Cardiovascular disease (CVD) represents the primary cause of death and disability globally, with elevated cholesterol as one of the leading risk factors for CVD. We describe the clinical characteristics, treatment patterns, and effectiveness of evolocumab in treating hyperlipidemia.
MethodsObservational study conducted through a chart review of patients with hyperlipidemia receiving evolocumab as part of clinical management in Colombia.
ResultsThis study included 115 patients treated with evolocumab. A total of 101 patients (87.8%) had a history of CVD, 13 (11.3%) familial hypercholesterolemia (FH), and 23 (20%) type 2 diabetes. Thirty-nine patients reported intolerance to any statin (33.9%). The median value of LDL-C before initiation of evolocumab was 147mg/dL (IQR: 122.5–183.7mg/dL). Within the first 3 months of treatment, LDL-C value dropped to a median value of 53mg/dL (IQR: 34.0–95.5mg/dL), showing a reduction of 63.9%. The median LDL-C values remained below 45mg/dL until the end of follow-up. Among the patients with available data, up to 61% achieved an LDL-C level below 55mg/dL at the 10–12-month follow-up. A total of 72% of patients were persistent with treatment. Safety results showed a low frequency of hospitalizations (≤2%) and treatment-emergent adverse drug reactions (5.2%). No serious adverse events were reported.
ConclusionsEvolocumab was associated with reductions in LDL-C levels, with a relative decrease of 63.9% within the first 3 months of treatment. Low rates of interruptions due to adverse events and adequate medication persistence was reported.
Las enfermedades cardiovasculares (ECV) representan la principal causa de muerte y discapacidad en todo el mundo, siendo el colesterol elevado uno de los principales factores de riesgo de ECV. El presente estudio describe las características clínicas, patrones de tratamiento y la efectividad de evolocumab en el tratamiento de la hiperlipidemia.
MétodosEstudio observacional de revisión de historias clínicas de pacientes con hiperlipidemia que reciben evolocumab como parte del manejo clínico en Colombia.
ResultadosSe incluyeron 115 pacientes tratados con evolocumab. Un total de 101 pacientes (87,8%) presentaron antecedentes de ECV, 13 (11,3%) de hipercolesterolemia familiar y 23 (20%) de diabetes tipo 2. De los pacientes estudiados, 39% declararon intolerancia a alguna estatina (33,9%). La mediana de C-LDL antes del inicio de evolocumab fue de 147mg/dL (IQR: 122,5-183,7mg/dL). En los primeros tres meses de tratamiento, el valor de C-LDL descendió a 53mg/dL (IQR: 34,0-95,5mg/dL), siendo una reducción de 63,9%. La mediana de C-LDL se mantuvo por debajo de 45mg/dL hasta el final del seguimiento. Entre los pacientes con datos disponibles, hasta 61% alcanzó un nivel de LDL-C inferior a 55mg/dL en el seguimiento de 10-12 meses. De los pacientes analizados, 72% fue persistente al tratamiento. Los resultados de seguridad mostraron una baja frecuencia de hospitalizaciones (≤2%) y de reacciones adversas relacionadas al tratamiento (5,2%). No se notificaron acontecimientos adversos graves.
ConclusionesEvolocumab se asoció con reducciones en los niveles de C-LDL, con una disminución relativa de 63,9% en los primeros tres meses de tratamiento. Se reportaron bajas tasas de interrupciones por eventos adversos y adecuada persistencia a la medicación.
Cardiovascular disease (CVD) represents the primary cause of death and disability globally,1,2 with elevated cholesterol as one of the leading risk factors for cardiovascular (CV) disease, with an estimated prevalence of 39% among all adults.2
According to a World Health Organization report, in 2019, there were 17.9 million deaths due to CVDs, representing 32% of all global deaths for that year.1 Of these deaths, 85% were secondary to heart attacks and strokes. Additionally, of the 17 million premature deaths of non-communicable diseases reported for 2019 (persons under 70 years old), 38% were caused by CVDs. In Colombia, 75,826 deaths were reported due to circulatory system diseases in 2019, with a mortality rate of 153 per 100,000 inhabitants.3
Treatment of dyslipidemia by reduction of low-density lipoprotein cholesterol (LDL-C) and other cholesterol measures (total cholesterol and non-high-density lipoprotein cholesterol [HDL-C]) has proved beneficial in lowering the risk of CV events.4 Evolocumab is a fully human monoclonal immunoglobulin G2 (IgG2) antibody directed against proprotein convertase subtilisin/kexin type 9 (PCSK9). It is indicated in adults with primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia, as an adjunct to diet, in combination with a statin or statin with other lipid-modifying therapies (LLTs) in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin, alone or in combination with other LLTs in statin-intolerant patients, or for whom a statin is contraindicated.4
Evolocumab has been shown to effectively reduce LDL-C levels and the incidence of CV events in clinical studies with large sample sizes. The OSLER-1 trial, which included 4465 patients, showed that evolocumab plus standard therapy vs standard therapy alone reduced the level of LDL-C by 61%, from a median of 120mg/dL to 48mg/dL.5 Similarly, the FOURIER trial with 27,564 patients showed a 59% least-squares mean percentage reduction in LDL-C levels with evolocumab compared with placebo at 48 weeks of treatment, and a significant reduction in the risk for major CV events (hazard ratio: 0.85; 95% confidence interval [CI]: 0.79–0.92).6
Regarding the reimbursement process in Colombia, at the time of the ZERBINI study, evolocumab was not included in the health benefits plan (PBS), so the prescribing physician had to fill out a form justifying the use of the drug (MIPRES). This form allowed verifying compliance with the indications for use approved by the local regulatory agency, after which its dispensation was approved; evolocumab has been included in the national benefit plan since January 2022, and the medication is no longer reimbursed but paid for directly by the healthcare provider.
However, despite the proven efficacy of treatments available to reduce the risk of CVDs in controlled clinical settings, there is a need for local, real-world data on patients receiving evolocumab for hyperlipidemia treatment. This is the first study to describe the clinical characteristics, treatment patterns, and effectiveness of evolocumab in patients at high risk of CVD in Colombia.
Patients and methodsThe MultiZonal obsERvational study conducted By clinIcal practitioners on Repatha® use iN patients with hyperlipIdemia (ZERBINI) is a multicountry, observational, ambispective study conducted through a chart review of patients receiving evolocumab as part of routine clinical management of their hyperlipidemia. The ZERBINI study has adhered to the STROBE ethical guidelines for observational studies. Here, we report the results for the Colombian population. Variable measurements, such as baseline LDL-C, were taken from data up to 6 months before evolocumab treatment (retrospective data). In contrast, follow-up measurements were taken prospectively according to a patient's entry to the study. The study protocol was reviewed and approved by each site's institutional ethics committee. Participants were required to sign an informed consent form for inclusion in the study. There were no additional exclusion criteria.
Data collected included demographic and clinical characteristics of patients receiving evolocumab as part of their hyperlipidemia treatment. Patients were followed up for 12 months after initiation of evolocumab, and information about treatment patterns and effectiveness of the clinical intervention was collected. Patients treated for hyperlipidemia who received at least one dose of evolocumab were included. Data were provided by study site staff, using patient's medical notes to abstract information to complete electronic case report forms in the study-specific database.
Primary outcomes included clinical characteristics before initiation of evolocumab, including familial hypercholesterolemia (FH) status (diagnosed/not diagnosed), based on the Dutch Lipid Clinic Network or the Simon Broome criteria; CVD history; diabetic status (diabetic/not diabetic; type 1 or type 2); LDL-C value (the last LDL-C measured within 6 months before the initiation of evolocumab will be regarded as the baseline LDL-C). The protocol did not require a specific method for LDL-C calculation, allowing calculation by Friedewald formula or by ultracentrifugation.
Additional outcomes included LDL-C and other cholesterol levels (over time; all values available during the 12-month follow-up period), use of evolocumab at treatment initiation and over 12 months of follow-up (dose, frequency, switching to another PCSK9 inhibitor), lipid-modifying therapy (LMT) use at baseline and over 12 months of follow-up after evolocumab initiation (type, dose, frequency, switching, augmentation), incidence of hospitalization (reason for admission/final diagnosis, admission date, and discharge date) over 12 months of follow-up after evolocumab initiation, incidence of physician visits over 12 months of follow-up after evolocumab initiation (date and reason for visit), incidence of LDL-C <55mg/dL at any time over 12 months of follow-up after evolocumab initiation,7 and change from baseline in LDL-C over 12 months of follow-up after evolocumab initiation. Medication persistence was defined as the percentage of patients who remained on evolocumab until the end of the observational period (12 months).
Statistical analysesStatistical analyses were descriptive. Categorical variables were described with frequencies and percentages. Continuous variables underwent the Shapiro–Wilks normality test, and medians and interquartile ranges were reported since the continuous variables did not have a normal distribution. Two sensitivity analyses were conducted to confirm the consistency of the results by using the mean LDL-C value for patients with more than one LDL-C measurement in a given period. Statistical analysis was performed in SAS System version 9.4.
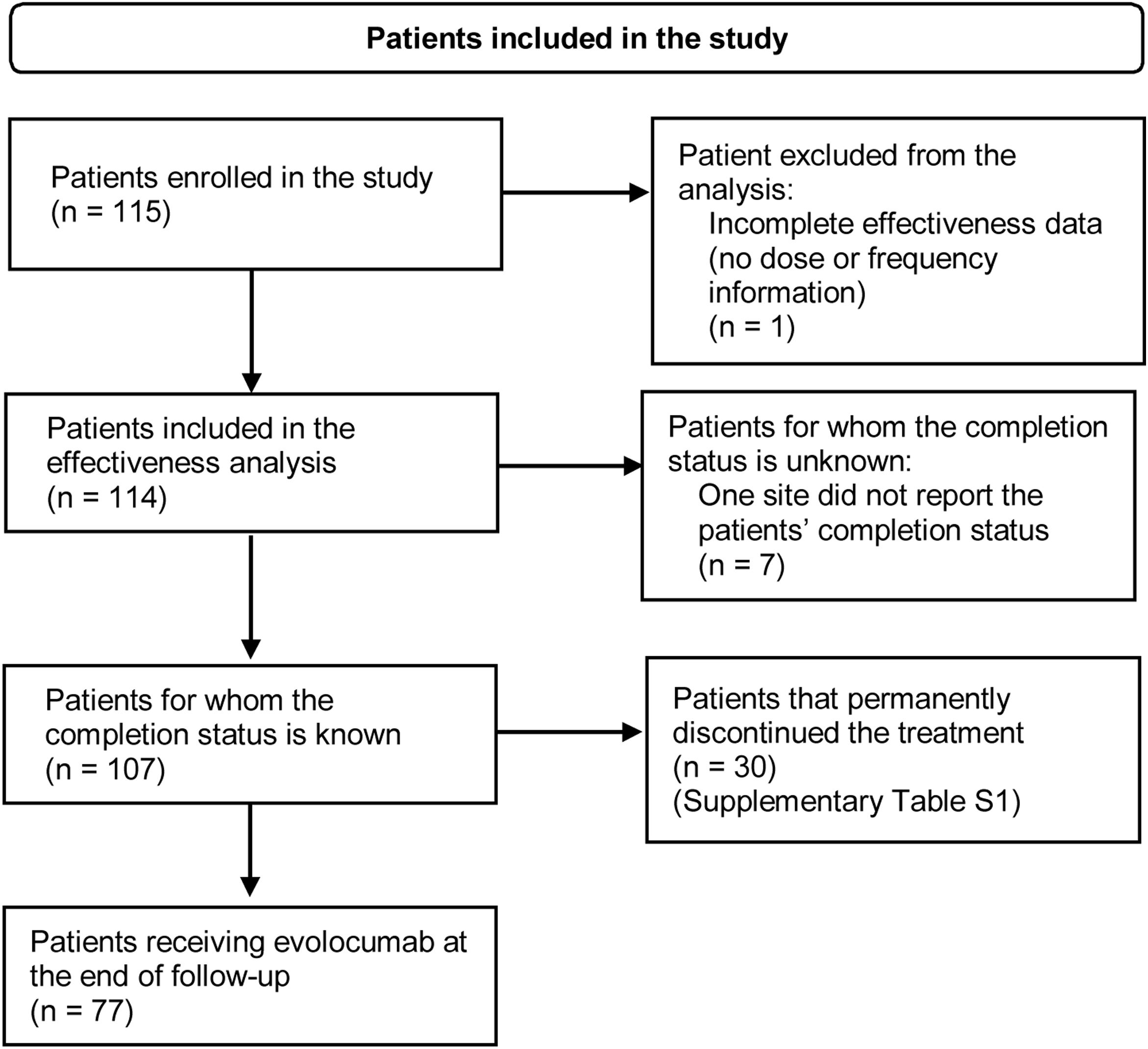
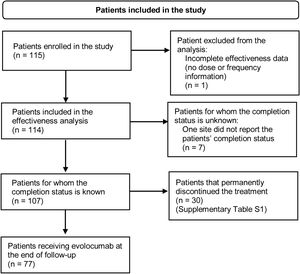
ResultsPatient characteristicsA total of 115 patients were enrolled in the study. At the end of the follow-up period, 77 patients were still receiving evolocumab. Thirty patients (26.1%) discontinued evolocumab by the end of the study, mainly due to the physician's request or decision, healthcare provider company not dispensing the medication, administrative decision or patients being lost to follow-up (Supplementary Table S1). Fig. 1 shows the flowchart of patients included in the study and their permanence.
Flowchart of patients included in the study. Inclusion criteria: Patients with dyslipidemia older than 18 years at the time of signing the informed consent form who initiated on evolocumab at physician's discretion after August 1, 2017. Patients should have received at least one dose of evolocumab before enrolment and were required to have less than 6 months of exposure to evolocumab. Although the patient has no information on dosage or treatment schedule, information on baseline characteristics was reported.
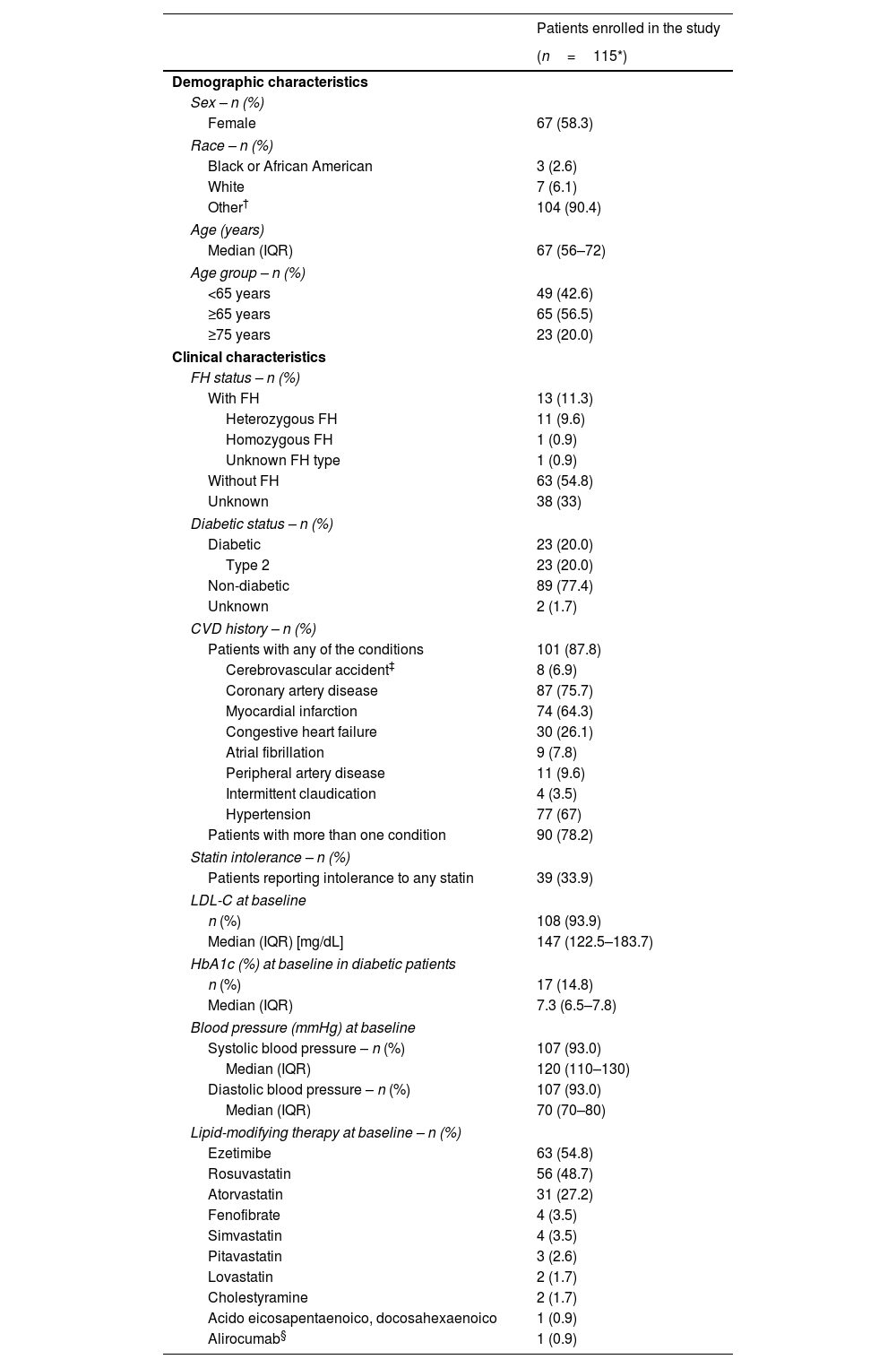
The demographic and clinical characteristics of patients are shown in Table 1. Most of the patients were female 58.3% (n=67). Patients had a median age of 67 (interquartile range [IQR]): 56–72 years); the range was 28–88 years. Most of the patients were aged 65 years and above (56.5%).
Demographic and clinical characteristics.
| Patients enrolled in the study | |
|---|---|
| (n=115*) | |
| Demographic characteristics | |
| Sex – n (%) | |
| Female | 67 (58.3) |
| Race – n (%) | |
| Black or African American | 3 (2.6) |
| White | 7 (6.1) |
| Other† | 104 (90.4) |
| Age (years) | |
| Median (IQR) | 67 (56–72) |
| Age group – n (%) | |
| <65 years | 49 (42.6) |
| ≥65 years | 65 (56.5) |
| ≥75 years | 23 (20.0) |
| Clinical characteristics | |
| FH status – n (%) | |
| With FH | 13 (11.3) |
| Heterozygous FH | 11 (9.6) |
| Homozygous FH | 1 (0.9) |
| Unknown FH type | 1 (0.9) |
| Without FH | 63 (54.8) |
| Unknown | 38 (33) |
| Diabetic status – n (%) | |
| Diabetic | 23 (20.0) |
| Type 2 | 23 (20.0) |
| Non-diabetic | 89 (77.4) |
| Unknown | 2 (1.7) |
| CVD history – n (%) | |
| Patients with any of the conditions | 101 (87.8) |
| Cerebrovascular accident‡ | 8 (6.9) |
| Coronary artery disease | 87 (75.7) |
| Myocardial infarction | 74 (64.3) |
| Congestive heart failure | 30 (26.1) |
| Atrial fibrillation | 9 (7.8) |
| Peripheral artery disease | 11 (9.6) |
| Intermittent claudication | 4 (3.5) |
| Hypertension | 77 (67) |
| Patients with more than one condition | 90 (78.2) |
| Statin intolerance – n (%) | |
| Patients reporting intolerance to any statin | 39 (33.9) |
| LDL-C at baseline | |
| n (%) | 108 (93.9) |
| Median (IQR) [mg/dL] | 147 (122.5–183.7) |
| HbA1c (%) at baseline in diabetic patients | |
| n (%) | 17 (14.8) |
| Median (IQR) | 7.3 (6.5–7.8) |
| Blood pressure (mmHg) at baseline | |
| Systolic blood pressure – n (%) | 107 (93.0) |
| Median (IQR) | 120 (110–130) |
| Diastolic blood pressure – n (%) | 107 (93.0) |
| Median (IQR) | 70 (70–80) |
| Lipid-modifying therapy at baseline – n (%) | |
| Ezetimibe | 63 (54.8) |
| Rosuvastatin | 56 (48.7) |
| Atorvastatin | 31 (27.2) |
| Fenofibrate | 4 (3.5) |
| Simvastatin | 4 (3.5) |
| Pitavastatin | 3 (2.6) |
| Lovastatin | 2 (1.7) |
| Cholestyramine | 2 (1.7) |
| Acido eicosapentaenoico, docosahexaenoico | 1 (0.9) |
| Alirocumab§ | 1 (0.9) |
CVD: cardiovascular disease; FH: familial hypercholesterolemia; HbA1c: hemoglobin A1c; LDL-C: low-density lipoprotein cholesterol; IQR: interquartile range; SD: standard deviation.
Table 1 presents the clinical characteristics of patients before initiation of evolocumab, including FH status, CVD history, diabetic status (diabetic/not diabetic; type 1 or type 2), and history of statin intolerance, assessed by the treating physician's criteria. Overall, 11.3% (n=13) patients had a diagnosis of FH, 87.8% (n=101) had a history of CVD, 20.0% (n=23) had type 2 diabetes and 33.9% (n=39) reported intolerance to any statin.
Baseline LDL-C values were reported for 108 patients (93.9%). Friedewald formula was the most frequent method for LDL-C calculation (>75%). The median value of LDL-C before initiation of evolocumab was 147mg/dL (IQR: 122.5–183.7mg/dL) (Table 1), with a minimum value of 63mg/dL in a patient with coronary artery disease, and a maximum value of 529mg/dL in a patient with FH (case classified as probable according to the criteria of the Dutch Lipid Clinic Network; it could not be confirmed genetically due to the death of the patient). Other clinical baseline values such as HbA1c and blood pressure are reported in Table 1.
Lipid-modifying therapyIn addition, LMTs were assessed at baseline. Possible combinations of LMTs with evolocumab and the demographic and clinical characteristics of patients in each treatment group are shown in Supplementary Table S2. Ezetimibe was the most used LMT in combination with statins and evolocumab (46 patients [40%]). A total of 42 patients (36.5%) were treated with evolocumab plus a statin and 12 patients (10.4%) were treated with evolocumab plus ezetimibe.
Rosuvastatin (48.7%) and atorvastatin (27.2%) were the most frequent statins used at baseline (Table 1). Among patients who received statins at baseline, 87% (n=80) received high-intensity statins, 10.9% (n=10) moderate-intensity, and 2.2% (n=2) low-intensity. At baseline, six patients (5.2%) they treated with evolocumab as monotherapy.
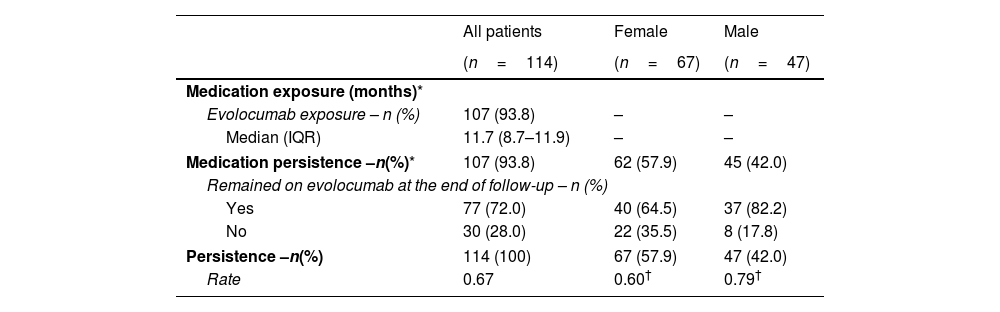
Evolocumab treatment patternsOne patient was excluded from the effectiveness analysis because of incomplete data (no information of dose, frequency, or administration device). All patients received evolocumab in the 140mg every other week dose regimen. Data of exposure to evolocumab were available for 107 patients. Median evolocumab exposure was 11.7 months (IQR: 8.7–11.9), with a minimum exposure of 1.4 months and a maximum exposure of 12 months (completed follow-up) (Table 2). No switch to another PCSK9 inhibitor was identified in the included population.
Treatment exposure and medication persistence.
| All patients | Female | Male | |
|---|---|---|---|
| (n=114) | (n=67) | (n=47) | |
| Medication exposure (months)* | |||
| Evolocumab exposure – n (%) | 107 (93.8) | – | – |
| Median (IQR) | 11.7 (8.7–11.9) | – | – |
| Medication persistence –n(%)* | 107 (93.8) | 62 (57.9) | 45 (42.0) |
| Remained on evolocumab at the end of follow-up – n (%) | |||
| Yes | 77 (72.0) | 40 (64.5) | 37 (82.2) |
| No | 30 (28.0) | 22 (35.5) | 8 (17.8) |
| Persistence –n(%) | 114 (100) | 67 (57.9) | 47 (42.0) |
| Rate | 0.67 | 0.60† | 0.79† |
Medication persistence was calculated on the basis of total number of patients with available information on completion status. Persistence rate=1−(number of discontinuations/total number of person-years of observation). Calculation was based on the total population included in the study analysis.
IQR: interquartile range; SD: standard deviation.
Temporary discontinuation (temporarily missed doses within a time window) was most frequent between months 4 and 6 of treatment. However, the frequencies for temporary discontinuation were less than 15% throughout the entire study period.
Of the patients for whom the study completion status was known (n=107), 72% remained on evolocumab at the end of the follow-up (n=77); hence, 72% of the patients were considered persistent to evolocumab treatment since it is a long-term medication.
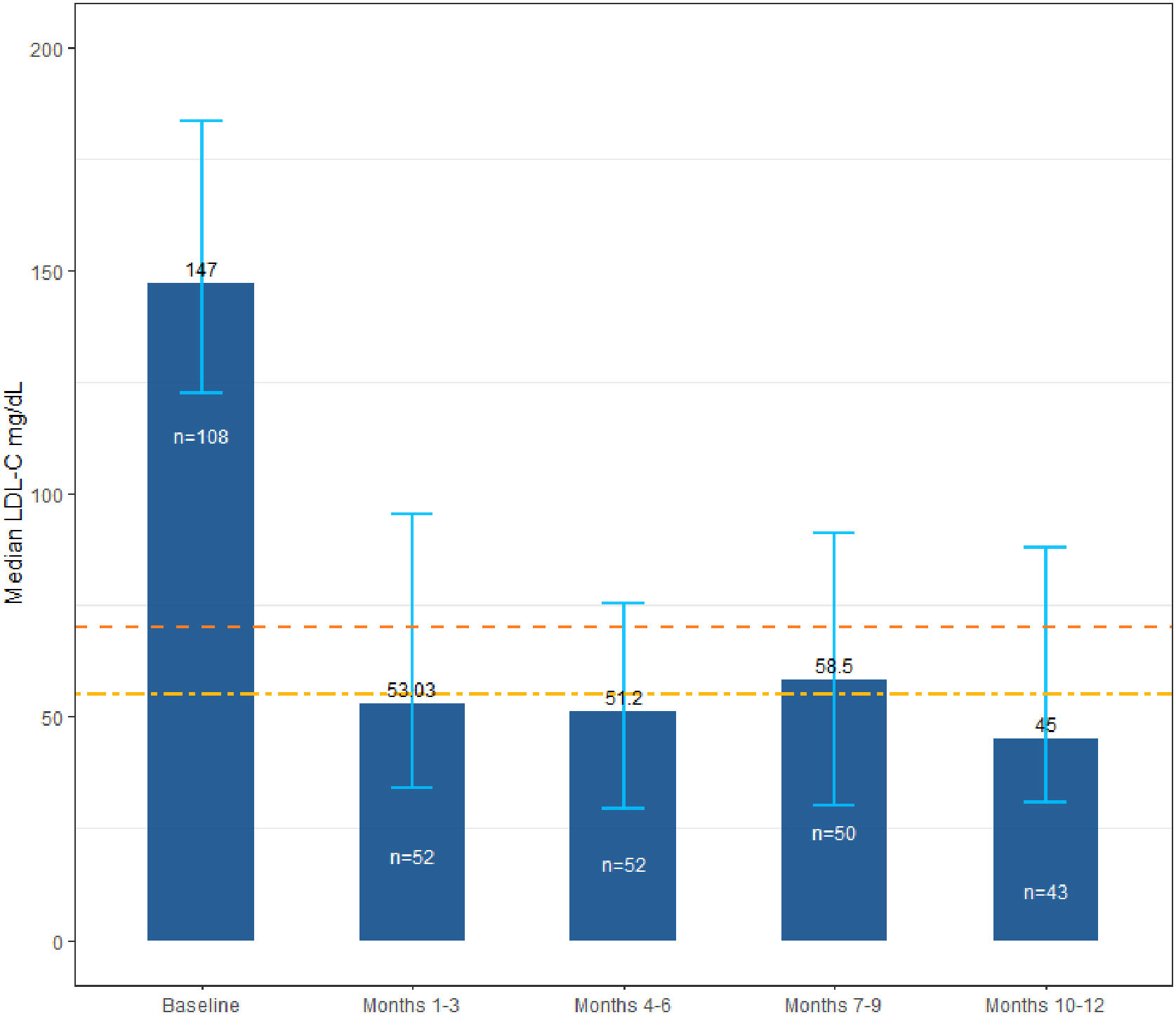
LDL-C changes over timeThe primary outcome was the LDL-C levels at baseline and during the 12-month follow-up. The results showed that the median LDL-C level following the initiation of evolocumab treatment dropped to 53mg/dL (IQR: 34.0–95.5mg/dL) within the first 3 months and remained in this range throughout the study's follow-up period (63.9% relative reduction) (Fig. 2). LDL-C and other cholesterol levels at baseline and over time are shown in Table 3.
LDL-C levels at baseline and over time. Dotted lines represent 1.4mmol/L [55mg/dL] and 1.8mmol/L [70mg/dL] levels for LDL-C goals, according to guidelines for the management of dyslipidemias.7,8 Error bars represent the interquartile range for the LDL-C median value. LDL-C, low-density lipoprotein cholesterol.
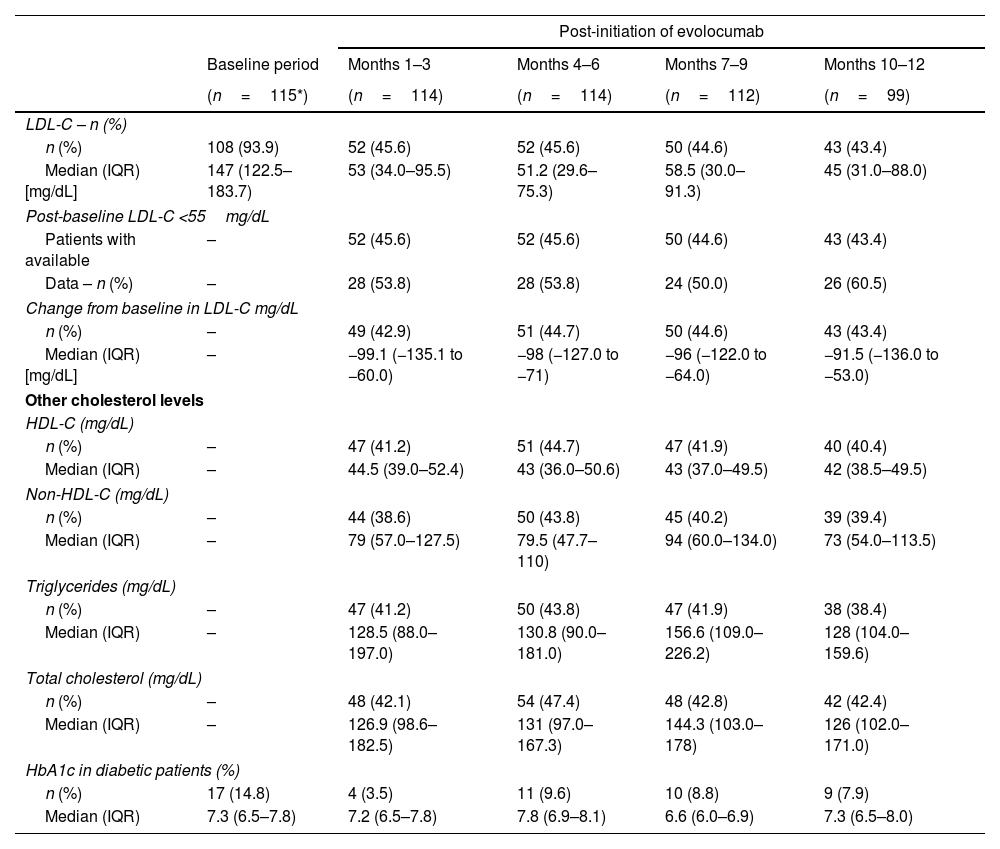
LDL-C and other cholesterol levels at baseline and over time.
| Post-initiation of evolocumab | |||||
|---|---|---|---|---|---|
| Baseline period | Months 1–3 | Months 4–6 | Months 7–9 | Months 10–12 | |
| (n=115*) | (n=114) | (n=114) | (n=112) | (n=99) | |
| LDL-C – n (%) | |||||
| n (%) | 108 (93.9) | 52 (45.6) | 52 (45.6) | 50 (44.6) | 43 (43.4) |
| Median (IQR) [mg/dL] | 147 (122.5–183.7) | 53 (34.0–95.5) | 51.2 (29.6–75.3) | 58.5 (30.0–91.3) | 45 (31.0–88.0) |
| Post-baseline LDL-C <55mg/dL | |||||
| Patients with available | – | 52 (45.6) | 52 (45.6) | 50 (44.6) | 43 (43.4) |
| Data – n (%) | – | 28 (53.8) | 28 (53.8) | 24 (50.0) | 26 (60.5) |
| Change from baseline in LDL-C mg/dL | |||||
| n (%) | – | 49 (42.9) | 51 (44.7) | 50 (44.6) | 43 (43.4) |
| Median (IQR) [mg/dL] | – | −99.1 (−135.1 to −60.0) | −98 (−127.0 to −71) | −96 (−122.0 to −64.0) | −91.5 (−136.0 to −53.0) |
| Other cholesterol levels | |||||
| HDL-C (mg/dL) | |||||
| n (%) | – | 47 (41.2) | 51 (44.7) | 47 (41.9) | 40 (40.4) |
| Median (IQR) | – | 44.5 (39.0–52.4) | 43 (36.0–50.6) | 43 (37.0–49.5) | 42 (38.5–49.5) |
| Non-HDL-C (mg/dL) | |||||
| n (%) | – | 44 (38.6) | 50 (43.8) | 45 (40.2) | 39 (39.4) |
| Median (IQR) | – | 79 (57.0–127.5) | 79.5 (47.7–110) | 94 (60.0–134.0) | 73 (54.0–113.5) |
| Triglycerides (mg/dL) | |||||
| n (%) | – | 47 (41.2) | 50 (43.8) | 47 (41.9) | 38 (38.4) |
| Median (IQR) | – | 128.5 (88.0–197.0) | 130.8 (90.0–181.0) | 156.6 (109.0–226.2) | 128 (104.0–159.6) |
| Total cholesterol (mg/dL) | |||||
| n (%) | – | 48 (42.1) | 54 (47.4) | 48 (42.8) | 42 (42.4) |
| Median (IQR) | – | 126.9 (98.6–182.5) | 131 (97.0–167.3) | 144.3 (103.0–178) | 126 (102.0–171.0) |
| HbA1c in diabetic patients (%) | |||||
| n (%) | 17 (14.8) | 4 (3.5) | 11 (9.6) | 10 (8.8) | 9 (7.9) |
| Median (IQR) | 7.3 (6.5–7.8) | 7.2 (6.5–7.8) | 7.8 (6.9–8.1) | 6.6 (6.0–6.9) | 7.3 (6.5–8.0) |
HDL-C: high-density lipoprotein cholesterol; HbA1c: hemoglobin A1c; LDL-C: low-density lipoprotein cholesterol; IQR: interquartile range; SD: standard deviation.
Per protocol, baseline measurements were not recorded for the other cholesterol indicators (HDL-C, non-HDL-C, triglycerides, and total cholesterol); however, post-initiation follow-up data for these are reported in Table 3.
Reduction in LDL-C according to the LLT combination is shown in Supplementary Table 3. The higher reduction within the first 3 months of treatment was found among patients who received evolocumab plus ezetimibe and statin (n=19), with a reduction from baseline of 72.2%, from 149.0mg/dL (IQR: 126.6–181.4mg/dL) to 41.36mg/dL (IQR: 35.0–69.0mg/dL), and among patients treated with evolocumab plus ezetimibe (n=9), with a reduction of 60.9%, from 124mg/dL (IQR: 105.0–149.6mg/dL) to 48.5mg/dL (IQR: 35.0–62.0mg/dL).
Patients with a value less than 55mg/dL in LDL-C levelsThe incidence of patients who achieved an LDL-C level of less than 55mg/dL at some point during the follow-up was assessed after initiation of evolocumab. The target lipid value was established in accordance with the 2019 ESC/EAS guidelines for the management of dyslipidemias.7 Since patients could have multiple LDL-C assessments within the same period (e.g., LDL-C value at months 1 and 3), the minimum value was considered within each period. Information about LDL-C <55mg/dL was available in 50–52 patients during the first 9 months and in 43 during the last measurement (months 10–12). Among the patients with available data, 60.5% achieved an LDL-C level below 55mg/dL at the 10–12-month follow-up.
Subgroup analysisThe representation of subgroups of interest in the sample studied allowed us to explore the analysis of effectiveness among usually unexplored populations. Concerning age groups, the study included 20.0% of patients aged 75 years or older. Median LDL-C at baseline was 146.0mg/dL (IQR: 130.0–160.0mg/dL); after three months of initiation with evolocumab, LDL-D dropped to 50.6mg/dL (IQR: 33.0–85.0mg/dL), remaining below 55.0mg/dL, and ending by months 10–12 at 64.2mg/dL (IQR 37.0–93.7mg/dL) (Supplementary Table S4). The persistence rate was not statistically different among adults 75 years or older (0.74) compared to patients younger than 75 years (0.66) (χ2p=0.46).
Most of the patients were females (58.3%). The baseline LDL-C value was higher in women (151.3mg/dL; IQR: 132.5–190.0mg/dL) than in men (136.3mg/dL; IQR: 104.8–166.5mg/dL). The most significant difference in LDL-C reduction was at three months of treatment, where women showed a level of 68.1mg/dL (IQR: 36.5–110.7mg/dL), compared to men whose levels dropped to 41.5mg/dL (IQR: 21.8–57.7mg/dL). The results for the other follow-ups were more similar in that both groups had levels below 55.0mg/dL for months 4–6 and 10–12 (Supplementary Table S4). Additionally, the treatment persistence rate was higher in men (0.79) than in women (0.60) (Table 2). The χ2 test showed that the persistence rate differed significantly depending on sex (p=0.04). However, the association between sex and statin intolerance was not significant; results showed that intolerance to statins was similar among female patients (34.3%) than in males (34.0%) (χ2p=0.975).
Finally, a total of 30 patients had a history of congestive heart failure (CHF) at baseline. Initial LDL-C among patients with CHF was similar to patients without CHF (145mg/dL [IQR: 130–169] and 149mg/dL [IQR: 121.4–190.0], respectively). After three months of treatment, LDL-C levels in patients with a history of CHF were 52.7mg/dL (IQR: 31.5–76.5mg/dL), similar to the rest of the study population (53.8; IQR: 35.0–112.4mg/dL); a similarity that was maintained at the end of follow-up where patients with CHF had LDL-C value of 39.6mg/dL (IQR: 28.0–85.0mg/dL) (Supplementary Table S4).
Sensitivity analysisConsidering that patients could have more than one measurement of LDL-C levels in the same time period, the primary analysis was estimated using the average value in patients with more than one LDL-C measurement in each time window. To assess whether using the mean value could underestimate or overestimate the results, two sensitivity analyses were performed: (1) using the maximum value of the LDL-C value in the time period and (2) using the minimum value of LDL-C in the time period. The results of both sensitivity analyses were consistent with the main analysis, showing effectiveness in LDL-C change after initiation of evolocumab treatment (Supplementary Table S5).
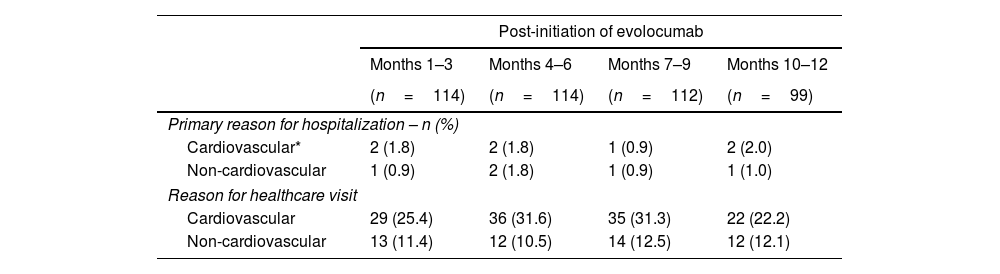
SafetySafety-related outcomes are shown in Table 4. During the 12-month follow-up, seven hospitalizations for CV reasons and five hospitalizations for non-CV reasons were recorded during the post-initiation of evolocumab period. The median length of stay (LoS) for CV-related hospitalizations was 4.5 days (IQR: 1.0–7.0). The median LoS for non-CV hospitalizations was 5 days (IQR: 3.0–6.0).
Safety-related outcomes.
| Post-initiation of evolocumab | ||||
|---|---|---|---|---|
| Months 1–3 | Months 4–6 | Months 7–9 | Months 10–12 | |
| (n=114) | (n=114) | (n=112) | (n=99) | |
| Primary reason for hospitalization – n (%) | ||||
| Cardiovascular* | 2 (1.8) | 2 (1.8) | 1 (0.9) | 2 (2.0) |
| Non-cardiovascular | 1 (0.9) | 2 (1.8) | 1 (0.9) | 1 (1.0) |
| Reason for healthcare visit | ||||
| Cardiovascular | 29 (25.4) | 36 (31.6) | 35 (31.3) | 22 (22.2) |
| Non-cardiovascular | 13 (11.4) | 12 (10.5) | 14 (12.5) | 12 (12.1) |
Primary Healthcare Visit: dental practices, visits to general practitioners, community pharmacies and optometrists.
Secondary Healthcare Visit: acute healthcare (elective care or emergency care) provided by medical specialists in a secondary care setting.
IQR: interquartile range; SD: standard deviation.
During the follow-up period, 10% (1–3 months) and 16% (7–9 months) of the patients consulted primary healthcare services (e.g., dental practices, general practitioners, optometrists), while up to 29% attended for acute healthcare (elective care or emergency care) at the 4–6-month follow-up, provided by medical specialists in a secondary care setting.
The safety results were based on the descriptive report of treatment-emergent adverse drug reactions, which included any adverse event related to treatment, with a particular interest in serious adverse events (death or events that threaten life, require hospitalization, extend hospitalization, or cause disability), and adverse events that led to the discontinuation of evolocumab.
Throughout the study follow-up time, six patients reported treatment-related adverse events (5.2%), of which none were serious. Likewise, only two adverse events led to discontinuation of evolocumab treatment; none of them was classified as a serious adverse event.
According to the system organ class and preferred term classification, the most frequent adverse events were general disorders and administration site conditions, followed by nervous system disorders. Dizziness and influenza (flu-like symptoms) were the only adverse events reported in more than one patient (Supplementary Table S6).
DiscussionCVDs are the leading cause of global mortality and a significant contributor to disability.1,2 Prevention and treatment of dyslipidemia are considered an integral part of individual CVD prevention interventions.4
Statins remain the first-choice therapy for dyslipidemia since they show reductions in the risk of major vascular events by lowering LDL-C.4 However, some patients require additional therapies to achieve lipid goals.4,8 This observational study using real-world data presents information on the routine clinical management of patients with hyperlipidemia in Colombia.
Our results are highly consistent with those of previous studies. Data on clinical trials for PCSK9 inhibitors have demonstrated their effectiveness and safety as LLTs. A systematic review and network meta-analysis conducted with 69 trials of LLTs that enrolled patients requiring further LDL-C reduction while on maximally tolerated medium- or high-intensity statin showed that PCSK9 inhibitors significantly reduced LDL-C by 54–74% compared with placebo.9
Likewise, results of the effectiveness and safety of evolocumab in the present study support the findings of clinical trials. In our study, the baseline median value of LDL-C was 147mg/dL (IQR: 122.5–183.7mg/dL), and after 3 months of treatment was 53mg/dL (IQR: 34.0–95.5mg/dL), showing a median LDL-C decrease of 99.1mg/dL (IQR 135.1–60.0mg/dL), i.e., an average reduction of 63.9%. The initial median LDL-C decrease was followed by reductions between 91.5mg/dL and 98.0mg/dL until the end of the 12-month follow-up; which is consistent with the data reported by Koren et al. in the OSLER-a trial, where the mean baseline LDL-C decreased from 140mg/dL to 61mg/dL on treatment in the first year of treatment.10
Data on Colombian patients in the present study are also highly consistent with those in other real-world studies. The RETOSS-CARDIO study, a multicenter, retrospective, and observational study of patients starting treatment with evolocumab in clinical practice in Spanish cardiology units included 186 patients across 31 cardiology units. The RETOSS-CARDIO study showed an LDL-C reduction of 57.6%, with a mean LDL-C value of 62.2mg/dL (95% CI: 50.2–74.3) at 12 weeks, similar to the one reported in our study with a mean LDL-C value of 69.6mg/dL (SD 57.3mg/dL; median 53.0mg/dL; IQR 34–95.5mg/dL) within the first 3 months of treatment.11
Recently, results of the ZERBINI study in the Canadian population were published.12 This study included 131 patients from 15 sites across Canada. Results showed that the mean LDL-C after evolocumab treatment decreased by 58.7% from baseline. Most patients (93.1%) did not experience an adverse event; effectiveness and safety results are highly consistent with our ZERBINI study findings in the Colombian population.
In addition to the consistency of the published results, our study explored populations not always represented in experimental studies. Our study had a majority of female participants, whose results showed a higher dropout rate, represented by a lower persistence to treatment; likewise, the female population showed higher LDL-C values at baseline; despite this, the treatment achieved a reduction after the fourth months of treatment that was maintained until the end of follow-up. The reasons for lower treatment persistence in the female population were not associated with statin tolerance. Although the present study covers some barriers identified in the literature regarding the lack of research on lipid management in the female population,10,13 future research could explore additional reasons leading to higher treatment dropout according to sex.
Likewise, our study showed favorable results in populations less well represented in experimental studies, such as adults older than 75 years or patients with a history of heart failure (e.g., the Fourier study excluded patients based on a low ventricular ejection fraction percentage6). For patients with congestive heart failure history, our results showed LDL-C levels below 55mg/dL obtained in the first three months and at the end of the one-year follow-up; similarly, patients with 75 years old or older managed to lower their levels below the target of 55mg/dL, maintaining it during the one-year follow-up.
These results provide evidence that even in populations not represented in experimental studies,14,15 with clinical or sociodemographic features associated with cardiovascular risk,14,16 evolocumab showed consistent effectiveness in reducing LDL-C levels from the first months of treatment.
Another important finding was the high number of patients who discontinued treatment. Although our cohort of patients reported high persistence to treatment (72%), 26.1% of patients discontinued evolocumab, a significantly higher percentage than the one reported in clinical extension studies where the annualized discontinuation rate at 1 year was 6.7% (95% CI: 6.4–7.4%).17,10 This difference can be explained by the high number of patients who discontinue treatment for administrative reasons, reimbursement conditions for evolocumab for the study period (including the need for special authorization for specific health technology prescriptions – MIPRES), problems with the healthcare system provider, or the decision of the treating physicians. These reasons associated with treatment discontinuation suggest the need for an awareness strategy to reduce the barriers that might affect the proven clinical benefit of using evolocumab to treat patients at a high risk of CVD. Additionally, external events such as the COVID-19 pandemic could have affected the continuity of treatment since some patients were admitted prior to the quarantine orders, and their follow-up could have been affected. However, data on adherence was not collected in the present study, limiting the possible associations between measurement as persistence and compliance to treatment.
In addition, real-world studies have limitations related to their design. Studies based on clinical practice information are limited to the quality of the report in the electronic medical records, which could lead to measurement errors18 or lack of evaluated or reported data (e.g. one FH subtype not reported, no collection on smoking). In this regard, the guidelines for the management of the disease may influence the data reported; at the time of the study, the available Clinical Practice Guide of the Colombian Ministry of Health for the Management of Dyslipidemias published in 2014 did not include the recommendation to measure lipoprotein a “Lp(a)”, which is why this value was not included in the study.19 The Lp(a) test has been available since 2022 in the benefit plan of the Colombian Ministry of Health.
Within our cohort, a high number of patients with intolerance to statins (34%) was identified, a percentage substantially higher than that reported in other studies,10,20 limiting the generalization of the results due to possible differences in the studied and target population. An explication for this measure could be the sample size or the absence of a standardized criterion to determine statin intolerance.
An additional limitation could be related to the possibility that some patients could have received evolocumab up to six months before enrollment in the study; the previous treatment experience may have increased the likelihood of persistence during the study.
Finally, considering that the frequency of cholesterol level measurements and the permanence in the study during the entire follow-up period depends mainly on variables not controlled in observational studies (e.g., changes in the treatment protocol or extensive periods between laboratory tests), this study has a moderate amount of missing data, especially for LDL-C levels in each of the time windows, as well as baseline levels for other cholesterol measures. Future studies of real-world evidence require greater standardization for the number of cholesterol measurements according to clinical criteria and according to the requirements of the study so that there is a more significant number of patients with available data that allows greater robustness in the findings.
Two sensitivity analyses using different values of LDL-C in cases where multiple LDL-C values existed in the same observational period were conducted to minimize the impact of differential measurements among patients and data lost. The findings of these analyses were consistent with those of the primary analysis; however, the limitations mentioned previously should be considered while interpreting the results.
ConclusionsOur results are consistent with those reported in other real-world studies and long-term clinical trials showing significant effectiveness for LDL-C-lowering, tolerance, and safety of evolocumab.
In particular, the benefits of treatment with evolocumab were maintained in subgroups such as those over 75 years of age, patients with a history of heart failure, or the female patients, even though the latter presented a lower rate of persistence to treatment. Similarly, these results reflect the potential impact of biological therapies in real-world settings in patients with high and very-high risk of CVDs.
FundingThis publication was funded by Amgen Inc. The funding source was involved in the study design. Collection, analysis and interpretation of data were conducted by a third-party company.
Conflict of interestH.M.R., J.R.L.-P., A.A.G., J.L., C.A.H., J.A.B., E.C. declare that they have no conflict of interest in the conduct of this study. C.L.C. and M.P.-P. are employees of Amgen Inc. J.A.B. and C.P.P. were employees of Amgen Biotecnológica SAS at the time of development of this publication. Data collection, analysis and interpretation were performed by IQVIA, a third party not affiliated with the funding company.
No honoraria or payments were made for authorship.
The authors thank the institutions Fundación Clínica Shaio, IPS Universitaria, Hospital Pablo Tobón Uribe, Fundación Valle del Lili, Angiografía de occidente, Clínica el Country, Hospital Universitario San Ignacio, Clínicos IPS, and Cardiocolombia for their participation in the study.
The authors thank IQVIA for their writing and editing support.






![LDL-C levels at baseline and over time. Dotted lines represent 1.4mmol/L [55mg/dL] and 1.8mmol/L [70mg/dL] levels for LDL-C goals, according to guidelines for the management of dyslipidemias.7,8 Error bars represent the interquartile range for the LDL-C median value. LDL-C, low-density lipoprotein cholesterol. LDL-C levels at baseline and over time. Dotted lines represent 1.4mmol/L [55mg/dL] and 1.8mmol/L [70mg/dL] levels for LDL-C goals, according to guidelines for the management of dyslipidemias.7,8 Error bars represent the interquartile range for the LDL-C median value. LDL-C, low-density lipoprotein cholesterol.](https://static.elsevier.es/multimedia/02149168/0000003600000001/v1_202402130516/S0214916823000517/v1_202402130516/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)