desarrollado para tratar la hiperlipidemia, junto con otros fármacos liporreductores, en aquellos pacientes que necesitan una reducción lipídica adicional.
Objetivos(1) Estudiar la eficacia anti-lipídica del ácido bempedoico; (2) analizar los efectos antiinflamatorios del ácido bempedoico, calculados a través de la proteína C reactiva de alta sensibilidad (hsCRP).
MétodosRealizamos un meta-análisis incluyendo ensayos aleatorios de terapia de ácido bempedoico, reportando colesterol de lipoproteína de baja densidad (LDL-C), colesterol de lipoproteína de no alta densidad (no-HDL-C), apolipoproteína B y hsCRP con un mínimo de 4 semanas de seguimiento. El objetivo primario se definió como el cambio porcentual de lípidos y niveles de hsCRP medidos desde el inicio hasta el seguimiento, comparando los sujetos de los grupos ácido bempedoico frente a placebo.
ResultadosSe incluyeron siete ensayos elegibles de ácido bempedoico (3.892 pacientes). La terapia de ácido bempedoico se asoció a una reducción significativa de los niveles de LDL-C [−20,3% (IC 95% de −23,5 a −17,1)]; I2 = 43%]. De igual modo, se demostró una reducción porcentual significativa de los niveles de apolipoproteína B [−14,3% (IC 95% de −16,4 a −12,1)]; p < 0,05; I2 = 46%], niveles de no-HDL-C [−15,5% (IC 95% de −18,1 a −13)]; p < 0,05; I2 = 53%] y hsCRP [−23.4% (IC 95% de −32,6 a −14,2)]; p < 0,05; I2 = 69%] con el uso de ácido bempedoico, reflejando el análisis de sensibilidad que los resultados eran sólidos.
ConclusiónNuestros datos sugieren que el uso de ácido bempedoico reduce significativamente los niveles de todos los marcadores lipídicos aterogénicos, incluyendo LDL-C, no-HDL-C y la apolipoproteína B. Además, considerando los niveles de hsCRP, el fármaco produce un efecto antiinflamatorio.
Many patients do not achieve the low-density lipoprotein cholesterol (LDL-C) targets recommended in the current guidelines, even with the use of statins and ezetimibe.1 The muscular effects could be one of the reasons that causes low adherence to statins. The introduction of proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors is an alternative to hypercholesterolemia therapies, but its use was limited by the high cost. Consequently, new lipid-lowering strategies are necessary in order to reduce the residual cardiovascular risk in patients who, despite receiving maximum tolerated lipid-lowering therapy, do not achieve the recommended lipid goals. Hence, there is a growing interest in develop drugs with a statin-like mechanism of action but associated with less or none muscular side effects. Consequently, new lipid-lowering strategies are necessary to reduce residual cardiovascular risk
Bempedoic acid is a non-statin lipid-lowering drug developed for the treatment of hypercholesterolaemia.2 It is a prodrug that requires activation by the enzyme very long-chain acyl-coA synthetase 1 present in the liver but absent in most other tissues. It lowers LDL-C by inhibiting ATP citrate lyase (ACL), an enzyme involved in cholesterol biosynthesis, which acts upstream of 3-hydroxy-3-methyl-glutaryl-CoA (HMGCoA) reductase.3
Mendelian randomization of large human study cohorts has validated ACL inhibition as a target for LDL-C lowering and atheroprotection.4 Several phase 2 and 3 clinical trials revealed that bempedoic acid effectively reduce LDL-C as monotherapy, combined with ezetimibe, added to statin therapy and in statin-intolerant hypercholesterolemic patients.5–11
Based on positive findings in these clinical trials, bempedoic acid was approved in the USA and in the EU as monotherapy and as a fixed-dose combination with ezetimibe.12
Accumulating evidence suggests that inflammation plays an important role in the pathophysiology of atherosclerotic plaque stabilization and thromboembolism, with inflammatory cells being involved in all stages of atherosclerosis development. Therefore, anti-inflammatory properties of lipid-lowering drugs would contribute in plaque stabilization and in the prevention of thromboembolic events.13
A previously published meta-analysis has evaluated the lipid effects of bempedoic acid use.14 However, only five phase 2 studies were included, many of which used lower doses of bempedoic acid than currently recommended. The multiple phase 3 studies that have been published in the last two years were not included. Also, the anti-inflammatory effect was not evaluated in the analysis.
Therefore, the objectives of the present meta-analysis were: (1) to investigate the lipid efficacy of bempedoic acid, analyzing all the evidence available to date; (2) to analyze the anti-inflammatory effects of bempedoic acid, estimated through high-sensitivity C-reactive protein (hsCRP).
Material and methodsData extraction and quality assessmentOur meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews.15 A literature search was performed that identified clinical trials of bempedoic acid (ETC-1002) and published between January 2000 and April 2020. Two independent reviewers searched the electronic PubMed/MEDLINE, Embase, Google Scholar, Scielo and Cochrane Controlled Trials databases using the following terms: “bempedoic acid”, “ETC-1002” and “non-statin lipid-lowering therapy”, “cholesterol”, “dyslipidemia”, “hypercholesterolemia”, “heterozygous familial hypercholesterolemia”, “combined familial hyperlipidemia”, “elevated cholesterol levels”, “elevated cholesterol”, “lipoproteins”, “LDL-C”, “non-high-density lipoprotein cholesterol” (non-HDL-C), “apolipoprotein B” and “hsCRP”.
All the analyzed studies meet the following inclusion criteria: (a) Comparisons of efficacy of bempedoic acid versus placebo; (b) Follow-up duration ≥4 weeks; (c) Randomized clinical trials; (d) Reporting of change in lipids and hsCRP values between baseline and follow-up. The lipids evaluated were LDL-C, non-HDL-C and apolipoprotein B.
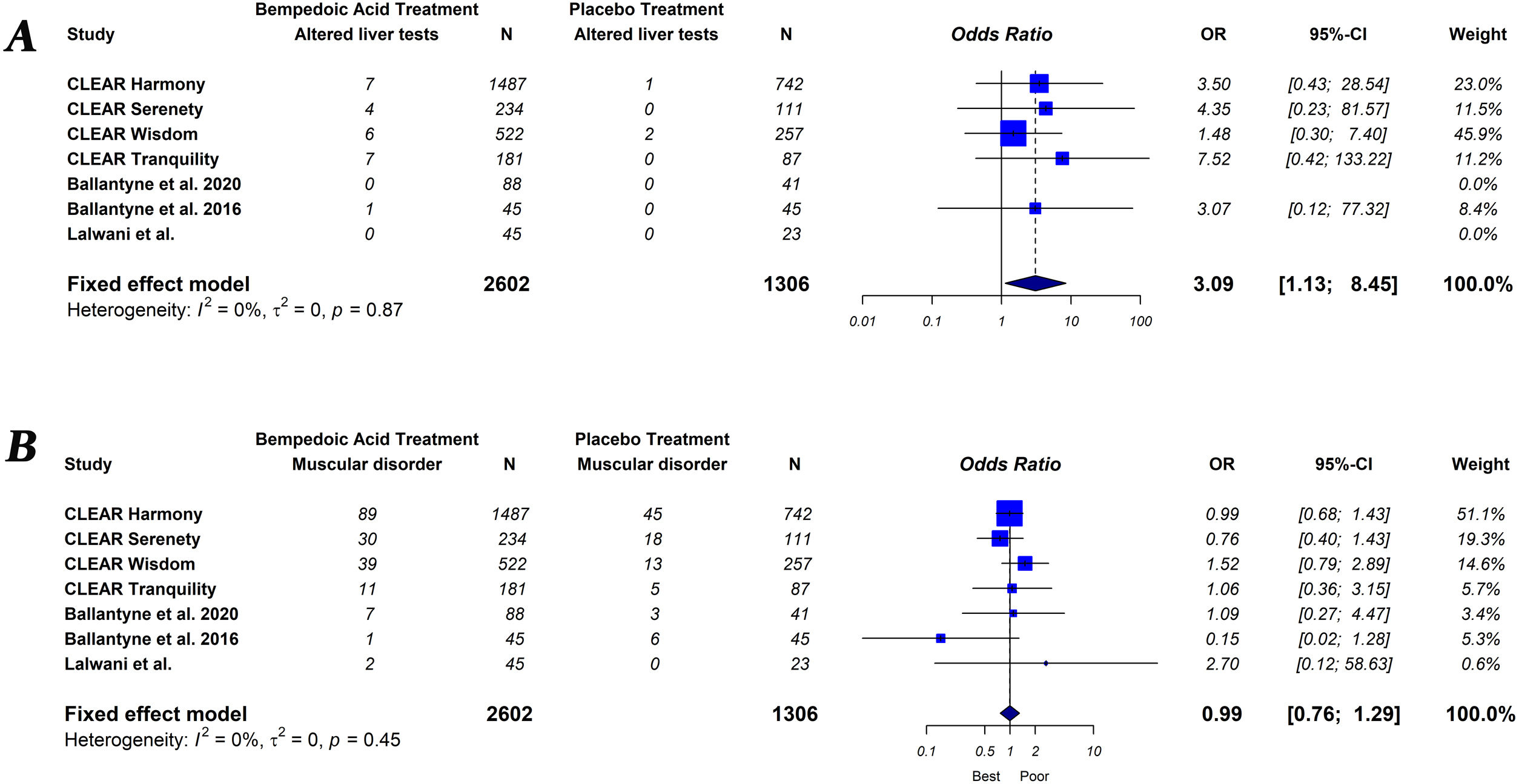
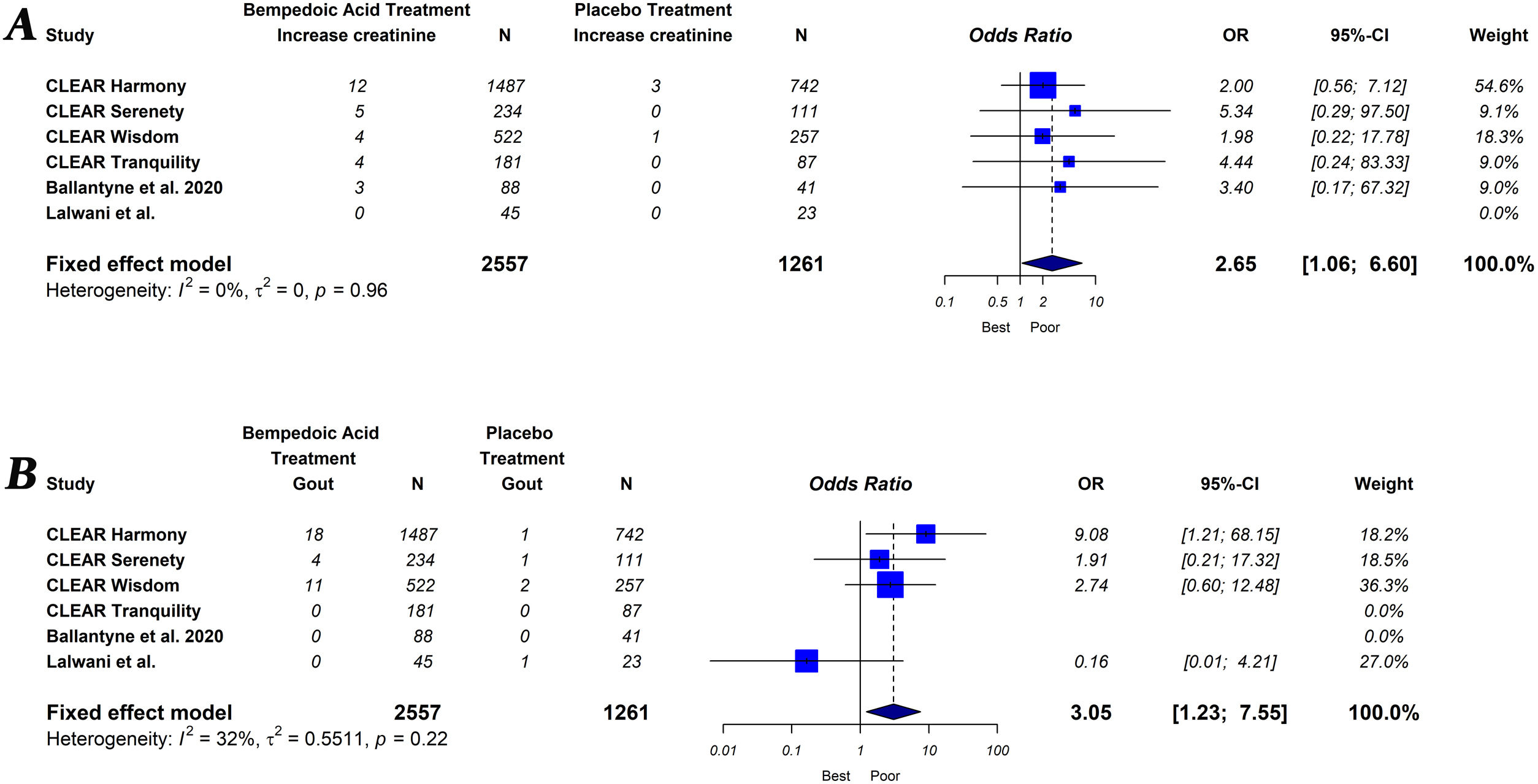
The primary endpoint of the study was defined as the percentage change in lipids and hsCRP levels measured from baseline to follow-up, comparing groups of subjects on bempedoic acid versus placebo. As a secondary exploratory endpoint, we evaluated the safety of bempedoic acid. The occurrence of muscular disorder (myalgia, muscle spasms, pain in extremity or muscular weakness), elevation of liver enzymes (alanine or aspartate aminotransferase level>3×ULN), increased creatinine level, occurrence of gout and serious adverse events (results in death, is life-threatening, requires in-patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, is a congenital abnormally defect or is an important medical event) were evaluated.
When the summary/dispersion measures were not mean and standard deviation, conversion tools previously suggested by the literature were used.16
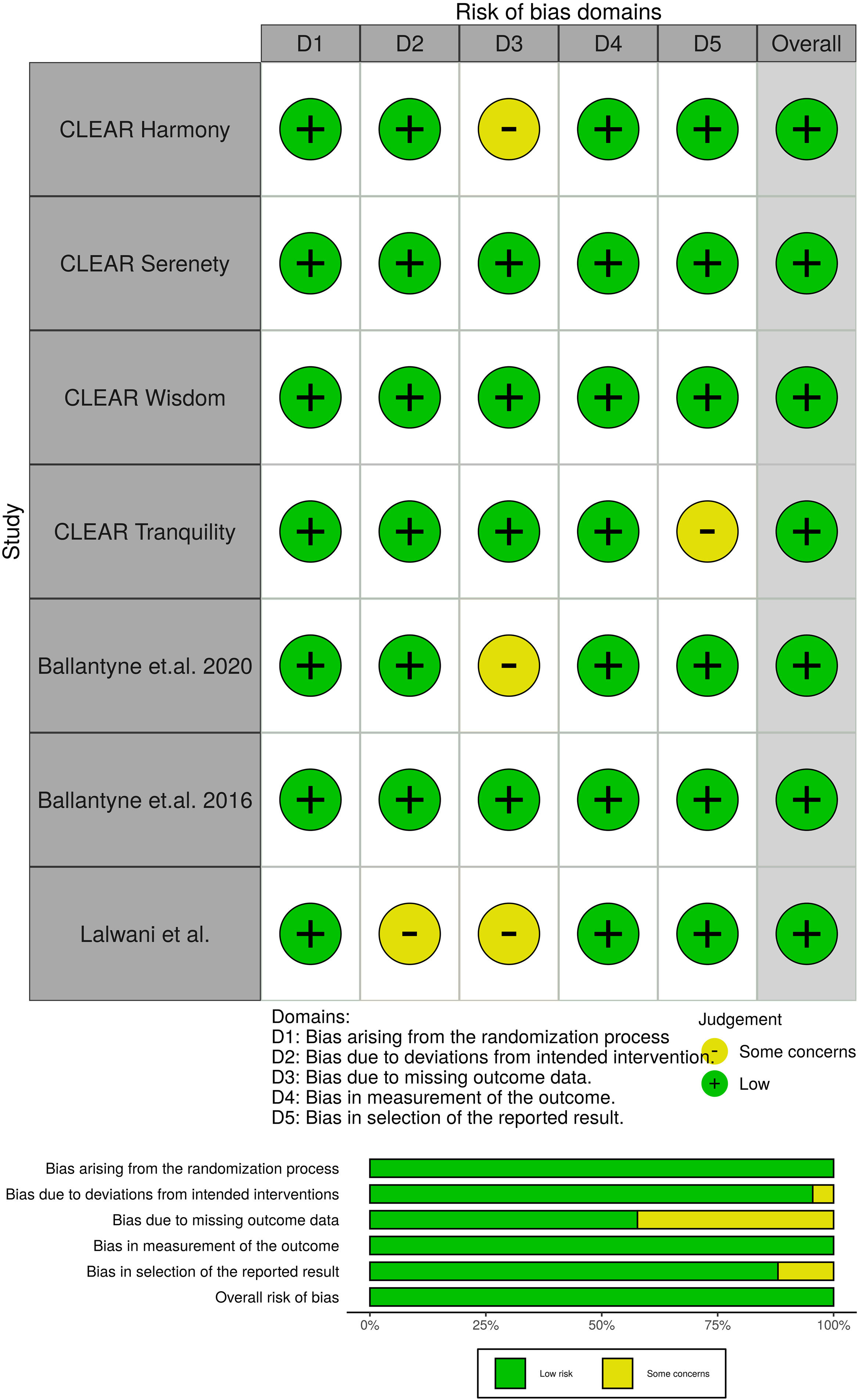
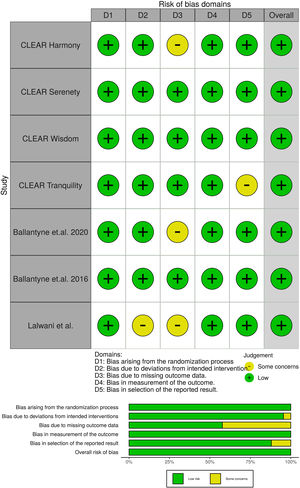
Potential risks of bias were evaluated for all included trials, using the Cochrane tool developed for this purpose.17 This tool assesses bias in five different domains: bias arising from the randomization, bias due to deviations from intended intervention, bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported result. Each domain was rated as “High”, “Low” or “Some concerns” depending on the judgment of each author following the recommendations.
Statistical analysisThe summary effect of bempedoic acid on lipids and hsCRP levels was estimated. Measures of effect size were expressed as mean difference for the primary endpoint and as Odds Ratio (OR) for the secondary exploratory analysis, and the I2 statistic was calculated to quantify between-trial heterogeneity and inconsistency. Meta-analyses were conducted using a fixed-effect model or a random-effect model based on the low (<40%) or moderately-high (>40%) inter-study heterogeneity. The level of statistical significance was set at a two-tailed alpha of 0.05. Statistical analyses were performed using the R software for statistical computing version 3.5.1 with additional specific packages.18
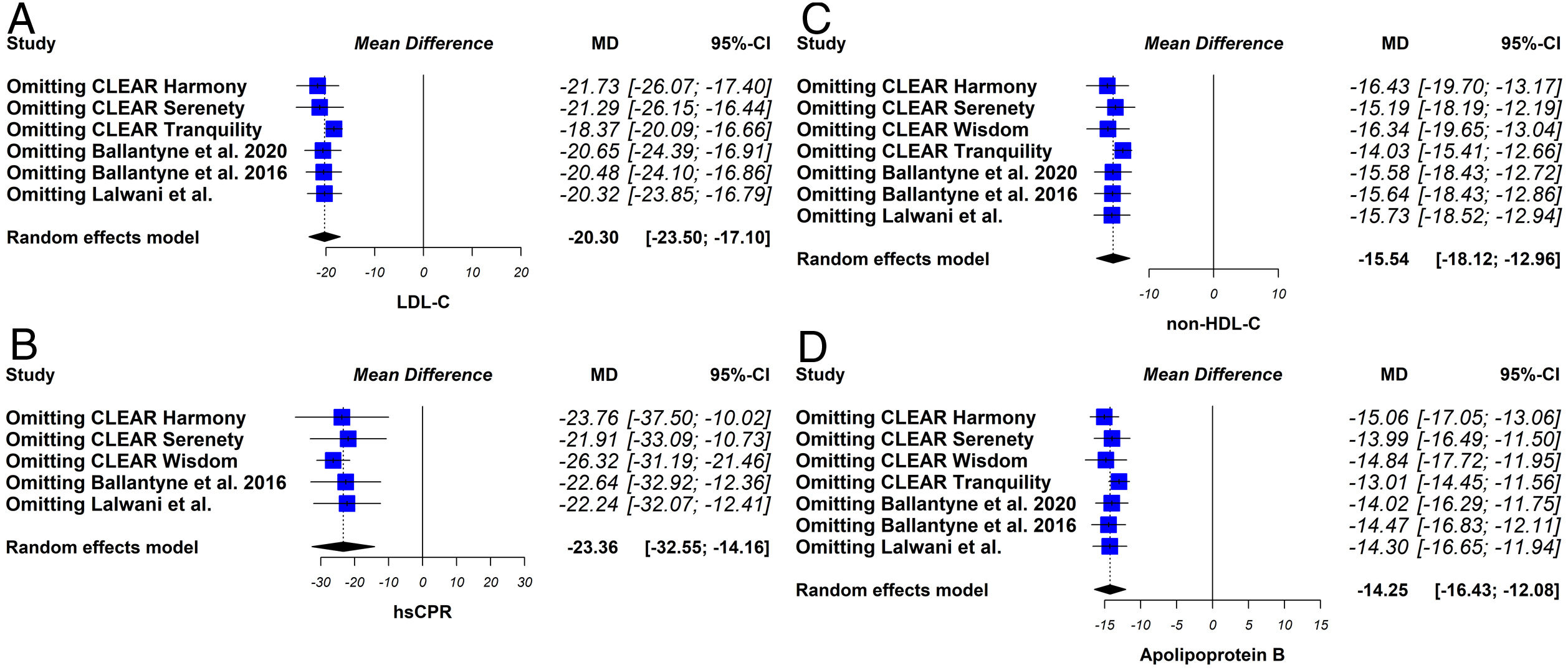
Sensitivity analysesThe sensitivity analysis consists of replicating the results of the meta-analysis, excluding in each step one of the studies included in the review. If the results obtained are similar, both in direction and magnitude of the effect and statistical significance, it indicates that the analysis is robust.
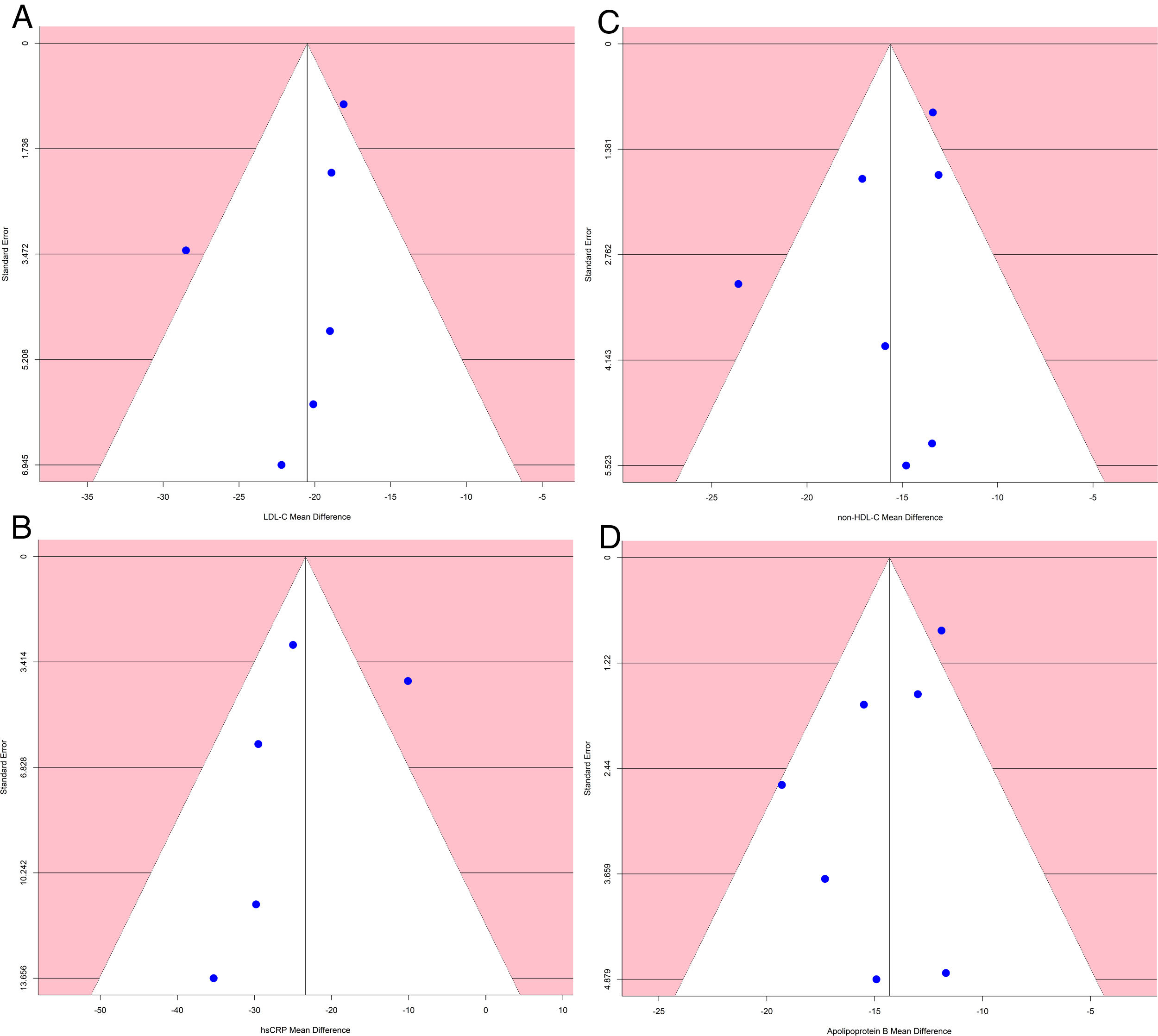
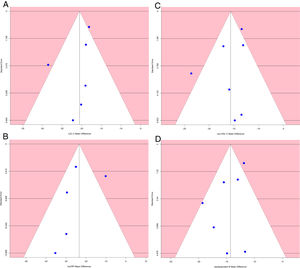
Analysis of publication biasA funnel plot using the standard error (SE) for mean difference was created, and Egger's regression intercept tests were done.
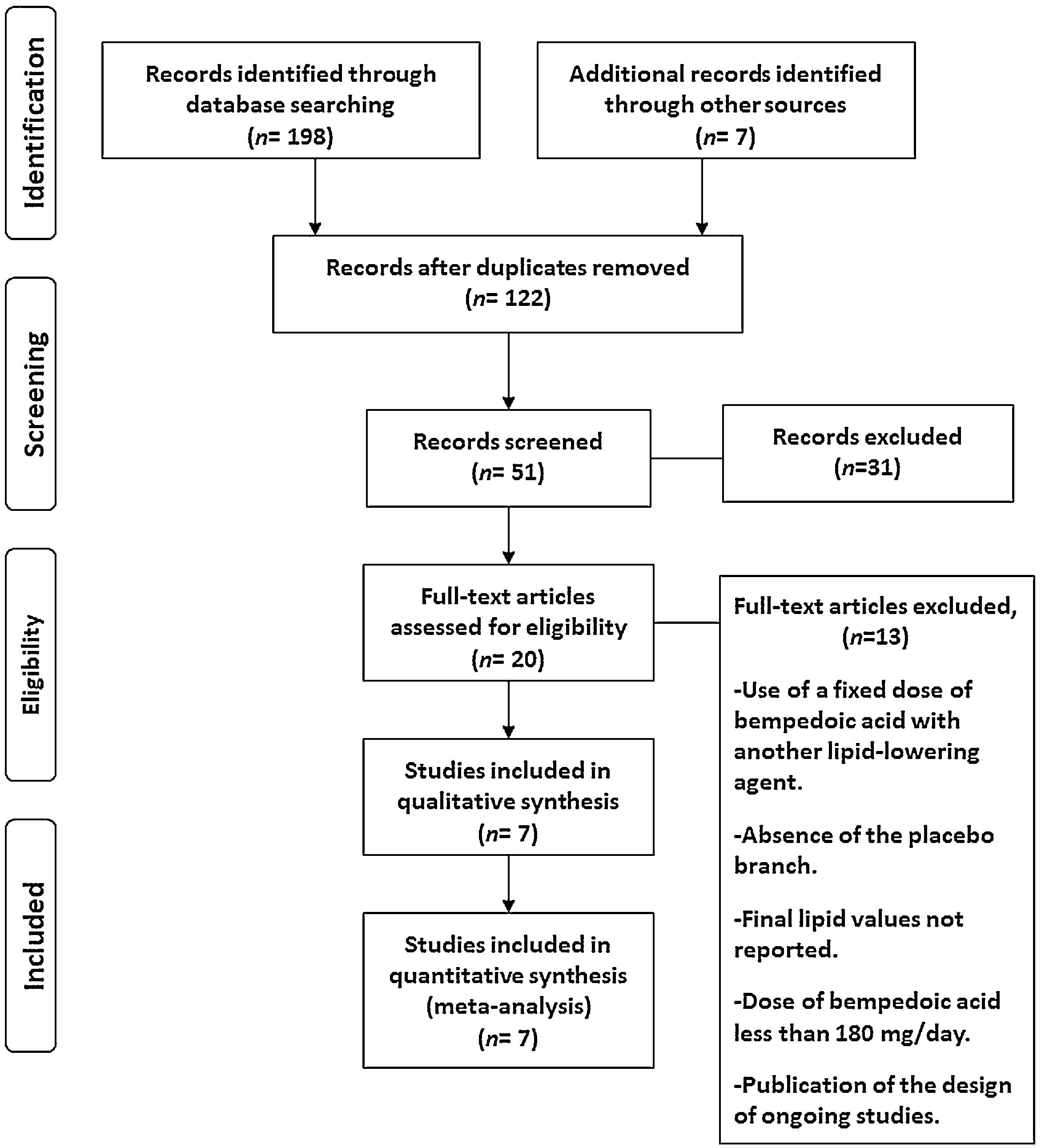
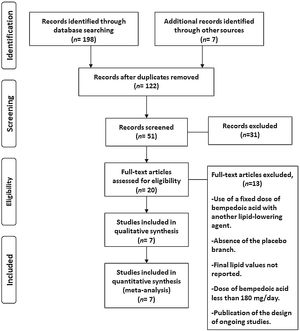
ResultsSeven eligible trials of bempedoic acid, including 3892 patients, were identified and considered eligible for the analyses. All these studies were taken into account for the analysis of non-HDL-C and apolipoprotein B. In this case, there was a total of 2589 subjects allocated to receive bempedoic acid and 1303 subjects allocated to the respective placebo group. Six studies were included for the analysis of LDL-C and five trials for hsCRP analysis because these data were reported. A flow diagram of the study's screening process has been shown in Fig. 1.
All studies evaluated were randomized clinical trials and had a placebo group. The quality of the studies evaluated can be seen in Fig. 2.
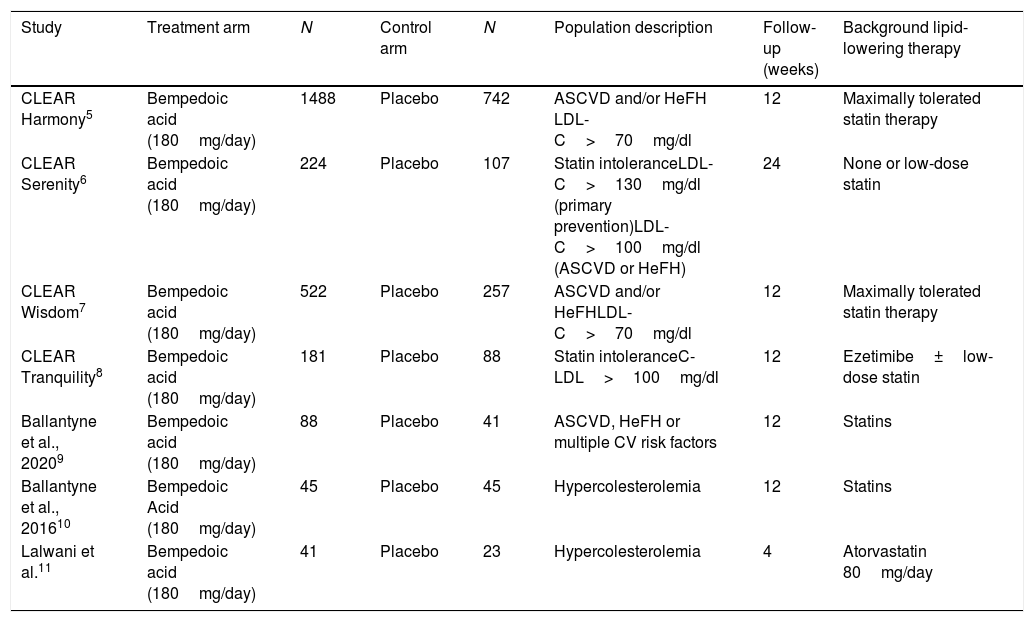
Of the total of studies, five were phase 3 and two were phase 2 studies. Two studies included dyslipidemic patients and two others included statin intolerant subjects. Likewise, two studies included adults with atherosclerotic cardiovascular disease, heterozygous familial hypercholesterolemia or both. One study included, in addition to these patients, subjects with multiple cardiovascular risk factors. In all trials, the patients were eligible to participate if they had been taking stable doses of maximally tolerated statin therapy either alone or in combination with other lipid-lowering therapies and if they had a LDL-C level above the threshold defined in each study. In most cases, this LDL-C threshold ranged from 70 to 100mg/dl in patients with cardiovascular disease or heterozygous familial hypercholesterolemia and from 100 to 130mg/dl in subjects in primary prevention. The follow-up ranged between 4 and 24 weeks. The characteristics of the studies included in the analysis can be seen in Table 1.
Characteristics of the studies included in the analysis.
| Study | Treatment arm | N | Control arm | N | Population description | Follow-up (weeks) | Background lipid-lowering therapy |
|---|---|---|---|---|---|---|---|
| CLEAR Harmony5 | Bempedoic acid (180mg/day) | 1488 | Placebo | 742 | ASCVD and/or HeFH LDL-C>70mg/dl | 12 | Maximally tolerated statin therapy |
| CLEAR Serenity6 | Bempedoic acid (180mg/day) | 224 | Placebo | 107 | Statin intoleranceLDL-C>130mg/dl (primary prevention)LDL-C>100mg/dl (ASCVD or HeFH) | 24 | None or low-dose statin |
| CLEAR Wisdom7 | Bempedoic acid (180mg/day) | 522 | Placebo | 257 | ASCVD and/or HeFHLDL-C>70mg/dl | 12 | Maximally tolerated statin therapy |
| CLEAR Tranquility8 | Bempedoic acid (180mg/day) | 181 | Placebo | 88 | Statin intoleranceC-LDL>100mg/dl | 12 | Ezetimibe±low-dose statin |
| Ballantyne et al., 20209 | Bempedoic acid (180mg/day) | 88 | Placebo | 41 | ASCVD, HeFH or multiple CV risk factors | 12 | Statins |
| Ballantyne et al., 201610 | Bempedoic Acid (180mg/day) | 45 | Placebo | 45 | Hypercolesterolemia | 12 | Statins |
| Lalwani et al.11 | Bempedoic acid (180mg/day) | 41 | Placebo | 23 | Hypercolesterolemia | 4 | Atorvastatin 80mg/day |
ASCVD: atherosclerotic cardiovascular disease; CV: cardiovascular; HeFH: Heterocygous Familial Hypercholesterolemia; LDL-C: low density lipoprotein cholesterol.
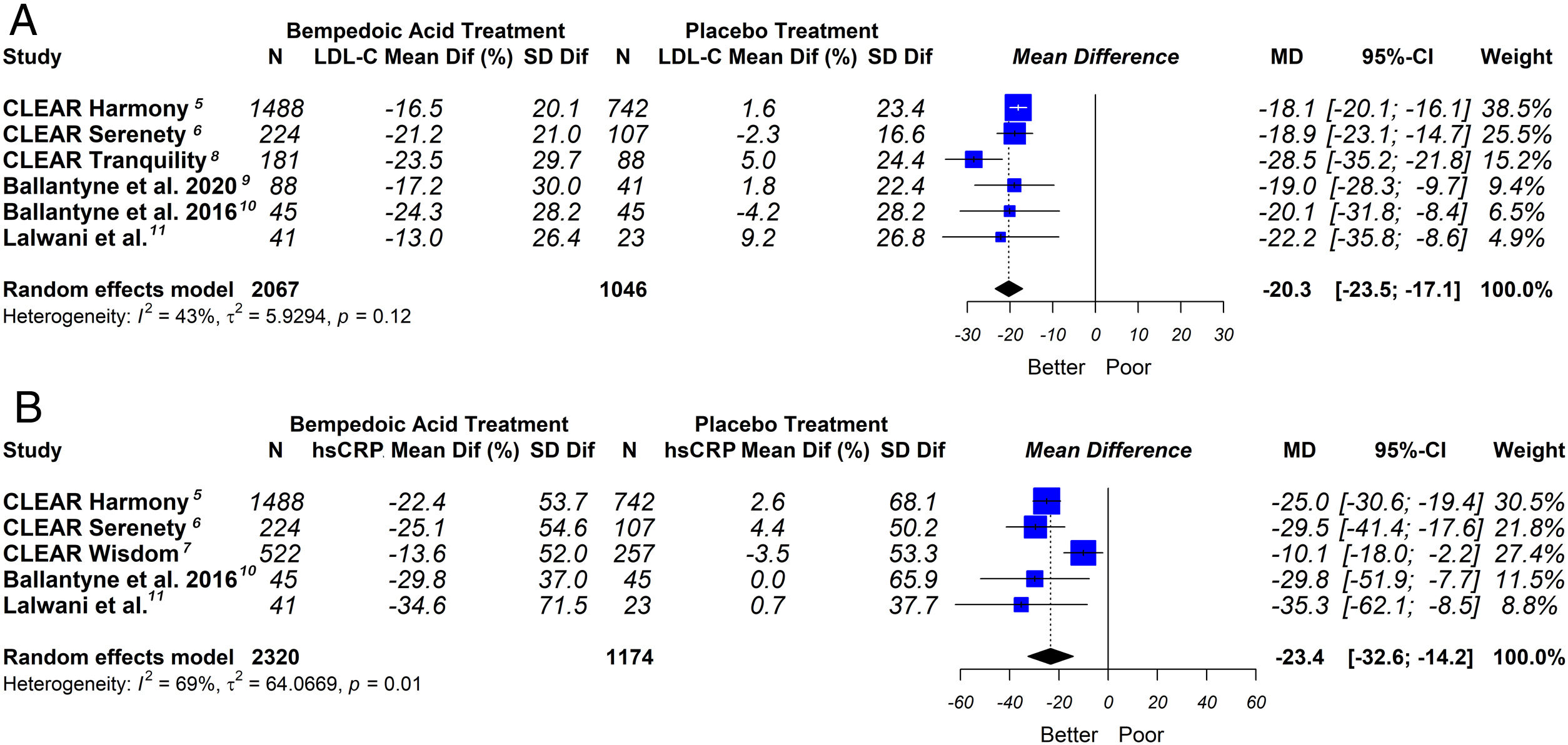
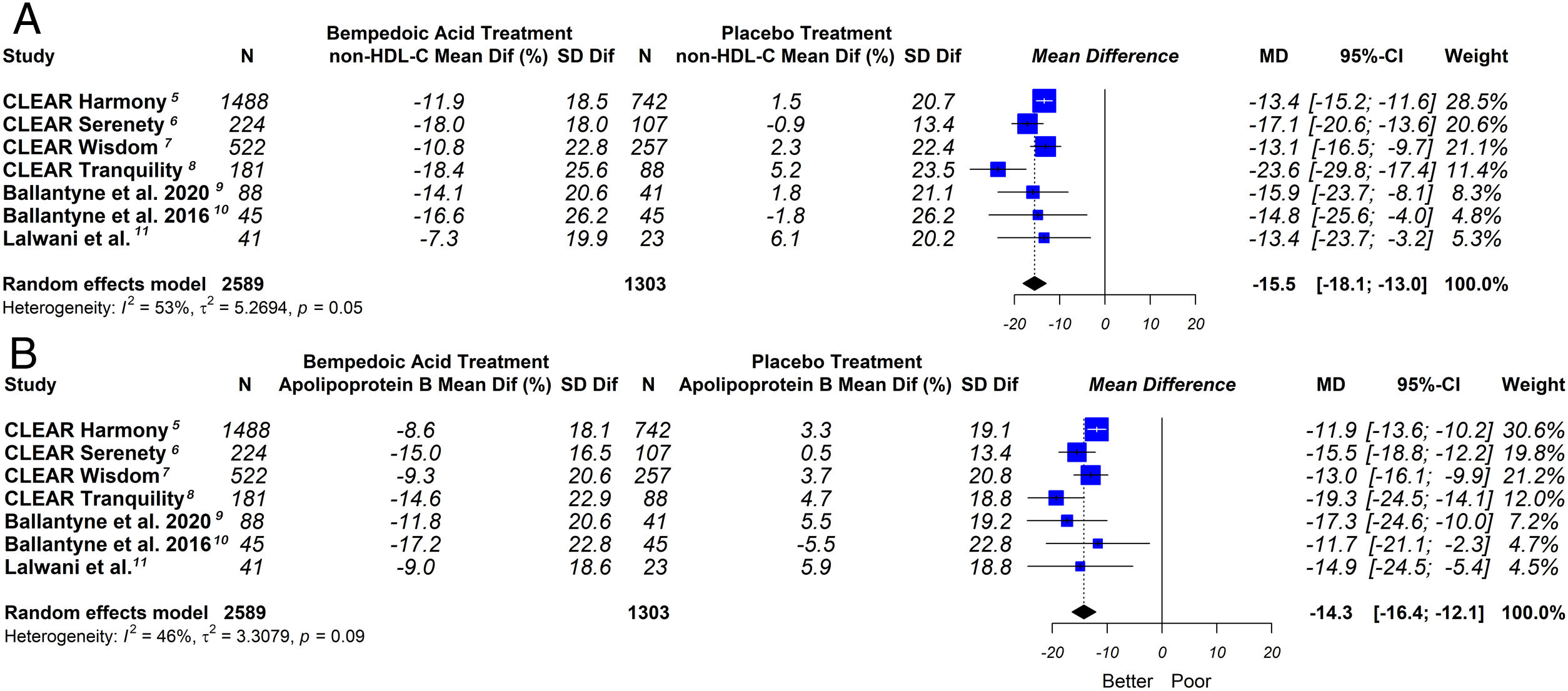
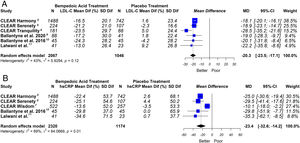
This meta-analysis showed that bempedoic acid was associated with a significant percentage reduction in LDL-C levels [−20.3% (CI 95% −23.5 to −17.1)]; p<0.05; I2=43%]. Similarly, a significant percentage reduction in the apolipoprotein B levels [−14.3% (CI 95% −16.4 to −12.1)]; p<0.05; I2=46%] and non-HDL-C levels [−15.5% (CI 95% −18.1 to −13.0)]; p<0.05; I2=53%] was demonstrated with the bempedoic acid use.
On the other hand, bempedoic acid was associated with a significant percentage reduction in hsCRP levels [−23.4% (CI 95% −32.6 to −14.2)]; p<0.05; I2=69%].
The graphic representation of the effect of bempedoic acid on lipid and inflammatory markers can be seen in Figs. 3 and 4.
Bempedoic acid therapy showed a similar rate of serious adverse events (OR, 1.09; 95% CI, 0.89–1.33; I2=0%) and muscle-related adverse events (OR, 0.99; 95% CI, 0.76–1.29; I2=0%) compared to placebo. However, the use of bempedoic acid was more frequently associated with an increase in liver enzymes (OR, 3.09; 95% CI, 1.13–8.45; I2=0%), creatinine level (OR, 2.65; 95% CI, 1.06–6.60; I2=0%) and the incidence of gout (OR, 3.05; 95% CI, 1.23–7.55; I2=32%). The graphic representation of the main adverse effects can be seen in the supplementary material (Figs. S1 and S2).
The funnel plot of standard error by mean difference of endpoints did not suggest publication bias (Fig. 5). In the same way, Egger's regression intercept tests gave a p value of 0.25, not indicating possible publication bias.
The sensitivity analysis showed that the results were robust (Fig. 6).
DiscussionIn this meta-analyses, which included the phase 3 studies from the CLEAR program, bempedoic acid therapy compared with placebo was associated with a significant reduction in the levels of all atherogenic lipoproteins and inflammatory markers such as hsCRP.
Dyslipidemia is a critical predisposing factor for the development of cardiovascular diseases. Statins competitively inhibit HMGCoA reductase, the rate-limiting enzyme in cholesterol synthesis. In response, a compensatory upregulation in hepatic LDL receptor cell surface expression occurs, leading to a reduction in circulating LDL-C by 30–50%.19 Recently published cholesterol treatment guidelines emphasize the use of statins as the preferred treatment strategy for both primary and secondary prevention of cardiovascular disease.1,20 However, despite the widespread prescription of these drugs, adherence to statin therapy is a major challenge worldwide. The most common adverse events of statins are muscle related and are the main reason for statin non-adherence and/or discontinuation.21,22 Thus, patients who cannot tolerate a statin-based treatment regimen present a challenge for lipid management and cardiovascular event risk reduction.
Medical societies have released guidelines to address the appropriate use of non-statin therapies.21–23 These guidelines incorporated new evidence, including IMPROVE-IT, FOURIER and ODYSSEY OUTCOMES clinical trials, which showed that the combination of statin therapy with other non-statin agents such as ezetimibe or PCSK9 inhibitors had a significant clinical benefit.24–26 However, the modest effect of ezetimibe or the high cost of PCSK9 inhibitors means that the problem has not yet been resolved.
Bempedoic acid is a novel, non-statin, oral drug being developed for the treatment of hyperlipidemia in combination with other lipid-lowering drugs in patients who need additional lipid lowering.2 Similar to statins, the predominant mechanism of action of bempedoic acid is through increased LDL receptor activity and consequent reduction in the plasma concentration of LDL-C. Unlike statins, bempedoic acid's mechanism of action is to impair cholesterol synthesis through ACL inhibition which acts upstream of HMGCoA reductase. Furthermore, bempedoic acid is a prodrug that becomes activated by an enzyme expressed primarily in the liver, allowing it to avoid the potential myotoxicity associated with statin therapy.
This meta-analysis jointly investigated the lipid-lowering effect on the main atherogenic particles and the anti-inflammatory effect of bempedoic acid. Our analysis showed that on average the use of bempedoic acid decreased LDL-C by 20%.
The efficacy of bempedoic acid depends on concomitant treatment. The lipid-lowering response is greater when it is used as monotherapy or in combination with ezetimibe than when it is combined with statins. This could explain why the efficacy in lowering LDL-C was greater in CLEAR-Tranquility and CLEAR-Serenity studies compared to the rest of the trials.6,8
A previously published meta-analysis found that the reduction of LDL-C was slightly higher (26.6%).14 However, this study included fewer patients (625) and analyzed different doses than those currently recommended (180mg/day). Likewise, in our study we included several recently published phase 3 studies.
Although LDL-C is the main lipid target, the non-HDL-C and apolipoprotein B represents the total atherogenic particles better than LDL-C.27,28 In our study we also evaluated the impact of bempedoic acid on these lipid markers, showing similar findings to the C-LDL analysis.
Another relevant point of our meta-analysis is that the effect of bempedoic acid on hsCRP was evaluated. Anti-inflammatory properties of bempedoic acid were further investigated in primary human monocyte derived macrophages and in vivo models of inflammation. In clinical studies, bempedoic acid has not only demonstrated improved lipid profiles but also revealed significantly attenuated levels of hsCRP, an independent risk factor for coronary artery disease.5–11 Likewise, bempedoic acid regulates immune response, leukocyte homing, and adipose tissue inflammation via LKB1-dependent activation of macrophage AMP-activated protein kinase.29
Statins studies showed a reduction in hsCRP levels. In a sample of 472 participants included in the CARE trial, the use of pravastatina 40mg exhibited a median reduction of 17.4% on hsCRP levels.30 Similar reduction was observed in the AFCAPS/TexCAPS where lovastatin 20mg therapy, reduced levels by 14.8%.31 In the JUPITER trial, compared to placebo, use of rosuvastatin 20mg showed a greater reduction (37%).32
Consequently, the 23.4% reduction in hsCRP levels with the use of bempedoic acid showed in this meta-analysis could be relevant since it would add an additional cardiovascular benefit beyond lipids.
Our results showed that bempedoic acid therapy no significant increase serious adverse effects. In addition, bempedoic acid, due to its mechanism of action, does not increase the risk of muscle-related side-effects. However, an increase in blood levels of transaminases, creatinine and uric acid were observed with the use of bempedoic acid. Likewise, the occurrence of gout was three times higher with bempedoic acid compared to placebo. The observed increase in uric acid may be attributable to a potential competition between uric acid and the glucuronide metabolite of bempedoic acid for the same renal transporters, resulting in less urinary excretion of these substance.33 In addition, the observed increase in uric acid might be due to glomerular filtration rate reduction by bempedoic acid. A recent meta-analysis specifically designed to analyze the association between bempedoic acid and elevated uric acid showed the same results.34 On the other hand, the observed signs of renal damage may involve the exposure of glomerular and tubular structures to high uric acid levels.35 New studies will be necessary to establish the clinical relevance of these adverse effects related to bempedoic acid.
This meta-analysis presents several limitations. First, they are related with clinical heterogeneity (popular characteristics, different schemes of lipid-lowering therapy, different follow-up). However, the statistical heterogeneity was low and the results were robust when performing the sensitivity analysis. Second, the analysis included only trial-level data without having the individual data. Finally, we did not perform the analysis with another primary endpoint, such as the absolute change of lipid and hsCRP levels, because we did not have these data in all the original publications.
ConclusionOur data suggests that the use of bempedoic acid significantly reduces the levels of atherogenic lipid markers, including LDL-C, non-HDL-C and apolipoprotein B. Furthermore, considering hsCRP levels, the drug produces an anti-inflammatory effect. Future studies will demonstrate whether the lipid and anti-inflammatory effects of bempedoic acid could be associated with a reduction in vascular events.
Authors’ contributionsMasson Walter was the main coordinator of the project and was responsible for the study design. Masson Walter and Lavalle-Cobo Augusto drafted the manuscript of the present paper. Masson Walter, Lobo Martín and Lavalle-Cobo Augusto were involved in the supervising of data collection and stratification. Lobo Martin and Masson Walter contributed to data assembly and analysis. Molinero Graciela contributed with manuscript revision. All authors contributed intellectually to this manuscript and have approved this final version.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestNone.