MEMOGAL study (NCT04319081) is aimed at evaluating changes in cognitive function in patients treated with PCSK9 inhibitors (PCSK9i). This is the first analysis: (1) discussion about the role of the Hospital Pharmacists during the pandemic, and also the assessment of the impact of COVID-19 in the lipid control; (2) descriptive analysis; (3) effectiveness in LDL cholesterol (LDL-c) reduction of alirocumab and evolocumab; (4) communicate PCSK9i safety.
Material and methodsIt is a prospective Real-World Evidence analysis of patients that take PCSK9i for the first time in the usual clinical practice, and they are included after the first dispensation in the public pharmacy consultations of 12 Hospitals in Galicia from May 2020 to April 2021. Baseline values of LDL-c are the previous values before taking PCSK9 and the follow-up values are in 6 months time.
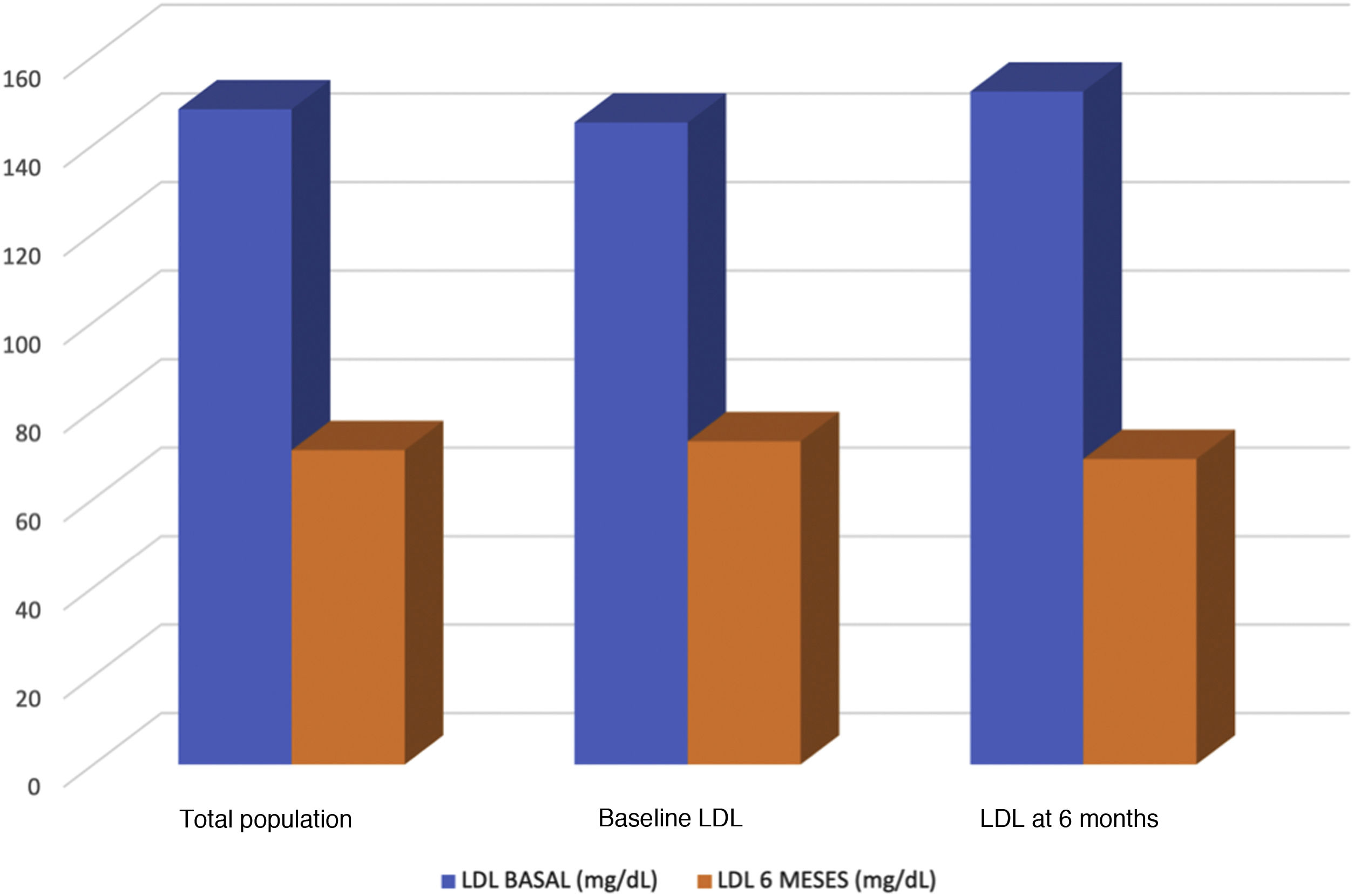
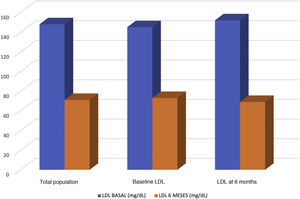
Results89 patients were included. 86.5% with cardiovascular disease and 53.9% with statin intolerances. 78.8% of the patients were treated with high intensity statins. Statins most used were rosuvastatin (34.1%) and atorvastatin (20.5%). Baseline value of LDL-c was 148mg/dl and the follow-up value was 71mg/dl. The baseline value of patients treated with alirocumab (N=43) was 144mg/dl and 73mg/dl in the follow-up. With evolocumab (N=46) was 151mg/dl in basaline and 69mg/dl in follow-up. The LDLc- reduction was 51.21% with evolocumab and 51.05% with alirocumab. 43.1% of the patients showed values >70mg/dl in six month time; 19.4% between 69mg/dl and 55mg/dl and 37.5% <55mg/dL. 58.3% of the patients achieved a reduction >50% of LDL-c. The adverse events were: injection point reaction (N=2), myalgias (N=1), flu-like symptoms (N=1) and neurocognitive worsening (N=1).
Conclusions(1) Despite the number of prescriptions was reduced because of the pandemic, the lipid control was not affected. (2) Half of the patients treated with PSCK9i is due to statins intolerance and the 86% is for secondary prevention. (2) The reduction results were similar to pivotal clinical trials. Despite this, 39% of the total of the patients and 60% of patients with dual teraphy did not reach the goal of ESC/EAS guidelines (<55mg/dl and/or reduction>50%). There were not significant differences between evolocumab and alirocumab: 51.21% vs 51.05% (P=.972). (3) There were not any adverse events of special interest. The possible neurocognitive worsening will be studied as the primary endpoint once the MEMOGAL study has been completed.
El estudio MEMOGAL (NCT04319081) está dirigido a evaluar cambios en la función cognitiva en pacientes tratados con inhibidores de la PCSK9 (iPCSK9). Se realiza primer análisis: 1) discutir el papel de los farmacéuticos hospitalarios durante de la pandemia, así como evaluar el impacto de la misma en el control lipídico; 2) análisis descriptivo; 3) eficacia en reducción de colesterol-LDL (c-LDL) de alirocumab y v; y 4) reportar seguridad de los iPCSK9.
Material y métodosSe trata de un análisis prospectivo en vida real de pacientes tratados por primera vez con iPCSK9 en la práctica clínica habitual e incluidos en su primera dispensación en las consultas de farmacia de 12 hospitales de Galicia desde mayo de 2020-abril de 2021. Los valores basales de c-LDL son los previos al inicio del tratamiento con iPCSK9 y como seguimiento los valores a los 6 meses.
ResultadosSe incluyeron 89 pacientes. El 86,5% con enfermedad cardiovascular y un 53,9% intolerancia a las estatinas. Un 78,8% de los pacientes fueron tratados con estatinas de alta intensidad. Las estatinas más usadas fueron rosuvastatina (34,1%) y atorvastatina (20,5%). El nivel basal de c-LDL fue 148mg/dl y de 71mg/dl al seguimiento. Los pacientes tratados con alirocumab (n=43) presentaban valores basales de 144mg/dl y de 73mg/dl al seguimiento y con evolocumab (n=46) de 151mg/dl basal y 69mg/dl al seguimiento. La reducción de c-LDL fue para evolocumab 51,21% y alirocumab 51,05%. El 43,1% presentaba a los 6 meses valores>70mg/dl, el 19,4% entre 55 y 69mg/dl y el 37,5%<55mg/dl. Los pacientes que obtuvieron una reducción>50% de c-LDL fueron el 58,3%. Los eventos adversos presentados fueron: reacción en el lugar de inyección (n=2), mialgias (n=1), síntomas pseudogripales (n=1) y deterioro neurocognitivo (n=1).
Conclusiones1) A pesar de haber disminuido el número de prescripciones de iPCSK9 durante la pandemia, el control lipídico de estos pacientes no se ha visto afectado; 2) la mitad de los pacientes tratados con iPSCK9 se debe a intolerancia a estatinas y el 86% es en prevención secundaria; 3) se presentaron valores de reducción similares a los ensayos clínicos pivotales. A pesar de esto, un 39% del total y un 60% en doble terapia no alcanzaron las recomendaciones de las guías ESC/EAS (<55mg/dl y/o reducción>50%). No hubo diferencias significativas entre evolocumab y alirocumab: 51,21% vs. 51,05% (p=0,972); y 4) no se observaron eventos de especial interés con el uso de estos fármacos. El posible deterioro cognitivo será analizado como variable principal una vez completado el estudio MEMOGAL.
Low-density lipoprotein or LDL-cholesterol (LDL-C) is associated with atherosclerosis, the leading cause of coronary heart disease.1 Reducing LDL-C has been strongly associated with a reduction in the incidence of cardiovascular disease, and is considered the primary target for cardiovascular prevention.2,3
PCSK9 belongs to the subtilisin family of serine proteases and is mainly expressed in the liver. PCSK9 is involved in the regulation of the protein levels of the low-density lipoprotein receptor (LDLR). Once secreted into plasma, PCSK9 binds directly to the LDLR and stimulates its lysosomal degradation after internalisation. The increased degradation of the LDLR results in less reduction of LDL-C and therefore higher levels of circulating LDL-C.3,4
Two monoclonal antibodies that inhibit PCSK9 (PCSK9i) have been developed: alirocumab and evolocumab. Both have been shown to reduce LDL-C levels by 50%, as well as the risk of myocardial infarction,5,6 and therefore have been licensed for the treatment of familial hypercholesterolaemia (FH) or cardiovascular atherosclerosis that requires further lowering of LDL-C, despite the use of maximum-dose statins.
The current SARS-CoV-2 pandemic has triggered an enormous worldwide economic, social and, above all, health crisis. The first cases were reported in mid-December 2019, and by the first half of 2021, 180 million cases and more than 4 million deaths had been reported.7 Cardiovascular diseases are a primary complication in patients hospitalised for COVID-19, giving rise to increased hospitalisations, in-hospital complications, and mortality.8 Therefore it is essential that these patients be followed up to reduce hospitalisations and complications.
Hospital Pharmacy outpatient clinics play a key role in the management of these patients. One of the objectives of this article is to evaluate the role played by hospital pharmacists during the pandemic, to determine how the pandemic may have influenced the prescription and dispensing of PCSK9i and the management of these patients.
The MEMOGAL study (NCT04319081) is an observational, multicentre, prospective study aimed at assessing changes in cognitive function in patients treated with PCSK9i.9 This study began in May 2020, in the midst of the pandemic, and we list below a number of points to be analysed in parallel to the study objectives:
- 1)
To discuss the role of hospital pharmacists during the pandemic and assess the impact of the pandemic on lipid control.
- 2)
Indications and frequency of PCSK9i prescription during the COVID-19 pandemic and a descriptive analysis of patients treated with PCSK9i.
- 3)
To assess the efficacy in reducing LDL-C and differences between alirocumab and evolocumab.
- 4)
To report the safety of PCSK9i.
This is an observational, multicentre, prospective analysis with a quasi-experimental design using Real-World Evidence of patients treated for the first time with PCSK9i under current funding conditions in the pharmacy outpatient clinics of 12 hospitals of the Public Health System from May 2020 to April 2021.
Patients were included in the study who had been diagnosed with homozygous or heterozygous FH and/or with established cardiovascular disease (ischaemic heart disease, ischaemic cerebrovascular disease, or peripheral artery disease) prescribed evolocumab or alirocumab for the first time in routine clinical practice, not achieving an LDL-C<100mg/dl after treatment with statins at the maximum tolerated dose, or intolerance to statins, and with correct therapeutic adherence to statins (≥80% per dispensing record).
The participating centres were Hospital Clínico Universitario de Santiago, Hospital Universitario de Ourense, Hospital Álvaro Cunqueiro (Vigo), Hospital Provincial de Pontevedra, Hospital Barco de Valdeorras, Hospital Virxe da Xunqueira (Cee), Hospital Arquitecto Macide de Ferrol, Hospital Universitario de A Coruña, Hospital da Costa (Burela), Hospital Universitario Lucus Au-gusti (Lugo), and Hospital Comacal de Monforte.
LDL-C values prior to starting treatment with PCSK9i once the informed consent form had been signed were taken as baseline, and those at 6 months were taken as follow-up values. The rest of the study variables were taken from the electronic medical records after signing the informed consent form and by direct interview with the patient in hospital pharmacy consultations.
Statistical analysisQuantitative variables are presented as mean or standard deviation or median and interquartile range, and qualitative variables as frequency and percentage. Differences between LDL-C levels were assessed using the Wilcoxon Sign test. In addition, percent reductions in LDL-C were calculated and the Student's t-test was used to determine differences between the patients taking alirocumab and evolocumab.
Statistical significance was accepted at p<.05. All analyses were performed with SPSS 19.0 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
ResultsAfter the first lockdown from March to April 2020, the most critical period in Spain, a total of 89 patients from Galician hospitals were included from May 2020 to April 2021 (mean age 60.44 years; 27% were women).
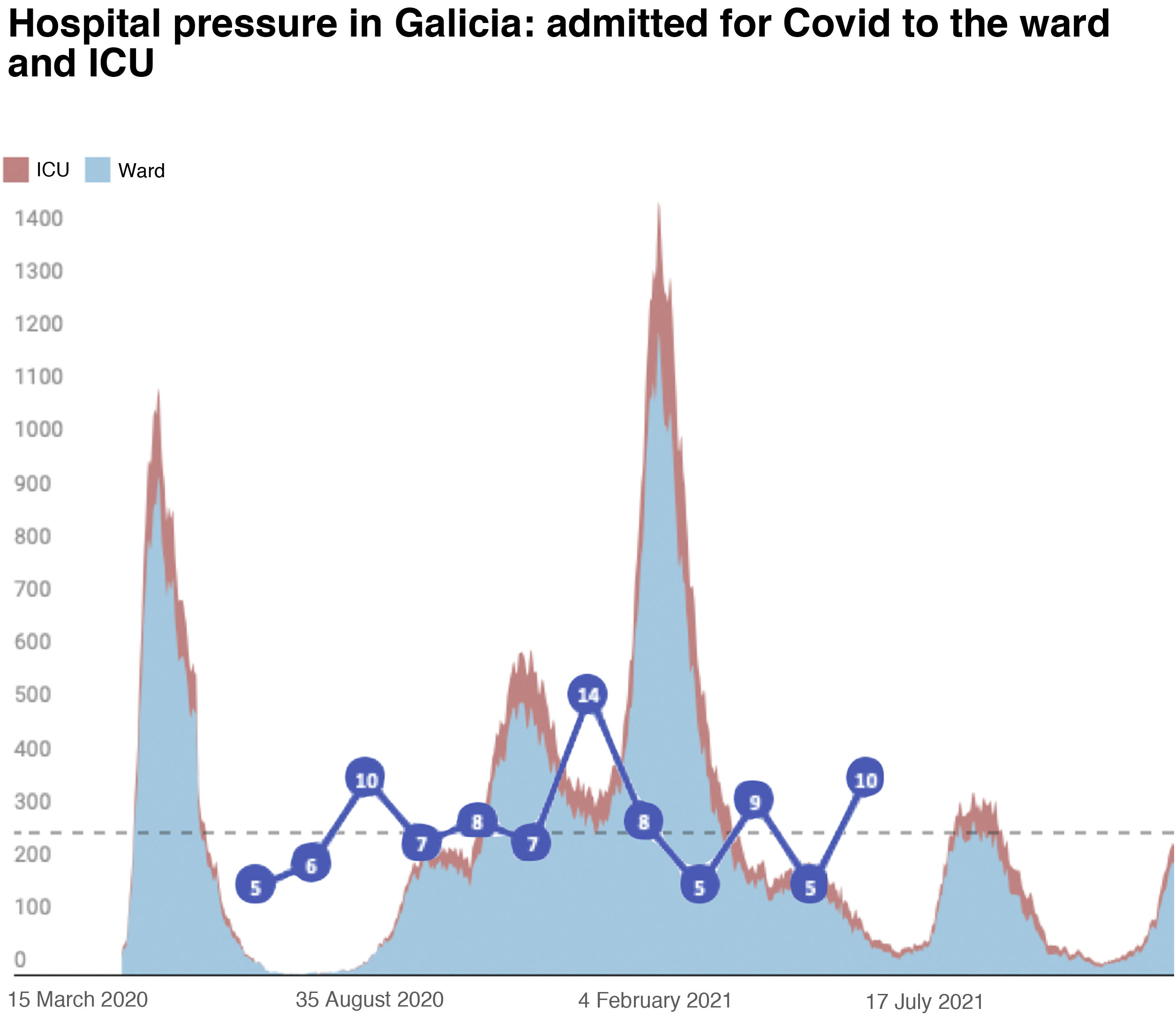
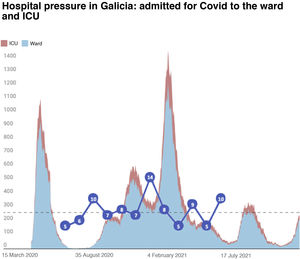
The inclusion rate was May 2020 5 patients; June 2020 6 patients; July 2020 10 patients; August 2020 7 patients; September 2020 8 patients; September 2020 8 patients; October 2020 7 patients; November 2020 14 patients; December 2020 8 patients; January 2021 5 patients; February 2021 9 patients; March 2021 5 patients; and April 2021 5 patients (Fig. 1).
Table 1 shows the baseline and demographic characteristics of the patients and changes in LDL-C are described in Table 1. The most relevant characteristics are 25.8% had FH, 86.5% had cardiovascular disease and 53.9% were intolerant to statins. A total of 78.8% of the patients were receiving high-intensity statin therapy, 19.2% medium-intensity, and 1.9% low-intensity statin therapy, 62.9% were on statin therapy, 62.9% were on ezetimibe.
Baseline and demographic characteristics of patients and changes in LDL-C.
| Variables | Patients included, n=89 |
|---|---|
| Sociodemographic characteristics | |
| Age (years)a | 60.44±9.973 |
| Sex (female), n(%) | 24 (27.0) |
| BMI (kg/m2)a | 28.47 (±4.531) |
| Previous medical history, n (%) | |
| Familiar hypercholesterolaemia | 23 (25.8) |
| Cardiovascular disease | 77 (86.5) |
| Diabetes mellitus | 18 (20.2) |
| Hypertension | 52 (58.4) |
| Intolerant to statins | 48 (53.9) |
| Type of statins, n (%) | |
| Atorvastatin | 18 (20.5) |
| Rosuvastatin | 30 (34.1) |
| Rosuvastatin | 1 (1.1) |
| Pitavastatin | 4 (4.5) |
| No statin | 35 (39.8) |
| Potency | |
| High potency | 41 (78.8) |
| Medium potency | 10 (19.2) |
| Low potency | 1 (1.9) |
| Ezetimibe | 56 (62.9) |
| PCSK9i (n,%) | |
| Evolocumab 140mg | 46 (51.7) |
| Alirocumab 75mg | 33 (37.1) |
| Alirocumab 150mg | 10 (11.2) |
| LDL levels (mg/dl) | |
| LDL at baseline (mg/dl)a | 148±53 |
| LDL at follow-up (mg/dl)a | 71±4 |
| Reduction in LDL (%) | 51±20 |
| Alirocumab | |
| LDL at baseline (mg/dl)a | 145±43 |
| LDL at follow-up (mg/dl)a | 73±36 |
| Reduction in LDL (%) | 51±15 |
| Evolocumab | |
| LDL at baseline (mg/dl)a | 152±62 |
| LDL at follow-up (mg/dl)a | 69±34 |
| Reduction in LDL (%) | 51±234 |
| LDL controls at follow-up, n (%) | |
| >70mg/dl | 31 (43.1) |
| 55–69mg/dl | 14 (19.4) |
| <55mg/dl | 27 (37.5) |
| Reduction >50% baseline | 42 (58.3) |
| Patients with LDL targets, n (%) | 44 (61.1) |
| EST+EZE+PCSK9i | 26 (59.1) |
| EZE+PCSK9i | 18 (40.9) |
The baseline LDL-C level was 148mg/dl and 71mg/dl at follow-up (up to 6 months). Forty-six patients were treated with evolocumab (51.7%) and 43 with alirocumab (49.3%). Patients treated with alirocumab had baseline values of 144mg/dl and 73mg/dl at follow-up, and those treated with evolocumab had baseline values of 151mg/dl and 69mg/dl at follow-up (Fig. 2). The reduction in LDL-C was 51.14%, with evolocumab 51.21% and with alirocumab 51.05% (Fig. 3). At 6 months, 43.1% had values>70mg/dl, 19.4% had values between 55 and 69mg/dl, and 37.5% had values<55mg/dl. Patients achieving a reduction>50% of LDL-C comprised 58.3%.
The adverse events presented were injection site reaction (n=2), myalgia (n=1), flu-like symptoms (n=1), and neurocognitive impairment (n=1).
DiscussionIn the present study we analysed PCSK9i prescription and lipid control in patients treated with these drugs during the COVID-19 pandemic. It is probably the first real-life study of patients treated with PCSK9i over that time. The results show that PCSK9i prescriptions cover a small proportion of patients, which may be due to the fewer face-to-face consultations or the non-implementation of telemedicine consultations. However, also taking into account that our PCSK9i -treated population is similar to all previous studies,6,10,12,13,20 the results in terms of efficacy are consistent with those published in pivotal and real-life clinical trials in non-COVID-19 times.5,6,10,11 These conclusions may be relevant when considering health policies in times of decreasing face-to-face activity so that the results of drugs with proven evidence can be applicable.
Pandemic and PCSK9i prescriptionThis may be mainly due to the work by healthcare professionals and the home dispensing system,14 which has resulted in good adherence to all treatments initiated in this period. This shows that applying these healthcare resources is effective in terms of efficacy and safety, and presumably cost-effective.15,16
However, it is worth noting that the number of inclusions per month in the study suggests that during the periods with more health restrictions, specifically the period of greater hospitalisation, the number of PCSK9i prescriptions decreased. The infection and inclusion curves are completely opposite in both graphs (Figs. 1 and 2). This may be because face-to-face medical consultations had been discontinued or decreased and/or teleconsultation was not implemented as efficiently in the different healthcare areas when prescribing these drugs17,30 (Fig. 1).
PCSK9i resultsIn the present study, more than half the patients were intolerant to statins and 86% were prescribed PCSK9i in secondary prevention, which highlights the large number of patients with cardiovascular disease and the importance of these drugs in this disease,1–3,5,6 for which only .89% of patients in Spain are currently prescribed them 12 months after an acute coronary syndrome.18 Moreover, the use of statins in monotherapy has been shown in many cases to be insufficient to achieve objectives in this group of patients.19–21
iPCSKs9 effectively and safely reduce LDL-C by around 50%.5,6,10,11 Evolocumab has been shown to be effective in homozygous FH.23 Alirocumab reduces the need for LDL-C apheresis in this type of patient, one of the few effective therapies for LDL-C control in FH.25 Other studies such as OSLER26 and ODYSSEY Long-term27 also demonstrated the efficacy of evolocumab and alirocumab in reducing LDL-C and major adverse cardiovascular events (MACE). Evolocumab and alirocumab show similar effects in reducing LDL-C at equipotent doses.5,6 Our results are coincide with these pivotal trials5,6and show that there is no significant difference in mean percentage reduction in LDL-C between evolocumab and alirocumab: 51.21% vs. 51.05% (p=.972).
The new European lipid guidelines (ESC guidelines)21 recommend that patients with FH or cardiovascular disease should reduce baseline LDL-C by 50% or target LDL-C by 50%. Furthermore, in cardiovascular disease after ACS the lipid profile should be reassessed at 4–6 weeks, and if targets have not been met PCSK9i (level of evidence I, class B) should be initiated.21
In the present analysis, statin-treated patients had 78% high intensity, 20% medium intensity and 2% low intensity, a percentage similar to the population studied in previous studies of statin monotherapy in secondary prevention, which concluded that 70% of patients did not reach targets below 70mg/dl LDL-C and only 11% did not reach the target of 50mg/dl LDL-C.22,23
Our results of PCSK9i use, both in monotherapy and in double or triple therapy (statins and/or ezetimibe) were that 43.1% of patients had LDL-C values above 70mg/dl at follow-up; 19.4% of patients between 55mg/dl and 69mg/dl, and 37.5% of all patients had values below 55mg/dl, therefore we have gone from 30% (in previous studies22,23) of patients below 70mg/dl to 60%, and also from 11% of patients below 55mg/dl (in previous studies22,23) to 37% in this study population.
In conclusion, we can see how the addition of PCSK9i improves the targets set by the guidelines, but nevertheless there are still around 40% of patients who do not reach the target of a 50% reduction in LDL-C or <55mg/dl.21 This happens essentially in triple therapy treatment (statins+ezetimibe+PCSK9i), however in patients totally intolerant to statins we see how the number of subjects who do not reach these targets increases to 60%, from which we can conclude and highlight the importance of maintaining statins as far as is possible.
Bempedoic acid, which at the time of publication of these data was in the process of approval in Spain, plays a key role in these patients. Several clinical trials28,29 have been published evaluating the reduction in LDL-C at 12 weeks. A reduction of 18% was observed when associated with statins and a reduction of 25% when used in monotherapy. The combination of bempedoic acid plus ezetimibe achieved a 38% reduction. For all these reasons, we believe it could be a very effective alternative in this type of patient who cannot tolerate any dose of statin and who do not meet the recommendations of the dyslipidaemia guidelines.
SafetyRegarding the safety of these drugs, excessive reduction of LDL-C has been related to the hypothesis of possible changes in cognitive function.
There is currently no evidence of this potential cognitive impairment as reported to date in only one publication: the EBBINGHAUS substudy.27 The adverse events reported in our study were injection site reaction (n=2), myalgia (n=1), flu-like symptoms (n=1), and neurocognitive impairment (n=1). Possible cognitive impairment is being studied as a main variable in the MEMOGAL study.9
Limitations include a small sample size as the analysis was conducted during the pandemic, when there may have been fewer PCSK9i prescriptions, therefore we believe that our results will provide important information on the current status of these drugs during the pandemic.
Conclusions- 1)
The COVID-19 pandemic has had a direct impact on PCSK9i prescription, which has decreased in the Galician population. Nevertheless, the results in terms of efficacy and safety are similar to previous studies, probably due to the work of healthcare professionals.
- 2)
More than half the patients were prescribed iPSCK9 due to statin intolerance, and 86% are in secondary prevention.
- 3)
These patients have similar reduction values to pivotal clinical trials. However, 39% of all patients on triple therapy and 60% of patients on dual therapy did not meet ESC/EAS guideline recommendations. There was no significant difference in the mean percentage reduction in LDL-C between evolocumab and alirocumab: 51.21% vs. 51.05% (p=.972).
- 4)
No events of special interest were observed with the use of these drugs.
This paper aims to contribute to the scientific literature with novel data and information on the current situation of patients treated with PCSK9i during the COVID-19 pandemic, in particular patients starting on PCSK9i for the first time. We seek to provide an overview of the current situation in terms of prescriptions, adherence to treatment in the context of health restrictions and different states of alarm and, above all, its effect in terms of efficacy and safety, and how these results conform to the current dyslipidaemia guidelines.
We would also like to highlight the multicentre, multidisciplinary, and prospective nature of the study.
Conflict of interestsThe authors have no conflict of interests to declare.24
We would like to thank the Fundación Instituto de Investigación Sanitaria de Santiago de Compostela (FIDIS) and all the principal investigators and sub-investigators of the centres participating in the study, belonging to the outpatient pharmacy clinics of the public hospitals of Galicia, and the Santiago and Alicante cardiology services.
Natalia Perez-Rodriguezj, María Anido-Garcíaj, Laura Villaverde-Piñeirok, Concepción Castro-Rubinesl, Yveth Michelle Tajes-Gonzalezl, Karina Lorenzo-Lorenzom, Cristina Casanova-Martinezm, Diego Rodriguez-Penasn, Iria Varela-Reyñ, Luis Margusino-Framiñáno, Carlos Crespo-Dizp, Irene Zarra-Ferroñ, Marisol Rodriguez-Cobosñ, Juan Rojo Valdésñ, Moisés Rodriguez-Mañeron,q, Héctor Mozo-Peñalverr, Esther Espino-Paisánr, Ana Rodriguez-Vazquezs, Jose Luis Rodriguez-Sanchezt, María Jesús García-Verdet, Alberto Cordero-Fortq,u, Jose Ramón Gonzalez-Juanateyn,q
j Servicio de Farmacia, Hospital Lucus Augusti, Lugo, Spain
k Servicio de Farmacia, Hospital Comarcal de Monforte de Lemos, Monforte de Lemos, Spain
l Servicio de Farmacia, Hospital da Costa de Burela, Burela, Spain
m Servicio de Farmacia, Hospital Álvaro Cunqueiro, Vigo, Spain
n Servicio de Cardiología, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, Spain
ñ Servicio de Farmacia, Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, Spain
o Servicio de Farmacia, Hospital Universitario de A Coruña, A Coruña, Spain
p Servicio de Farmacia, Complexo Hospitalario Universitario de Pontevedra, Pontevedra, Spain
q Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
r Servicio de Farmacia, Hospital do Barbanza, Ribeira, Spain
s Servicio de Farmacia, Hospital Comarcal de Valdeorras, O Barco de Valdeorras, Spain
t Servicio de Farmacia, Hospital Virxe da Xunqueira, Cee, Spain
u Servicio de Cardiología, Hospital San Juan de Alicante, San Juan de Alicante, Spain