In patients who have achieved optimal LDL-C control, there remains a residual risk of atherothrombotic cardiovascular disease (ACVD) related to alterations in lipid metabolism, where alterations in triglyceride-rich lipoproteins (TGRLP) and the cholesterol they contain, called remnant cholesterol, play a major role. Remnant cholesterol has an association with residual risk of CVD that is independent of LDL-C and has been demonstrated in epidemiological and Mendelian randomisation studies, and in analyses of clinical trials of lipid-lowering drugs. Remnant TGRLP particles are highly atherogenic, due to their ability to enter and be retained in the arterial wall, their high cholesterol content, their ability to generate “foam cells” and an inflammatory response. Assessment of remnant cholesterol may provide information on residual risk of ACVD beyond the information provided by LDL-C, Non-HDL-C, and apoB, particularly in individuals with hypertriglyceridaemia, type 2 diabetes, or metabolic syndrome. In the Reduce-It study, icosapent ethyl was shown to have a preventive effect against CVD in very high cardiovascular risk patients with hypertriglyceridaemia treated with statins and target LDL-C. New lipid-lowering drugs will help to define efficacy and criteria in the treatment of excess remnant cholesterol and hypertriglyceridaemia in the prevention of ACVD.
En los pacientes que han alcanzado un control óptimo del c-LDL persiste un riesgo residual de enfermedad cardiovascular aterotrombótica (ECVA) relacionado con alteraciones del metabolismo lipídico, entre las que las alteraciones de las lipoproteínas ricas en triglicéridos (LPRTG) y del colesterol que contienen, denominado colesterol remanente, juegan un papel principal. El colesterol remanente tiene una relación con el riesgo residual de ECVA que es independiente del c-LDL y ha sido demostrada en los estudios epidemiológicos y de aleatorización mendeliana, y en los análisis de los ensayos clínicos con fármacos hipolipemiantes. Las partículas remanentes de las LPRTG son altamente aterogénicas, por su capacidad de entrar y ser retenidas en la pared arterial, por alto contenido en colesterol su capacidad de generar células espumosas y una respuesta inflamatoria. La valoración del colesterol remanente puede aportar información sobre el riesgo residual de ECVA más allá de la información aportada por el c-LDL, el c-No HDL y la apoB, en particular en los individuos con hipertrigliceridemia, diabetes tipo 2 o síndrome metabólico. En el estudio Reduce-It se demostró que el icosapento de etilo tiene un efecto preventivo frente a la ECVA en los pacientes de muy alto riesgo cardiovascular con hipertrigliceridemia tratados con estatinas y con un c-LDL en objetivos. Los nuevos fármacos hipolipemiantes contribuirán a definir la eficacia y los criterios en el tratamiento del exceso de colesterol remanente y de la hipertrigliceridemia en la prevención de la ECVA.
Current guidelines on the treatment of dyslipidaemias in the prevention of atherothrombotic cardiovascular disease (atherothrombotic CVD) focus on lowering low-density lipoprotein cholesterol (LDL-C).1–3 In recent decades this approach has brought great benefit in years and quality of life.4 However, in large clinical trials of hypercholesterolaemia treatment with statins as monotherapy, or associated with ezetimibe or monoclonal antibodies against PCSK9 protein (iPCSK9), it has been observed that despite strict control of LDL-C5–8 there is still a residual risk that can be explained by lipid and non-lipid factors.9 With reference to the former, a large evidence base has shown that triglyceride-rich lipoproteins (TGRLP) and the particles resulting from their metabolisation, the so-called remnant particles, play a major role in this residual risk.10 Hypertriglyceridaemia is a very common problem in the general population due to genetic polymorphisms related to TGRLP metabolism and the increasing prevalence of obesity and diabetes mellitus. Around 25% of statin-treated patients have hypertriglyceridaemia11 despite maintaining a target LDL-C, and this is even more common in the population with CVD or diabetes.12 However, hypertriglyceridaemia is a clinical problem that goes undetected or unaddressed in most patients.13 Hypertriglyceridaemia and excess remnant cholesterol can therefore be considered as one of the major unresolved issues in the broad and complex field of cardiovascular prevention.
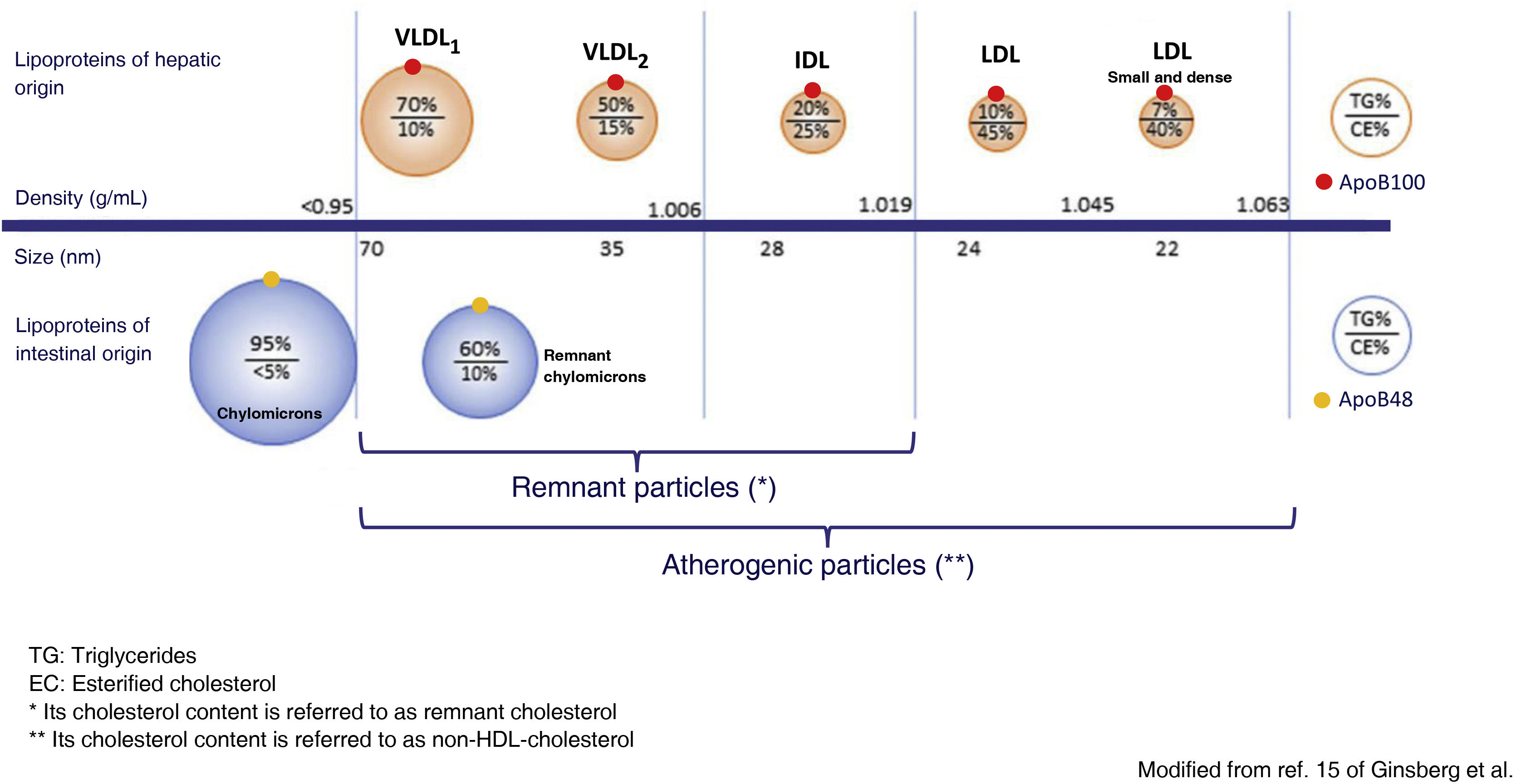
Metabolism of the TGRLP and its relationship with artherosclerosisTGRLPs include chylomicrons, which carry triglycerides of intestinal origin, and very low density lipoproteins (VLDL), which carry triglycerides mostly of hepatic origin, although up to 25% of VLDLs have been reported to be of intestinal origin.14 TGRLPs of hepatic origin contain apoB100 and those of intestinal origin a truncated form of apoB with 48% of its molecular weight, apoB48. As soon as they enter the bloodstream, TGRLPs begin to lose triglycerides under the action of lipoprotein lipase (LPL) and also apolipoprotein C, and become enriched in cholesterol and apoE, transforming them into the aforementioned remnant particles.15 In this process, TGRLPs decrease in size and can be eliminated in the liver or, in the case of VLDL, transformed into intermediate density lipoproteins (IDL) and finally, under the action of hepatic lipase (LH), into LDL LDL.16 particles. Thus, a heterogeneous population of remnant TGRLP particles is found in blood plasma and the cholesterol they contain is called remnant cholesterol. VLDL are very rapidly processed to remnant particles, so that most of the lipoproteins found in plasma can be considered remnant. They have a diameter < 70 nm, which allows them to cross the arterial wall by transcytosis, where they will exert their atherogenic effect. Chylomicrons, being larger in size, require greater exposure to LPL to decrease in size sufficiently to enter the arterial wall, so that although apoB48 particles can be incorporated into the atheroma plaque, those containing apoB100 predominate.15
The action of LPL and the hepatic clearance of TGRLP depends on different enzymes and lipid transfer proteins, and their composition in lipids and apolipoproteins. Both excess TGRLP production and decreased catabolism of TGRLP lead to an increase in the plasma concentration of remaining particles. In severe hypertriglyceridaemia, defined as concentrations above 880 mg/dL (10 mmol/L),1 the predominant mechanism is insufficient clearance of these lipoprotein particles. In this situation, larger VLDL particles, rich in triglycerides (VLDL1), and chylomicrons, particles which, due to their larger size, have more difficulty entering the arterial wall, a lower atherogenic potential (Fig. 1) and a higher risk of triggering pancreatitis predominate.
Modified from Ginsberg et al.15
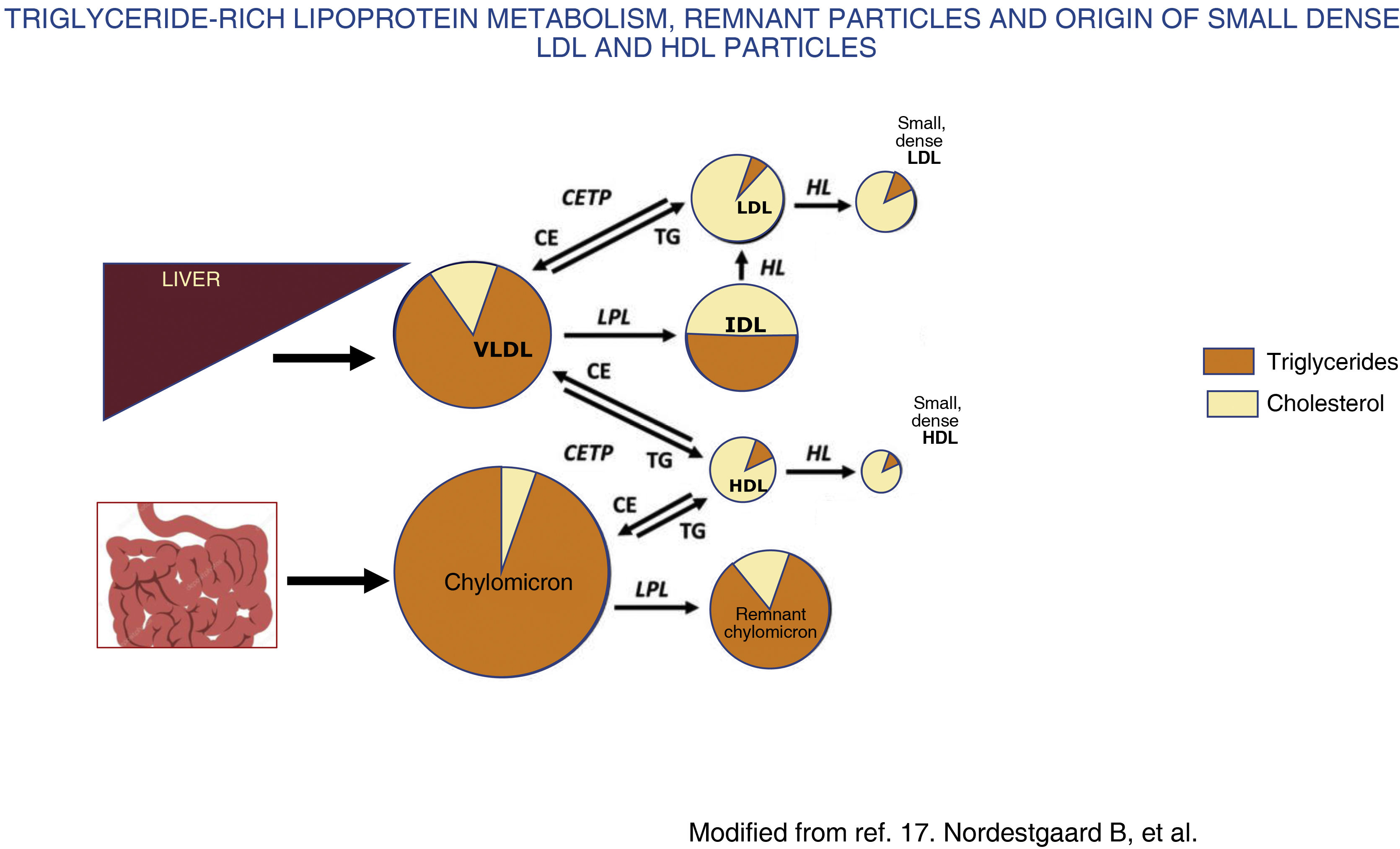
In contrast, in moderate hypertriglyceridaemia, defined as concentrations between 150 and 880 mg/dL (1.7−10 mmol/L),1,3 there is an increase in VLDL1 and remnant particles, a highly atherogenic situation due to several mechanisms. Firstly, there is an exchange of triglycerides for cholesterol between TGRLP, LDL and high-density lipoproteins (HDL), mediated by cholesterol ester transfer protein, which leads to an alteration in the structure, composition and functionality of LDL and HDL,17 resulting in small, dense LDL (Fig. 2) that is more atherogenic, and HDL in turn loses its anti-atherogenic properties.
Chylomicrons and VLDL are secreted from the intestine and liver, respectively, into the bloodstream, where they undergo rapid delipidation by lipoprotein lipase and hepatic lipase that induce the release of free fatty acids and the formation of remnant particles of both chylomicrons and VLDL. In conditions with increased fatty acid flux to the liver, such as metabolic syndrome and insulin resistance, there is increased production and secretion of hepatic VLDL, which increases the activity of cholesteryl ester transfer protein (CETP). This results in the remnant particles acquiring cholesterol from HDL in exchange for triglycerides, and this, together with the delipidation of triglycerides by lipases, results in the formation of small, cholesterol-rich, triglyceride-depleted remnant particles, with an increase in plasma remnant cholesterol. Likewise, activation of CETP induces the transfer of triglycerides from TGRLP to HDL and LDL and these cholesterol-depleted, triglyceride-rich particles, once they undergo the action of hepatic lipase, are transformed into small, dense LDL and HDL. Small, dense LDL has a high atherogenic potential and small, dense HDL has a lower protective effect against atherosclerosis, resulting in a highly atherogenic situation.
On the other hand, remnant particles easily enter the arterial wall and are retained in the subendothelial space with greater affinity than LDL, among other factors because of their ability to bind to proteoglycans. Thirdly, by virtue of their apoE content, they are taken up by macrophage scavenger receptors without modification, giving them a high capacity to generate foam cells, superior to LDL. The remaining particles have a high cholesterol content, which is at least 4 times higher per particle than LDL, and a cholesterol/apoB molar ratio 2 times higher than LDL,18 which also favours foam cell formation.19 Nevertheless, the main suppliers of cholesterol to the arterial wall are LDL particles in both fasting and postprandial situations, as they are 3–10 times more numerous in blood plasma than TGRLP and remain in the circulation for longer, between 2.5 and 3.5 days, unlike chylomicrons and VLDL, which in hypertriglyceridaemia remain for between 4 and 13 h. It has been estimated that the remaining particles, meaning the VLDL and IDL fractions, carry about 30% of the cholesterol load contained in the apoB lipoprotein pool.
Among the different plasmatic lipids, cholesterol is the main agent in the atherogenic process since, unlike triglycerides which may be degraded by most cells,20 cholesterol cannot process itself and is retained in the artery wall. To a lesser extent triglycerides are also atherogenic because with LPL action, the phospholipase and other enzymes release free fatty acids and lisolipids which trigger an inflammatory response.21 In a Mendelian randomised study an increase in cholesterol plasmatic concentration was observed of the remnant particles of 39 mg/dL (1 mmol/L) and three-fold associated increase in reactive C protein concentrations.22 Furthermore, fats in the diet may condition the inflammatory response triggered by the triglycerides, since this depends on the type of associated fatty acids to the glycerol molecule, and becomes more evident with saturated fatty acids.21 Remnant cholesterol atherogenicity may also be affected by the intervention of several key proteins in lipid metabolic regulation, such as apoCIII and ANGPTL3, which act through different apoB23 mechanisms.
Remnant cholesterol measurementRemnant cholesterol can be calculated from the conventional lipid profile by subtracting the value of LDL-C and HDL-C24 from the value of total cholesterol. In cases where LDL-C is obtained using the Friedewald equation, the remnant cholesterol value is simply equivalent to dividing the triglyceride concentration by 5 (when the units of measurement are mg/dL), which is the ratio assumed to exist between triglycerides and cholesterol in VLDL particles. Hypertriglyceridaemia is defined as a triglyceride concentration greater than 150 mg/dL (1.8 mmol/L)1–3; therefore, remaining remnant cholesterol is defined as a concentration greater than 30 mg/dL (.8 mmol/L). Another more precise way of calculating the remnant cholesterol consists of obtaining the LDL-C value by means of equations in which a variable adjustable factor is used depending on the plasmatic concentration of triglycerides and non-HDL-cholesterol (non-HDL-C).25 These more precise equations are particularly necessary in patients with hypertriglyceridaemia in whom the Friedewald equation has a wide margin of error, since it underestimates LDL-C as triglycerides increase, especially from triglyceride concentration of 200 mf/dL, and it is not usable from triglyceride concentrations of 400 mg/dL.26 Other methods of direct measurement of remnant cholesterol also exist but they lack applicability in clinical practice due to their complexity and lack of standardisation,27 although recently a homogenous totally automated method28 has been marketed, despite the fact it is not standardised. For some years now a nuclear resonance spectroscopy method has also existed. This technique facilitates rapid analysis and without the need to pre-treat the serum samples, which informs us of the quantity, composition and size of the lipoproteins and it provides us with a deeper insight into the status of lipid metabolism and the risk of ACVD in dyslipidaemic patients.29
Given this diversity of options for calculating or measuring remnant cholesterol, a consensus needs to be reached on the most appropriate and affordable method for clinical laboratories. Current guidelines and documents from both the United States2 and Europe1,3,30 indicate that the profile of the study of lipid metabolism should include total cholesterol and plasma triglycerides, LDL-C, non-HDL-C and lipoprotein (a), the latter at least once throughout life. Likewise, and although the non-HDL-C value includes both LDL-C and remnant cholesterol, it seems reasonable that the latter should also be included in any lipid profile, since, as they are 2 clearly differentiated components from the point of view of metabolism, they may require different therapeutic strategies.
Another parameter to which less attention has been paid, but which is no less relevant, is apoB. ApoB is currently considered the most accurate marker of ASCVD risk, above LDL-C and non-HDL-C.31
The mass of cholesterol contained in the atherogenic particles (VLDL, IDL, and LDL) is variable, but each lipoprotein particle contains a single apoB molecule, so the plasma apoB concentration is a good indicator of the number of atherogenic particles in the plasma. It has been postulated, as described below, that this number of particles is the main causative factor of artherosclerosis, above the concentration of plasmatic cholesterol and triglycerides,32 and that each particle with apoB is the fundamental unit with the capacity to damage the arterial wall.33 In line with this concept, a recent study using data from the UK biobank and 2 large clinical trials, the Fourier study and IMPROVE-IT, has shown that apoB is a better predictor of myocardial infarction risk than LDL-C and non-HDL-C, and even than the triglyceride content of said lipoproteins.34 These data support the concept that the main objective of treating dyslipidaemia to prevent ACVD is to reduce apoB and that more aggressive strategies should be carried out if, despite optimal control of LDL-C and non-HDL-C, it is still elevated. Furthermore the 2019 ESC/EAS guidelines, those of the American Association of Clinical Chemistry and those of the European Federation of Laboratory Medicine indicate that apoB can be measured with greater precision than LDL-C and non-HDL-C, particularly in hypertriglyceridaemia and in the presence of low total cholesterol.35
In keeping with the above, the ideal lipid profile, in addition to that of LDL-C, HDL-C, non-HDL-C and triglycerides, should include remnant cholesterol and apoB, regardless of whether on at least one occasion lipoprotein (a) is measured.
Remnant cholesterol and ACVD riskThe relationship between remnant cholesterol and the risk of ACVD has been well demonstrated from a large database that has emerged from prospective epidemiological studies in very large samples of the general population, Mendelian randomisation studies and post hoc analyses of randomised clinical trials.36
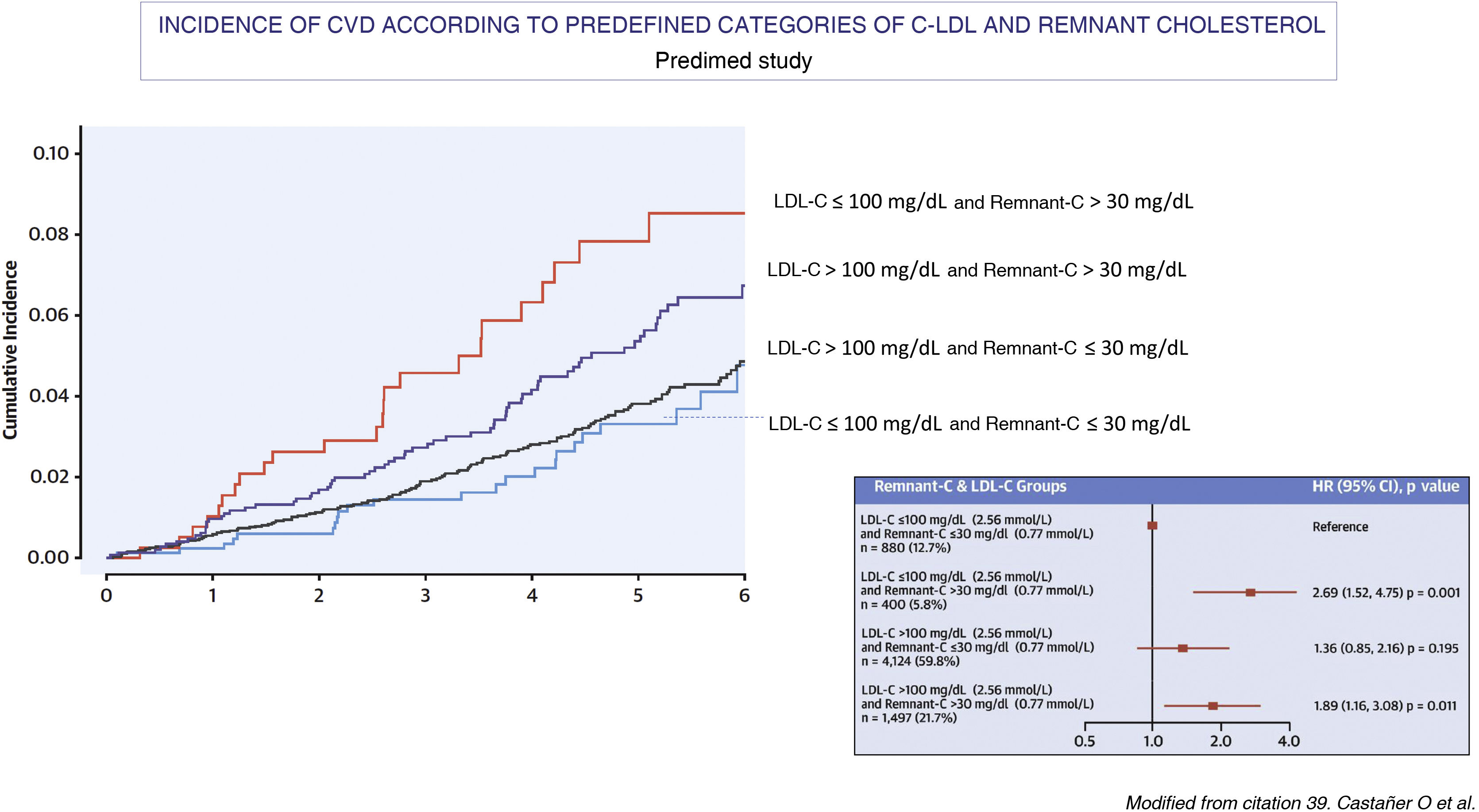
Several large-scale prospective epidemiological studies, such as the Copenhagen City Heart Study and the Copenhagen Ischemic Heart Disease Study, which were conducted primarily without fasting,20,37 and an intervention study with a Mediterranean diet, the Predimet study, in which samples were obtained on an empty stomach37 (Fig. 3) have demonstrated the relationship between the plasma concentration of triglycerides, remnant cholesterol and the risk of ACVD. Based on data from the Danish population, it has also been shown that remnant cholesterol is a better predictor of all-cause mortality than LDL-C.38 Likewise, in a study of 17,532 individuals without ASCVD, who were followed up for a mean time of 18.7 years and who had been included in the Atherosclerosis Risk in Communities study (n = 9.748), the Multi-Ethnic Study of Atherosclerosis (n = 3.049) and the Coronary Artery Risk Development in Young Adults (n = 4.735),39 it was observed that the logarithm of remnant cholesterol, which was calculated by subtracting the value of LDL-C from the estimated non-HDL-C, was an independent predictor of ASCVD risk in analyses adjusted for non-HDL-C, apoB and other ASCVD risk in analyses adjusted from non-HDL-C, apoB and other risk factors. It was also observed that among individuals with a discrepancy between remnant cholesterol and LDL-C, only those with high remnant cholesterol and low LDL-C had an increased risk of ASCVD when compared to those with no discrepancy between both values (HR 1,21, 95% CI 1.08–1.34).
The figure shows the relationship between baseline lipid profile and the incidence of major episodes of atherothrombotic cardiovascular disease (ACVD) in the Mediterranean Diet Prevention study population (Predimed; mean age 67 years, body mass index 30 kg/m2, 43% male and 48% with diabetes) during a follow-up of 4.8 years. The relationship between lipid concentrations as categorical variables and the incidence of major episodes of CVD was analysed using adjusted and unadjusted Cox proportional hazard models (n = 6,901; CVD cases = 263). In each LDL-C subgroup (>100 o ≤ 100 mg/dL [2.59 mmol/L]), a high basal remaining cholesterol (>30 mg/dL [0.78 mmol/L]) identified individuals at higher risk of ACVD compared with those with lower concentrations. The lower incidence of major episodes of CVD was observed in the groups with low remaining cholesterol, irrespective of LDL-C levels and other cardiovascular risk factors.
There are also data from 17 published reports from 13 independent cohorts describing a statistically significant association between excess remnant cholesterol and ACVD,10 both in ischaemic and non-ischaemic individuals,40 and in both sexes.41 In turn, patients treated with statins who have achieved optimal control of LDL-C, but have elevated triglyceride concentrations, have a higher risk of ASCVD that remains despite adjusting for other lipid factors, such as HDL-C and LDL-C.42,43 In these patients treated with statins, the decrease in remnant cholesterol has been associated with a decrease in the risk of ACVD with an independent relationship to the decrease in LDL-C.44
There are also data on the relationship of remnant cholesterol with the evolution of coronary artery lesions. In a pooled analysis of studies with intracoronary ultrasound, it was observed that remnant cholesterol concentrations during follow-up and changes in cholesterol were associated with the progression of artherosclerosis regardless of apoB concentrations and other ASCVD risk factors.45
It has also been questioned whether the statistical models used to analyse the relationship between the metabolism of TGRLP and the risk of ACVD should include, HDL-C in addition to triglycerides and it has been argued that the metabolism of both are closely linked, which can lead to an excess of adjustment that interferes with the assessment of said relationship. In the same sense, it has been suggested that the alterations in triglycerides and HDL are both the reflection of an alteration in the metabolism of the remnant particles of the TGRLP rather than independent causal factors.46
The relationship between TGRLP and the risk of ACVD has also been demonstrated in Mendelian randomization genetic studies.17 In them, variants of genes related to triglyceride metabolism, associated with increased lipolytic activity and lower concentrations of triglycerides and remnant cholesterol, including the LPL gene, apoCIII, ANGPTL3, and ANGPTL4, have been associated with a lower risk of ACVD.46–48 Conversely, genetic variants associated with decreased LPL and LH activity and increased triglycerides and remaining cholesterol are associated with a greater risk of ASCVD.49,50 In contrast, Mendelian randomization studies have not shown a relationship between the genetic variants associated with lower HDL-C concentrations and the risk of ACVD. Thus, Varbo et al., using a large sample of the Copenhagen population, analyzed coronary risk in relation to 3 groups of genetic variants, those associated more specifically with remnant cholesterol, those associated both with remnant cholesterol as with HDL-C, and those related to LDL-C. They observed that the variants linked to remnant cholesterol, and also to LDL-C, but not to HDL-C, were related to the risk of heart disease.22 In said study, it was estimated that for each increase of 1.0 mmol/L in remnant cholesterol, the risk of ischaemic heart disease increased 2.8 times, regardless of the values of LDL-C and HDL-C, and the risk increased 1.5 times for every 1 mmol/L increase in LDL-C.
Another large Mendelian randomization study has shown the relevance of apoB in the prediction of ASCVD risk.32 This is a study with 654,783 participants, including 91,129 cases of coronary disease, in which the influence of 2 genetic scores was compared, one based on the LPL variants associated with triglyceride concentrations and the other with LDL receptor variants associated with LDL-C concentrations. It was observed that for every 10 mg/dL decrease in the apoB concentration associated with the LPL genetic score, the triglyceride concentration was 70 mg/dL lower and without being associated with variations in LDL-C. Likewise, for every 10 mg/dL decrease in apoB associated with the LDL receptor genetic score, LDL-C decreased 14 mg/dL, without variations in triglyceride concentration. It was observed that the decrease in coronary risk associated with a decrease of 10 mg/dL in the apoB concentration was similar, regardless of whether it was associated with a decrease in LDL-C or triglycerides (OR .771 and OR .773, respectively). Furthermore, in multivariate analyses, the association between triglycerides and LDL-C with coronary risk disappeared when adjusting for differences in apoB. Therefore, the absolute change in apoB concentrations was what determined the benefit associated with lower triglyceride concentrations.23 However, cholesterol and triglycerides are not the only proatherogenic components that apoB lipoproteins contain, but also contain oxidized phospholipids that exert potent proinflammatory action and have a high potential to damage the arterial wall.
Treatment of hypertriglyceridaemia and of remnant cholesterol excessTo control hypertriglyceridaemia and reduce remnant cholesterol, it is a priority to improve lifestyle habits, particularly an inadequate diet and a sedentary lifestyle, and to correct the possible causes of secondary hypertriglyceridemia.51,52 Except in patients with severe hypertriglyceridaemia, where the first goal is to lower triglycerides to prevent acute pancreatitis, in moderate hypertriglyceridaemia the first step is to control non-HDL-C with a statin. Statins increase the plasmatic clearance of the remaining particles of hepatic and intestinal origin, decrease postprandial lipidemia and, as occurs with other drugs, their effect on triglycerides is all the more pronounced the higher the degree of hypertriglyceridemia.15 If the HDL-C targets are not achieved with statins in monotherapy, it is necessary to associate ezetimibe.1–3 Ezetimibe inhibits the Niemann-Pick C1L1 protein, decreases the intestinal absorption of cholesterol and the cholesterol content of the remaining chylomicrons, and increases the catabolism of apoB100 and of the remaining particles of hepatic origin.53 In very high-risk ASCVD patients who do not achieve LDL-C and non-HDL-C targets with statins in monotherapy or associated with ezetimibe, iPCSK9 may be indicated. These, like the statins, increase the activity of LDL receptors, but their effect on triglyceride concentrations is more modest and seems to be limited to the remnant particles of smaller hepatic origin, but not the intestine, in situations of moderate hypertriglyceridemia.15 Adding a fibrate can only be considered in high-risk patients who, after achieving LDL-C and non-HDL-C control, have a triglyceride concentration > 200 mg/dL, or when triglycerides are maintained for above 135 mg/dL, ethyl icosapent,1 taking into account that the objective is to reduce the risk of ACVD, rather than reaching a certain concentration of triglycerides or remaining particles, since the evidence on the benefit of achieving certain objectives of remaining triglycerides or cholesterol in intervention studies is low. However, in pooled analyses of multiple clinical trials with drugs that lower triglycerides to a greater extent than LDL-C (fibrates, nicotinic acid, and omega-3 fatty acids [n3-AG]) and also of statin trials, it has been observed that lowering triglycerides is associated with a lower risk of vascular disease episodes, even after adjusting for lowering LDL-C, although the effect is less than that obtained by lowering LDL-C. Thus, a decrease in LDL-C of 40 mg/dL was associated with a 20% lower cardiovascular risk, but when triglycerides decreased by 40 mg/dL, the risk was only 4%–5% lower.54
The drugs currently available with a greater effect on TGRLP than on LDL are fibrates and AG-n3.55
The main fibrates or derivatives of fibric acid are fenofibrate, gemfibrozil and bezafibrate, and they act by activating peroxisome proliferator-activated receptors alpha (PPAR-α) leading to increased expression of LPL and other genes related to lipolysis and HDL function. These drugs activate the clearance of VLDL, especially the largest particles and those rich in triglycerides (VLDL1), and reduce remnant cholesterol, postprandial lipidemia, and triglyceride concentration by between 30% and 50%, with a variable response that largely depends on the degree of hypertriglyceridemia.56
Fibrates moderately decrease apoB and LDL-C in moderate hypertriglyceridemia, but they can also moderately increase LDL-C in patients with severe hypertriglyceridaemia.15 The efficacy of fibrates to prevent ACVD has been evaluated in different randomized clinical trials in patients with high cardiovascular risk treated with these drugs in monotherapy and used in monotherapy or combined with statins, with controversial results.57,58 However, these trials suffer from the fact that hypertriglyceridaemia was not a mandatory inclusion criterion in any of them. Sacks et al.,59 when analyzing the results of the 5 main clinical trials with fibrates, observed that among patients with atherogenic dyslipidemia, defined as a concentration of triglycerides ≥ 204 mg/dL and HDL-C ≤ 34 mg/dL, treatment with these drugs was associated with a 35% decrease (95% CI 22–46) in the risk of presenting episodes of coronary disease, while in those who did not present said dyslipidemia, no significant change was observed. These results have been reaffirmed in larger meta-analyses of trials with fibrates,60 but as they are secondary analyses no firm recommendations on their indications in the treatment of hypertriglyceridaemia.1–3 have been established. Data from a recent study have added further uncertainty about the role of fibrates in cardiovascular prevention. This is the Prominent study, carried out with pemafibrate, a drug defined as the first selective modulator of PPAR-α receptors with the capacity to reduce the concentration of plasma triglycerides by more than 50%,61 in which 10,000 diabetic patients with hypertriglyceridaemia and HDL-C62 deficiency were included. This trial was interrupted before completion due to futility63 and other clinical trials with pemafibrate in patients with severe hypertriglyceridaemia and hepatic steatosis are currently underway.64 Ference et al.32 have postulated that the negative results of the trials with fibrates may be due to the fact that much more pronounced decreases in triglyceride concentrations are necessary than those achieved in the trials carried out to date. These authors suggest that if we consider that the cholesterol concentration of TGRLP is calculated by dividing the triglyceride concentration (expressed in mg/dL) by 5, and if we accept that all lipoproteins with apoB have a similar atherogenic effect, to reduce the risk cardiovascular disease by 20%, which is what is achieved by reducing the LDL-C 40 mg/dL, the concentration of triglycerides would have to be reduced 5 times more, that is, about 200 mg/dL. However, in clinical trials conducted to date, reductions greater than 50 mg/dL have not been achieved. We will have to wait for the results of the trials with the new drugs with greater hypotriglyceridaemic potency that are currently under investigation to assess this hypothesis.
Omega-3The n3-FAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) exert their lipid effects through PPAR65-mediated metabolic pathways and increase fatty acid catabolism, decrease lipogenesis and increase hepatic lipolysis. In addition, they improve the stability of the vascular endothelial cell membrane and have anti-inflammatory, antioxidant, and antithrombotic effects.66
In moderate hypertriglyceridaemia, triglycerides decrease by about 20% and their effects on plasma cholesterol concentration are limited. While the EPA induces a slight decrease, the DHA causes an increase, also slight. The main indication for GA-n3 is moderate or severe hypertriglyceridemia. Most of the studies with the combination of EPA and DHA have not shown efficacy in the primary or secondary prevention of ACVD.63 However, a preventive effect has been observed in trials with EPA. In the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial REDUCE-IT),67 clinical trial, which included patients with ACVD or diabetics with at least one additional cardiovascular risk factor, who following statin treatment, had concentrations triglycerides from 135 to 500 mg/dL and LDL-C from 41 to 100 mg/dL, patients were treated with icosapent ethyl, an EPA ethyl ester, 2 g/12 h, or placebo for 5 years. In the former, a 25% reduction in the risk of the main cardiovascular endpoint was observed (HR 0.75; 95%CI 0.68−0.83). In the active treatment group, triglycerides decreased by 18.3% and in the placebo group they increased by 2.2%, while LDL-C increased by 3.1% and 10.2%, respectively. The preventive effect against ACVD was barely related to the decrease in triglycerides, with serum EPA concentrations being the ones that were most clearly related to said effect. For this reason, it has been suggested that the favourable effects of ethyl icosapent are due to other biological effects, beyond lipid metabolism, including inflammation, oxidation or thrombosis. However, this is an aspect that is currently being studied. It would also be interesting to analyse whether there was any relationship between remnant cholesterol, non-HDL-C and apoB with said preventive effect. A controversial aspect of the REDUCE-IT study is that in the control group a mineral oil was used as placebo, which was associated with an increase in LDL-C and C-reactive protein, which could have led to an overestimation of the effect of EPA.68 However, pooled analyses of other clinical trials in which this same placebo has been used and the study of the influence that changes in LDL-C and C-reactive protein in the placebo group might have had on cardiovascular outcomes rule out that this has a relevant influence, taking into account the magnitude of the preventive effect observed.69 In another study with EPA, an open study in a Japanese population with a large number of individuals undergoing primary and secondary prevention who were treated with low doses of statins, the Japan EPA Lipid Intervention Study,70 a preventive effect against ACVD was also observed with lower doses of EPA (1.8 g/day). However, the serum EPA concentrations in the patients in this study were similar to those in REDUCE-IT, which has been attributed to the fact that they started with high EPA concentrations due to the high consumption of fish, typical of the Japanese population. The results of the REDUCE-IT study have justified the inclusion of treatment with 2g ethyl icosapent 2 times a day in the European guidelines for cardiovascular71 and dyslipidaemia1 prevention as a therapeutic option to prevent ACVD in patients with hypertriglyceridemia and high cardiovascular risk.
New drugsIn the next few years we will have new highly effective therapies to control alterations in the metabolism of TGRLP and remaining cholesterol. These include apoCIII and ANGPTL3 inhibitors, proteins that, among other effects, decrease LPL activity, inhibit lipolysis, and increase plasma triglyceride concentrations.72 Volanesorsen (ISIS 304810; Akcea-Ionis Pharmaceuticals) is an apoCIII antisense oligonucleotide that inhibits apoCIII mRNA translation and decreases apoCIII synthesis. It reduces plasma triglycerides by more than 80% and its financing has already been approved in patients with a genetic diagnosis of familial chylomicronemia syndrome, but not in the less severe forms of chylomicronemia of multifactorial origin. Its main side effect is thrombocytopenia, which can be severe.73
Other drugs are also in advanced stages of research, including an antisense oligonucleotide against apoCIII with selective hepatic action and with a lower risk of thrombocytopenia (AKCEA-APOCIII-LRx), evinacumab, which is an anti-ANGPTL3 antibody, and vupanorsen (AKCEA- ANGPTL3-LRx), an antisense oligonucleotide against ANGPTL3. ANGPTL3 inhibitors lower triglycerides by more than 70%–80%, and in addition to lowering remaining cholesterol, they induce marked decreases in LDL-C, non-HDL-C, and apoB.74,75
It is highly likely that these drugs will contribute to defining the efficacy of residual risk control related to TGRLP and remaining cholesterol in patients who have already achieved adequate control of LDL-C, and to establishing therapeutic targets for hypertriglyceridaemia and remnant cholesterol. Olezarsen (Akcea-Ionis Pharmaceuticals; formerly AKCEA-APOCIII-LRx) is the same as volanesorsen, an apoCIII antisense oligonucleotide, but conjugated to an N-acetylgalactosamine bead, giving it greater hepatic affinity and therefore it presents lower plasmatic concentrations, which is expected to be accompanied by a decrease in side effects.76
Phase III clinical trials are currently underway in patients with familial chylomicronemia syndrome (NCT04568434) and patients with multifactorial chylomicronemia (NCT05079919) to evaluate triglyceride lowering, and studies will be initiated to evaluate its efficacy in lowering triglycerides. Cardiovascular risk in patients with hypertriglyceridaemia. There is another apoCIII antagonist that is under investigation and that is ARO-APOC-III; this is RNA interference directed to apoCIII RNA. In phase I clinical trials it has shown reductions in triglyceride concentrations of between 78%–92% and phase III studies are underway in patients with polygenic hypertriglyceridaemia (NCT04720534). Evinacumab is an anti-ANGPTL3 monoclonal antibody that has been approved for the treatment of patients with homozygous familial hypercholesterolaemia, having shown decreases in LDL-C of 47.1% and triglycerides of 55% in phase III trials.77 Evinacumab has not shown efficacy in reducing triglycerides in patients with familial chylomicronaemia syndrome, but in patients with multifactorial chylomicronaemia, in whom triglyceride concentration decreases by 64.8%–81.7% and varies accordingly, depending on the genotype they present.78 A targeted study is currently underway to evaluate the efficacy of evinacumab in patients with multifactorial chylomicronaemia (NCT03175367). Finally, vuparnorsen, an N-acetylgalactosamine-conjugated antisense oligonucleotide directed against ANGPTL3 mRNA, showed promising results in early clinical trials; however, the phase III trial was suspended when insufficient lipid lowering was demonstrated, which was associated with a higher liver fat content and elevation of transaminases.79
ConclusionsIn patients who have achieved optimal LDL-C control, a residual lipid-mediated risk persists in which alterations in TGRLP and remnant cholesterol play a major role.
The relationship between remnant cholesterol and residual risk of CVD is independent of LDL-C and has been demonstrated in epidemiological studies, Mendelian randomisation studies and analyses of clinical trials of lipid-lowering drugs.
Remnant TGRLP particles are highly atherogenic because of their ease of entry and retention in the arterial wall, their high cholesterol content and their ability to generate foam cells and an inflammatory response.
Assessment of remnant cholesterol may provide information on residual risk of AVCD beyond the information provided by LDL-C, non-HDL-C and apoB, particularly in individuals with hypertriglyceridaemia.
The REDUCE-IT study has shown that ethyl icosapent has a preventive effect against CVD in very high cardiovascular risk patients with hypertriglyceridaemia treated with statins and target LDL-C.
New lipid-lowering drugs will help to define the efficacy and criteria for the treatment of excess remnant cholesterol and hypertriglyceridaemia in the prevention of AVCD.
Conflict of interestsThe authors have no conflict of interests to declare.
Our thanks to Emili Corbella for her review of the manuscript and her help in the??
Agustín Blanco (Servicio de Medicina Interna, Hospital Universitario 12 de Octubre, Madrid, Spain); Mariano Blasco (Centro de Salud Delicias Sur, Área Sanitaria III, Zaragoza, Spain); José Luís Díaz Díaz (Unidad de Lípidos y Riesgo Cardiovascular, Medicina Interna, Hospital Universitario A Coruña, A Coruña, Spain); Ángel Díaz Rodríguez (Centro de Salud de Bembibre, Universidad de León, León, Spain); Alipio Mangas (Internal Medicine Department, School of Medicine, Institute of Research and Innovation in Biomedical Sciences [INiBICA], University Hospital Puerta del Mar, University of Cadiz, Cádiz, Spain); Vicente Pascual (Centro de Salud Palleter, Universidad CEU-Cardenal Herrera, Castellón, Spain); Juan Pedro Botet (Unidad de Lípidos y Riesgo Vascular, Servicio Endocrinología y Nutrición, Hospital del Mar; Universitat Autònoma de Barcelona, Barcelona, Spain); Pablo Pérez Martínez (Grupo de Trabajo Nutrición y Estilo de Vida, Sociedad Española de Arteriosclerosis [SEA], Spain; Unidad de Lípidos y Arterioesclerosis, Universidad de Córdoba/Hospital Universitario Reina Sofía/Instituto Maimónides de Investigación Biomédica de Córdoba [IMIBIC], Córdoba, Spain; CIBER Fisiopatología de la Obesidad y Nutrición [CIBEROBN], Instituto de Salud Carlos III, Madrid, Spain. Email address: pabloperez@uco.es).








![The figure shows the relationship between baseline lipid profile and the incidence of major episodes of atherothrombotic cardiovascular disease (ACVD) in the Mediterranean Diet Prevention study population (Predimed; mean age 67 years, body mass index 30 kg/m2, 43% male and 48% with diabetes) during a follow-up of 4.8 years. The relationship between lipid concentrations as categorical variables and the incidence of major episodes of CVD was analysed using adjusted and unadjusted Cox proportional hazard models (n = 6,901; CVD cases = 263). In each LDL-C subgroup (>100 o ≤ 100 mg/dL [2.59 mmol/L]), a high basal remaining cholesterol (>30 mg/dL [0.78 mmol/L]) identified individuals at higher risk of ACVD compared with those with lower concentrations. The lower incidence of major episodes of CVD was observed in the groups with low remaining cholesterol, irrespective of LDL-C levels and other cardiovascular risk factors. The figure shows the relationship between baseline lipid profile and the incidence of major episodes of atherothrombotic cardiovascular disease (ACVD) in the Mediterranean Diet Prevention study population (Predimed; mean age 67 years, body mass index 30 kg/m2, 43% male and 48% with diabetes) during a follow-up of 4.8 years. The relationship between lipid concentrations as categorical variables and the incidence of major episodes of CVD was analysed using adjusted and unadjusted Cox proportional hazard models (n = 6,901; CVD cases = 263). In each LDL-C subgroup (>100 o ≤ 100 mg/dL [2.59 mmol/L]), a high basal remaining cholesterol (>30 mg/dL [0.78 mmol/L]) identified individuals at higher risk of ACVD compared with those with lower concentrations. The lower incidence of major episodes of CVD was observed in the groups with low remaining cholesterol, irrespective of LDL-C levels and other cardiovascular risk factors.](https://static.elsevier.es/multimedia/25299123/0000003500000004/v3_202311141613/S2529912323000499/v3_202311141613/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)