Hepatic steatosis is a public health problem with increased incidence and prevalence
ObjectiveTo determine whether the liver steatosis, as measured by the Fatty Liver Index (FLI), is related to metabolic risk and vascular factors and, if so, to identify the clinical-metabolic factor that explains the higher vascular risk.
MethodsCross-sectional study including a sample of 531 men who came to the University of Navarra Clinic Check-up Unit. The degree of steatosis was determined by the FLI. The metabolic risk was assessed using a scale based on determinations of HDL, LDL, triglycerides, blood glucose, HOMA-IR, neutrophil/lymphocyte index, and systolic blood pressure. The vascular risk was assessed by the presence of carotid and/or femoral atheromatous plaques. The dose-response association between FLI and both risks was analysed using non-parametric models (splines) and logistic regression.
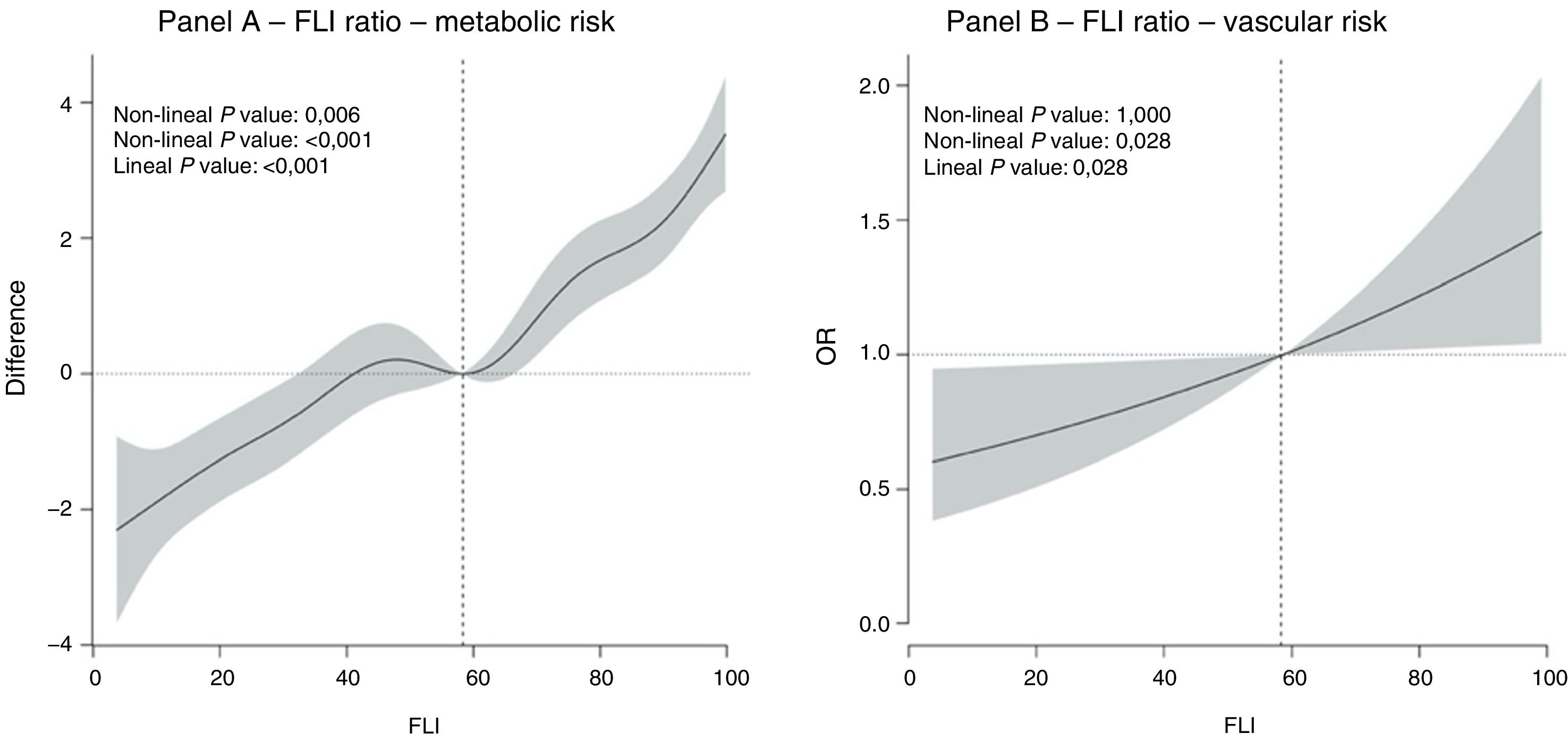
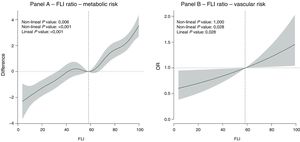
ResultsThe sample studied had a mean age of 52.70 years, with 49.3% having an FLI ≥ 60, as well as 33.6% with metabolic syndrome, and 43.9% with carotid and/or femoral atheromatous plaques. The relationship between FLI and metabolic risk and vascular was linear (Metabolic: non-linear P = .097; linear P < .001; Vascular: non-linear P = 1.000; linear P = .028). For every 10 units of increase in FLI, the odds of presenting with atheroma plaques increased by 9.7% (OR=1.097; 95% confidence interval 1.010–1.191). When adjusting for triglyceridaemia, the association disappeared (OR=1.001).
ConclusionsPatients with fatty liver disease had an increased metabolic and vascular risk. The increased vascular risk is associated with the triglyceride level. On a clinical level, this study suggests that these patients could benefit from treatment of hypertriglyceridaemia.
La esteatosis hepática es un problema de salud pública de incidencia y prevalencia crecientes en nuestra sociedad.
ObjetivoDeterminar si la esteatosis hepática, medida mediante el Fatty Liver Index (FLI), se relaciona con el riesgo metabólico y vascular y, de ser así, identificar qué factor clínico-metabólico explica el mayor riesgo vascular de estos pacientes.
MétodosEstudio transversal que incluye una muestra de 531 varones que acudieron a la Unidad de Chequeos de la Clínica Universitaria de Navarra. Se determinó el grado de esteatosis mediante el FLI. El riesgo metabólico fue evaluado mediante una escala basada en determinaciones de HDL, LDL, triglicéridos, glucemia, HOMA-IR, índice neutrófilo/linfocitario y presión sistólica; el riesgo vascular mediante la presencia de placas ateromatosas en carótidas y/o femorales. La asociación dosis respuesta entre el FLI y ambos riesgos se analizó mediante modelos no paramétricos (splines) y regresión logística.
ResultadosLa muestra estudiada presenta una edad media de 52,70 años, con un 49,3% de ellos presentando un FLI ≥ 60, 33,6% con síndrome metabólico y 43,9% con placas ateromatosas en carótidas o femorales. La relación entre FLI y el riesgo metabólico y vascular fue lineal (Metabólico: valor-p no lineal = 0,097; valor-p lineal <0.001; Vascular: valor-p no lineal = 1,000; valor-p lineal = 0,028). Por cada 10 unidades de incremento de FLI la odds de presentar placas de ateroma aumentaba en un 9,7% (OR = 1,097; Intervalo de confianza 95%: 1,010-1,191). Al ajustar por trigliceridemia la asociación desaparecía (OR = 1,001).
ConclusionesLos pacientes con esteatosis hepática presentan un mayor riesgo metabólico y vascular. El mayor riesgo vascular está asociado con el nivel de triglicéridos. A nivel clínico, este estudio sugiere que estos pacientes podrían beneficiarse del tratamiento de la hipertrigliceridemia.
Non-alcoholic fatty liver disease (NAFLD) is the first cause of chronic hepatic disease worldwide, and it is characterised by the hepatocytic infiltration of triglycerides and free fatty acids. The prevalence of NAFLD in the general population, at world level as well as in Spain, stands at 25%.1
In clinical practice several models combine multiple clinical and biochemical parameters which make it possible to determine the diagnosis of NAFLD quickly and effectively. One hypothesis is that NAFLD is a hepatic manifestation of metabolic syndrome, and cardiovascular disease (CVD) is one of the main causes of death.2 Several studies suggest that NAFLD is an independent risk factor for type 2 diabetes mellitus (DM2), CVD and chronic kidney disease (CKD).2 A meta-analysis by Wu et al.3 concludes that NAFLD is associated with a higher risk of total mortality, with a hazard ratio (HR) of 1.27 (confidence interval (CI) 95%: 1.03–1.57). When cardiovascular mortality is analysed this too was found to increase, although it does not attain statistical significance (HR = 1.70; CI 95%: 0.97–2.99). Similarly, the meta-analysis by Targher et al.4 concluded that NAFLD is significantly associated with an increase in the risk of mortal and non-mortal cardiovascular events. They also state that the most severe forms of NAFLD have a higher risk of mortality due to CVD. In spite of this, they consider that the underlying physiopathological mechanism of this association has yet to be clearly defined because of the many metabolic processes that are common to CVD and NAFLD. Some authors have suggested that this association is mediated by insulin resistance, which is hypothesised to be one of the causes of the metabolic changes that occur in the liver, giving rise to hyperglycaemia, hypertriglyceridaemia and an altered lipid profile.5 In a similar way, NAFLD has been found to be more prevalent in obese individuals, and the development of insulin resistance is one of the factors that favours this association.5
The aim of this study was to determine the metabolic and vascular risk associated with NAFLD and, if an increase in vascular risk were observed, to identify which clinical – metabolic factor explains this association.
MethodsDesignThis transversal study was designed with a sample of patients who had visited the check-up unit of the Clínica Universitaria de Navarra to evaluate their state of health in general and estimate their vascular risk. It included 531 men after excluding the patients with excessive alcohol consumption (≥ 30 g per day), viral hepatitis or other hepatic pathologies. The study obeyed the Helsinki Declaration and all of the participants gave their informed consent.
Evaluation of hepatic steatosisThe presence of hepatic steatosis was determined by the Fatty Liver Index (FLI; equation 1).6 This is one of the most widely used algorithms in clinical practice, and it has been validated in different populations as a method of diagnosing hepatic steatosis detected by ultrasound scan.7
Equation 1:
The FLI value allows us to classify individuals as those who have hepatic steatosis (≥ 60), who do not have steatosis (< 30), or who are in an intermediate situation (30–59).
Evaluation of metabolic riskMetabolic risk was determined by a score that included 7 metabolic parameters. The values of each parameter were categorised to award each subject 0, 1 or 2 points, establishing a range of metabolic risk from 0 to 14 points. The parameters studied and their cut-off points were:: LDL (< 116 mg/dl = 0; 116−159 mg/dl = 1; ≥ 160 mg/dl = 2); HDL (> 50 mg/dl = 0; 35−49 mg/dl = 1; < 35 mg/dl = 2); triglycerides (< 150 mg/dl = 0; 150−199 mg/dl = 1; ≥ 200 mg/dl = 2); glycaemia (< 100 mg/dl = 0; 100−125 mg/dl = 1; ≥ 126 mg/dl = 2); HOMA-IR (< 3,7 = 0; ≥ 3,7 = 2); systolic arterial pressure (< 120 mmHg = 0; 120–139 mmHg = 1; ≥ 140 mmHg = 2), and neutrophil-lymphocyte ratio (< 1.5 = 0; 1.5–2.99 = 1; ≥ 3 = 2).
The different cut-off points were set according to the European cardiovascular prevention guides.8 The cut-off point for HOMA-IR and the neutrophil-lymphocyte ratio was determined according to clinical criteria or those used previously in the literature.
A group of trained nurses carried out the physical examination following a standard protocol. Weight and height were measured with the patient shoeless and not fully clothed. Arterial pressure was taken after 5 min. resting using a calibrated sphygmomanometer, taking the average of 3 determinations. Abdominal circumference was measured at the mid-point between the iliac crest and the last rib, after breathing out normally.
Vascular risk evaluationVascular risk was determined by the presence of atheromatous plaques in the femoral and/or carotid arteries, detected using ultrasound scan imaging techniques. Carotid atheromas were determined by bilateral ultrasound study of the common carotid territories, carotid bulb and internal carotid. Femoral atheromas were determined by bilateral ultrasound scan of 20 mm segment proximal to the femoral bifurcation. An atheromatous plaque was defined as a structure invading the arterial span > 0.5 mm, with a > 50% increase in the thickness of the intima-media (IMG) respecting the adjacent wall or with an IMG > 1.5 mm.9
Statistical analysisContinuous variables are shown as averages with standard deviation if they follow a normal distribution, or as a median and interquartile range if they do not follow a normal distribution. Categorical variables are shown as frequency and percentage. Comparison of continuous variables between groups was studied using variance analysis if they followed a normal distribution (ANOVA), and by Kruskall-Wallis if they did not. Chi-squared analysis was used to compare categorical variables between groups. The lineal and non-lineal relationship between FLI and metabolic and vascular risk was determined using generalised additive models [GAM] and smooth age-adjusted splines were obtained. To analyse which metabolic or clinical factor explained the higher vascular risk associated with FLI several logistic regression models were defined, in which the presence of atheromatous plaque was the dependent variable, and the independent variables were the different relevant clinical-metabolic factors. Variables that did not follow a normal distribution were transformed logarithmically to introduce them into a logistic regression model (serum triglycerides and albumin coefficient /urine creatinine). The logistic regression model was used to study the vascular risk associated with an increase of 10 units of the FLI. A Spearman correlation study was performed between the variables of FLI, serum triglycerides, GGT, BMI, abdominal circumference and HOMA-IR to study their collinearity. A P value <.05 was considered to be statistically significant. Version 23 of the SPSS program was used for statistical analysis, together with R Core Team (2013). R: A language and environment for Statistical Computing, Vienna, Austria.
ResultsThe sample studied consisted of 531 men with an average age of 52.70 years; 49.3% of them had a FLI ≥ 60, 31.6% had a FLI of 30–59 and 19% had a FLI < 30. 33.6% of these subjects had metabolic syndrome, 43.9% had atheromatous plaques in the carotid and/or femoral arteries; more specifically, we found 15.1% had carotid plaques and 40.8% had femoral plaques. 32% of our sample were smokers, 50.9% were hypertensive, 27.8% had hypertriglyceridaemia > 150 and with an average coronary risk at 10 years of 4.98%, calculating using the REGCIOR risk function.
The participants’ characteristics grouped according to FLI value are shown in Appendix A, supplementary tables 1 and 2.
Fig. 1 shows the dose-response relationship between the FLI value and the risk of metabolic and vascular risk. A predominantly lineal relationship was found between the FLI and metabolic risk (non-lineal P value: .006; lineal P value: < .001) (Fig. 1A) and the relationship between the FLI and vascular risk was completely lineal (non-lineal P value: 1.000; lineal P value: .028) (Fig. 1B).
The age-adjusted logistic regression model shows that for each 10 unit increase in the FLI the odds of having atheromatous plaques in the carotid and/or femoral arteries increases by 9.0% (OR = 1.09; CI 95%: 1.01−1.18) (Table 1, model 1). Table 1 also shows the results of the different logistic regression models adjusted for the relevant clinical-metabolic factors. It can be seen that the most decisive parameter for increased vascular risk in these patients is the level of triglycerides in serum (Table 1, model 7), as this reduces the regression coefficient from 0.09 to 0.00. This fall is even greater than the one observed when coronary risk at 10 years is included, estimated as a REGCIOR risk function (Table 1, model 2).
Logistic regression models that analyse the relationship between the Fatty Liver Index (FLI) and vascular risk (the presence of atheromatous plaques in the carotid and/or femoral arteries), adjusted for different clinical and metabolic parameters.
| Coefficient B | Standard deviation | OR | CI 95% | P | |
|---|---|---|---|---|---|
| Model 1 | 0.09 | 0.04 | 1.09 | 1.01−1.18 | 0.028 |
| Model 2 | 0.04 | 0.05 | 1.04 | 0.99−1.13 | 0.427 |
| Model 3 | 0.09 | 0.05 | 1.09 | 0.99−1.18 | 0.078 |
| Model 4 | 0.24 | 0.07 | 1.28 | 1.11−1.47 | 0.001 |
| Model 5 | 0.21 | 0.07 | 1.23 | 1.07−1.42 | 0.004 |
| Model 6 | 0.05 | 0.05 | 1.05 | 0.96−1.16 | 0.721 |
| Model 7 | 0.00 | 0.06 | 1.00 | 0.90−1.11 | 0.985 |
Model 1: age; Model 2: age + REGCIOR risk function; Model 3: age + HOMA-IR; Model 4: age + abdominal circumference; Model 5: age + BMI; Model 6: age + GGT; Model 7: age + ln(TG).
When the relationship between FLI and atheromatous plaques in the carotid and femoral arteries is studied separately (Appendix A, supplementary tables 3 and 4) a stronger association is found with femoral plaques, and triglycerides are still the metabolic factor that explains the greater part of this relationship. Supplementary table 5 in Appendix A shows the results of the correlation between the different variables introduced in the logistic regression, to make it possible to study the collinearity between them.
DiscussionIn this transversal study we found that the degree of hepatic steatosis determined by the FLI is associated with greater metabolic as well as vascular risk. The higher vascular risk of hepatic steatosis is associated with hypertriglyceridaemia.
These results are consistent with recent publications on this subject. To focus on metabolic risk, transversal studies have suggested that there is a hepatic fat threshold (6%) over which metabolic changes occur, such as insulin-resistance in the muscles, hypertriglyceridaemia and low levels of HDL.10 On the other hand, study of a cohort of 1205 patients showed that the FLI is associated with a higher risk of a CVD event, independently of metabolic, anthropometric, lifestyle and pro-inflammatory risk factors.11 A recent meta-analysis concluded that NAFLD is an independent predictive factor for mortal and non-mortal cardiovascular events and the presence of metabolic syndrome, and that the severity of NAFLD is probably related to a higher risk of incidence.4
In our study, the higher level of vascular risk found in these patients was mediated by hypertriglyceridaemia. This difference may be explained because in our analysis we adjusted for levels of triglycerides in serum as a continuous variable, while in other previous cohort studies either did not include this variable or included it within the definition of metabolic syndrome, as a categorical variable.
The most important limitation of this study is its transversal design, as this prevents establishing any causal relationship between hepatic steatosis and greater metabolic and vascular risk. On the other hand, the mediating role of triglyceride levels and the risk of atheromatous plaque in this population may also be questioned due to the absence of a temporal sequence. Nevertheless, the results are consistent with those observed in Mendelian randomised studies. On the other hand, Mendelian randomised studies that analysed the causal relationship between NAFLD and coronary disease are controversial:12 some studies have observed that genetic variants associated with NAFLD are also associated with a lower risk of coronary disease (in PNPLA3, TM6SF2, PEMT, MTTP), and others show that different variants (in GCKR, MBOAT7, LYPLAL1, PPP1R3B, TRIB1, FADS1-2-3, ERLIN1-CHUK-CWF19L1, ADIPOQ) are associated with greater risk of coronary disease. This discordance seems to be associated with the different effect of these variants on the lipid profile, triglycerides and LDL cholesterol, suggesting the existence of a pleiotropy that limits the conclusions and interpretability of the results of Mendelian randomisation. In any case, these studies indicate that triglycerides are a possible mediating mechanism in the association between NAFLD and coronary risk. On the other hand, Mendelian randomisation studies support the causal relationship between level of triglycerides in serum and cardiovascular risk in the general population, independently of LDL and HDL levels.13 As a whole, these results support the hypothesis that, effectively, triglycerides are a decisive factor in the cardiovascular risk of patients with hepatic steatosis. Insulin resistance and high levels of glycaemia have traditionally been considered to play an important mediating role in the vascular risk of these patients.5 In our study these variables do not explain the association between hepatic steatosis and vascular risk. In a similar way, observational studies such as the one by Jin et al.14 suggest that hepatic fat content, independently of insulin resistance, plays a decisive role in the vascular risk of these patients by altering the lipid profile.
One of the limitations of this study is that a scale that has not been validated for the Spanish population was used to evaluate metabolic risk. However, cut-off points proposed in clinical practice guides or ones used in normal clinical practice have been used. Another limitation is that in our study hepatic steatosis was measured indirectly using the FLI. Although this index is widely used in clinical practice and its correlation with hepatic steatosis has been found to be high by ultrasound scan imaging, it is weaker when compared with hepatic steatosis quantification by means of hepatic biopsy.15 Likewise, the fact that only men were studied prevents knowledge of whether a similar relationship also exists in women, or whether there is any difference. Finally, this study also has the limitation of having studied vascular risk in the form of atheromatous plaques and not clinical cardiovascular events.
ConclusionTo conclude, our study shows that the presence of NAFLD is associated with higher metabolic and vascular risk, both of which have a lineal relationship with hepatic steatosis. Our results also suggest that the factor which could explain this higher vascular risk is the level of triglycerides in serum. These results are based on a transversal study, so that we are unable to infer causality, although at a clinical level this seems to suggest that patients with NAFLD would be good candidates for treatment of hypertriglyceridaemia to reduce their vascular risk. Further studies would be required, firstly to confirm the conclusion of this work and subsequently to examine if treating hypertriglyceridaemia is beneficial for this group of patients.
FinancingThis research received no specific aid from private, public or commercial sector agencies, or from not-for-profit bodies.
AuthorsManuscript concept and design: Iker Elosua-Bayés; Óscar Beloqui Ruiz.
Data gathering: Iker Elosua-Bayés; Óscar Beloqui Ruiz.
Data analysis and interpretation: Iker Elosua-Bayés; Óscar Beloqui Ruiz.
Writing, revising and approving the sent manuscript: Iker Elosua-Bayés; Óscar Beloqui Ruiz.
Conflict of interestThe authors have no conflict of interests to declare.
Please cite this article as: Elosua-Bayés I, Ruiz ÓB. Asociación entre hígado graso no alcohólico, riesgo metabólico y vascular. Clin Investig Arterioscler. 2020;32:200–205.