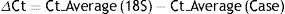NRP1 inflammasome is crucial in endothelial dysfunction. Platelets are mandatory for the inflammation that precedes it. Aspirin could inhibit NLRP1 inflammasome in endothelial cells, and clopidogrel could also provoke a reduction in vascular inflammation.
A study was carried out on the influence of platelet inflammatory inhibition by P2Y receptor inhibition versus COX enzyme inhibition on the transcription of NLRP1 inflammasome in endothelial cells.
MethodsAn open-label, prospective, randomised crossover study with two periods of platelet inhibition enrolled 20 healthy volunteers. They received clopidogrel 75mg/day/7days and aspirin 100mg/day/7days. A venous blood sample was collected from all participants before and after this period. Human aortic endothelial cells (HAECs) were exposed for 2h in cultures. NLRP1 gene expression was then analysed in these cultures.
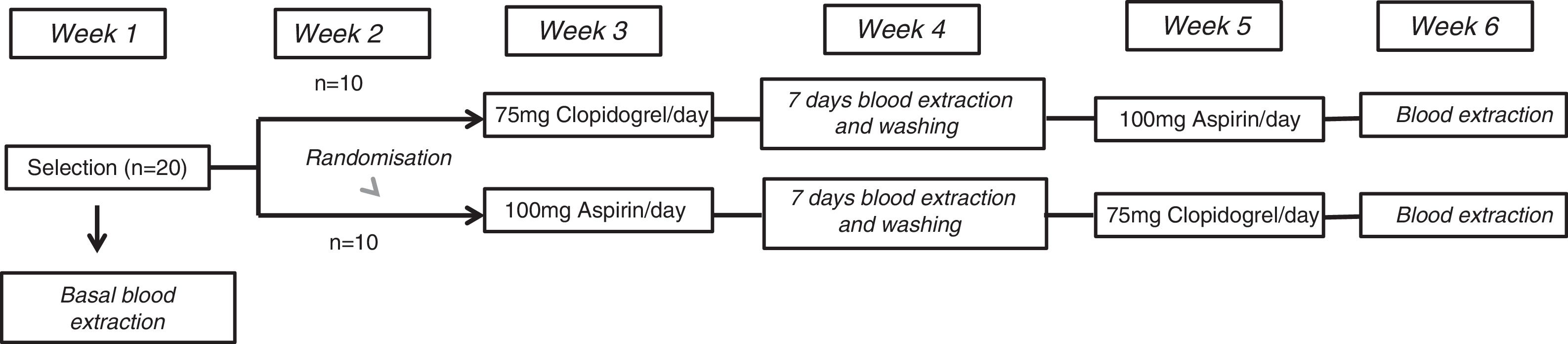
ResultsHAEC cultures that were exposed to baseline plasma showed higher expression of NLRP1 than HAECs exposed to plasma after one week of aspirin or clopidogrel intake [relative quantification (RQ), 1.077±0.05 vs. 1.002±0.06; OR, 1.8; 95% CI, 1.1-2.9; P<.01 and 1.077±0.05 vs. 1.04±0.03; OR, 1.7; 95% CI, 1.2-2.6; P<.001, respectively]. NLRP1 expression in HAEC cultures exposed to plasma after one week of aspirin or clopidogrel was similar to that observed in control HAECs that was no exposed to human plasma (PBS) [RQ; 1.002±0.06 vs. 1.009±0.03; OR, 0.9; 95% CI, 0.5-1.4; P=.7, and 1.04±0.03 vs. 1.009±0.03; OR, 0.8; 95% CI, 0.3-1.2; P=.5, respectively]. No difference was observed in NLRP1 percentage reduction in HAEC after aspirin or clopidogrel exposure (3.8% vs. 2.8%, P=.3, respectively).
ConclusionsPlatelet inhibition by P2Y pathway is similar to COX pathway in NLRP1 expression inhibition in HAECs.
Se ha demostrado que el inflamasoma NLRP1 es clave en la disfunción endotelial, estando las plaquetas implicadas en las reacciones inflamatorias que la desencadenan.
Investigamos la inhibición in vivo de la inflamación plaquetario-dependiente mediante inhibición del receptor P2Y vía ADP comparada con la de la enzima COX sobre la transcripción del NLRP1 en las células endoteliales.
MétodosEstudio prospectivo, aleatorizado, abierto y cruzado con 2 periodos de inhibición plaquetaria en 20 voluntarios sanos, administrando clopidogrel 75mg/día/7días y aspirina 100mg/día/7días de forma cruzada tras un periodo de lavado de una semana.
Las células endoteliales aórticas humanas (HAEC) fueron estimuladas 2h con plasma obtenido de los pacientes antes y después de la inhibición plaquetaria. La cuantificación de la expresión de NLRP1 se determinó mediante análisis qRT PCR.
ResultadosLas HAEC expuestas a plasma basal de individuos sanos presentaron niveles más elevados del NLRP1 que las expuestas a plasma de los participantes tras la administración de aspirina o clopidogrel [cuantificación relativa (CR), 1,077±0,05 vs. 1,002±0,06; OR, 1,8; IC95, 1,1-2,9; p<0,01 y 1,077±0,05 vs. 1,04±0,03; OR, 1,7; IC95, 1,2-2,6; p<0,001, respectivamente]. La expresión del NLRP1 en HAEC expuestas a plasma de los participantes tras la administración de aspirina o clopidogrel fue similar a las HAEC sin exposición a plasma humano (PBS) [CR 1,002±0,06 vs. 1,009±0,03; OR, 0,9; IC95, 0,5-1,4; p=0,7 y 1,04±0,03 vs. 1,009±0,03; OR, 0,8; IC95, 0,3-1,2; p=0,5, respectivamente].
No hubo diferencias en el porcentaje de reducción del NLRP1 en las HAEC expuestas al plasma tras la toma de aspirina comparado con la provocada por el plasma de estos mismos sujetos tras clopidogrel (3,8% vs. 2,8%, p=0,3, respectivamente).
ConclusionesLa inhibición plaquetaria por vías P2Y y COX provoca similar efecto en la inhibición del inflamasoma proaterogénico NLRP1 en las HAEC.
Atherosclerosis is currently considered to be a systemic inflammatory disease that starts due to an immune-inflammatory process which leads to an endothelial dysfunction.1 This endothelial dysfunction is an early atherosclerosis marker which emerges even before it is possible to detect atheromatous plaques using diagnostic imaging techniques.
There is abundant evidence for the importance of the innate as well as the adaptive immune response in the atherosclerotic process.2 In fact, the activation of T lymphocytes is essential in the progression of atherosclerosis.3 Several autoantigens have been located in the atheromatous plaque that are able to trigger an immune response through T lymphocyte activation and the generation of autoantibodies.4 All of these mechanisms are essential in the development of the endothelial dysfunction that causes the origin of atherosclerosis and the formation of the atheromatous plaque.5
Moreover, this endothelial dysfunction reduces the bioavailability of prostaglandins and nitric oxide, thereby causing platelet activation.6 Platelets are small anucleate cells produced in the bone marrow, and they have important functions at a vascular level. Over and above their specific haemostatic functions, there is evidence that indicates platelets play an important role in the inflammatory reactions that occur in the vascular wall. Platelet adhesion causes an inflammation and endothelial dysfunction which precede leukocyte adhesion. The activation of platelet adhesion is also fundamental for leukocyte recruitment to be triggered in the arterial wall during the initial stages of atherosclerosis:7
Inflammasomes have recently been said to be a key piece in the regulation of the immune and inflammatory response in the aetiopathogenesis of atherosclerosis.8 Inflammasomes are responsible for processing pro-interleukin beta (pro-IL-β) into its active form and the subsequent caspase-1 secretion and activation. 9 IL-1-β is a powerful proinflammatory cytokine that is crucial in the proatherogenic effect of vascular disease.
Inflammasome NLRP1 is able to trigger caspase-1 activation, causing an innate and inflammatory immune response.10 Although the exact mechanisms which trigger activation of this inflammasome have yet to be completely elucidated, recent studies have supplied information on the role that inflammasome NLRP1 plays in endothelial dysfunction.11 Patients with peripheral arterial disease who were treated with aspirin had less expression of inflammasome NLRP1 in the endothelial cells, and this reduction in expression was possibly caused by an inhibition in the kappa B nuclear factor pathway.12,13 A subsequent study undertaken in healthy subjects who received this drug added to knowledge of the role played by aspirin in the inhibition of inflammasome NLRP1 expression in endothelial cells. Data from this study indicated that in endothelial cells inflammasome NLRP1 intracytosolic expression is attenuated by the inhibitory auto/paracrine action of aspirin on platelets, without any direct interaction between the platelet and the endothelial cell. This modifies the immune-inflammatory process that triggers the inflammatory mechanisms which cause atherosclerosis.14
Likewise, recent studies suggest that clopidogrel, a P2V receptor inhibitor that block platelet function through adenosine diphosphate (ADP) may reduce vascular inflammation secondarily to the paracrine action of platelet activation inhibition.15 Nevertheless, to date the action that treatment with clopidogrel may exert over inflammasome NLRP1 expression on endothelial cells is unknown.
This study therefore studies the effect of invivo inhibition of platelet-dependent inflammation by inhibition of the P2Y receptor via ADP compared with inhibition of the COX enzyme over intracytosolic transcription of the NLRP1 inflammasome in endothelial cells.
MethodsStudy designThe ECLOAS study is prospective, randomised, open and crossed, with 2 periods of platelet inhibition in 20 healthy volunteers from the Universidad Europea, Madrid. The study participants had no relevant medical or surgical history or any associated cardiovascular risk factors, with vascular examination results within normal limits. None of the participants had any concomitant indication for pharmacological treatment, and smoking or substance abuse were exclusion criteria. The average age of the volunteers in the study was 21±1years. 60% (n=12) of them were women. The average sample body mass index was 24±3. The study was completed after concluding a 6 week protocol. All of the participants were assigned to one of 2 arms in the study under 2 experimental conditions. These conditions consisted of receiving a daily dose of 75mg clopidogrel during 7days and 100mg aspirin crossed, after a washout period of one week. The study design is shown in Figure 1.
Venous blood was extracted from each participant basally and after finishing each one of the weeks of administering the corresponding drug. Blood samples were centrifuged at 1,500rpm during 10min., and the resulting plasma was frozen at −20°C until it was analysed.
The study was undertaken in Getafe Hospital Universitario. All of the participants in the study signed an informed consent document, and they were able to abandon the study whenever they wished. The study complied with the ethical requisites established in the Helsinki Declaration, and it was approved by the Hospital Ethics Committee.
The fourth week of the protocol, in which the participants received neither of the drugs, was used as a washout period between the drugs, to eliminate any effect of the previous medication administered. At the end of each week of drug administration, the participants were evaluated in a medical visit to assess any possible adverse effects which may have arisen.
Selection visit and randomisationThe selection visit took place during the first week of the protocol, and 20 healthy volunteers were recruited who fulfilled the inclusion-exclusion criteria described above. They signed the informed consent document in this first visit and the first basal blood extraction from each participant took place, before they took the antiaggregant drugs to be tested.
The patients were randomised using a digital application.16 The researchers who recruited and randomised the volunteers were not involved in either data gathering or the subsequent data analysis.
Preparation of cell culturesHuman aorta endothelial cells (HAEC) (Lonza, Workingham, United Kingdom) were cultured at 37̊C on plates with 5% carbon dioxide, in an endothelial cells growth medium composed of 10ng/ml human epidermal growth factor, 1.0mg/ml hydrocortisone, 50mg/ml gentamycin, 50mg/ml amphotericin-B, 3mg/ml bovine brain extract and 5% foetal serum (Clonetics; Lonza).
Assays took place on single layers of HAEC until 70%-90% confluence was attained, using cells harvested from passage 3 to passage 6 for the study. The plasma samples obtained from the healthy volunteers in each phase of the protocol were then unfrozen and added the cell cultures to stimulate them during 2 hrs. Each cell tissue was exposed to a plasma sample from each participant in the study, extracted from each one at the moments previously described in the protocol.
Determination of NLRP1 inflammasomeThe total ribonucleic acid (RNA) total of the sown cultures was obtained using the RNAeasy Fibrous MiniKit commercial kit (Qiagen, Hilden, Germany) following the manufacturer's recommendations. We determined the amount of purified RNA using 260nm spectrometry in an ND-100 Nanodrop analyser (Nanodrop Technologies, Wilmington, DE). The purity of the samples was verified by 260/280nm measurement ratio, with values of from 1.8 to 2.1 indicating that the quality of RNA obtained is optimum for quantitative analysis by real time polymerase chain reaction (qRT PCR).
Quantification of NLRP1 inflammasome transcription was undertaken using qRT PCR (Real Time PCR 7500 Fast. Version 2.0, Applied Biosystems, Carlsbad, CA). For the study, 1μg of all the RNA was transcribed in its complementary deoxyribonucleic acid (cADN) using the High Capacity cADN Reverse Transcription commercial kit (Applied Biosystems, Carlsbad, CA).
Subsequently, in a final volume of 12μl, 30ng of cADN were used as the mould to analyse the PCR in real time of the specific gene for NLRP1 inflammasome and for the RN18S1 constitutive gene using the TaqMan Master Mix Universal Fast (2×) test (Roche Diagnostics, Indianápolis, IN). The steps for gene amplification were as follows: first denaturalisation at 95̊C, followed by 40 further denaturalisation cycles at 95̊C lasting 15 seconds and finally a 1 min. extension at 60̊C.
Relative quantification of NLRP1 inflammasome expression was obtained using the ΔΔCT17 comparative method. To eliminate the possibility of technique variability, each one of the samples was analysed in triplicate. The results were standardised using an endogenic control without reverse transcription to thereby control PCR amplification of contaminant genome DNA. The program calculated the ΔCts and the ΔΔCT using the following formulas:
Level of inflammasome expression=2−DDCt
Statistical analysisThe SPSS 17.0 for Windows (SPSS, Chicago, IL) program was used for statistical analysis. Continuous variable normalcy was analysed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. The distribution of the NLRP1 inflammasome was Gaussian, and its value was expressed as an average ± standard deviation. Comparison of the levels of NLRP1 inflammasome transcription was expressed in odds ratio (OR) form, with a 95% confidence interval (CI 95). P values <.05 were considered to be statistically significant.
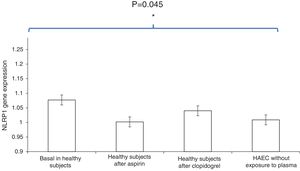
ResultsThe HAEC cell cultures exposed to the basal plasma of the healthy subjects had higher levels of NLRP1 inflammasome expression than those which had been stimulated with healthy subjects’ plasma after one week of aspirin or clopidogrel administration [relative quantification (RQ), 1.077±0.05 vs. 1.002±0.06; OR, 1.8; CI 95, 1.1-2.9; P<.01 and 1.077±0.05 vs. 1.04±0.03; OR, 1.7; CI 95, 1.2-2.6; P<.001, respectively].
Moreover, NLRP1 inflammasome expression in HAEC cultures exposed to healthy subjects’ plasma after one week of antiaggregant treatment with aspirin or clopidogrel was similar to the expression in HAEC without exposure to human plasma (PBS) [RQ, 1.002±0.06 vs. 1.009±0.03; OR, 0.9; CI 95, 0.5-1.4; P=.7 and 1.04±0.03 vs. 1.009±0.03; OR, 0.8; CI 95, 0.3-1.2; P=.5, respectively].
No significant differences were found in the percentages of NLRP1 inflammasome expression reduction in the HAEC cells exposed to the plasma of healthy subjects who had taken aspirin during one week compared with the NLRP1 inflammasome expression reduction caused by the serum of the same subjects after taking clopidogrel (3.8% vs. 2.8%, P=.3, respectively) (Fig. 2).
Comparison of NLRP1 inflammasome expression in HAEC cells exposed to basal plasma from healthy subjects, plasma from healthy subject after one week of aspirin administration, plasma from healthy subjects after one week of clopidogrel administration, and HAEC cultures without exposure to human plasma. Histogram data are expressed as an average ± standard deviation.
Likewise, we found no individual differences between the participants respecting the specific induced response over NLRP1 inflammasome expression in the HAEC cells when they were exposed to their plasma samples after the administration of aspirin and clopidogrel (difference in expression in RQ: 0.031±0.011; CI 95%, −0.005 to 0.041).
DiscussionThe importance of inflammatory mediators in the origin as well as in the progression of atherosclerotic plaques has been widely proven.1,2 In this study we found that HAEC cells exposed to the plasma of healthy volunteers who had been administered antiaggregant drugs during one week (aspirin and clopidogrel) had lower levels of NLRP1 inflammasome activation.
Additionally, NLRP1 inflammasome expression levels by aortic endothelial cells after exposure to the plasma of healthy subjects after receiving either of the 2 antiaggregant drugs during one week was comparable to the levels in HAEC cells prior to exposure to either plasma. This finding is of crucial importance, as it shows that aspirin as well as clopidogrel, by auto/paracrine action on platelets, are able to relieve endothelial vascular damage through inhibition of NLRP1 inflammasome activation in the said endothelium. Both of these antiaggregants therefore directly protect the integrity of the vascular endothelium.
It can be deduced from our results that aspirin as well as clopidogrel is able to produce significant auto/paracrine inhibition of NLRP1 inflammasome expression in the endothelium; the percentage of reduction is similar with both drugs, and so platelet inhibition by the P2Y and COX routes causes a similar effect in inhibiting proatherogenic NLRP1 inflammasome in arterial endothelial cells.
All of these data confirm the results of previous studies about whether the auto/paracine action of platelets is directly involved in modulating the immune-inflammatory process which eventually triggers the inflammatory mechanisms that cause endothelial dysfunction.12,14 It also suggests the existence of a close relationship between aspirin and clopidogrel and their anti-inflammatory effects in the treatment of cardiovascular diseases.
Long-term treatment with aspirin or clopidogrel has often been clinically demonstrated to effectively prevent the appearance of cardiovascular events.18,19 Nevertheless, the exact mechanism by which both antiaggregants are able to inhibit NLRP1 inflammasome expression in endothelial cells is unknown. Nevertheless, aspirin seems to act through the inhibiting action of acetylsalicylic acid on the nuclear kappa B factor, and the inhibition of the reactive oxygen species/thioredoxin protein interaction.13,20
Inflammasome activation contributes to the start of the inflammatory response which triggers the development and progression of atherosclerosis.8–10 This association between inflammation and atherosclerosis has opened up an important line of research into new treatments for the group of diseases included in atherosclerosis.
Recently the CANTOS study demonstrated that the monoclonal antibody canakinumab is able to reduce the incidence of new cardiovascular events in patients with a high inflammatory burden, as this drug is able to bring about a significant reduction in protein C reactive (PCR) levels.21,22 Canakinumab binds with high specific affinity to human IL-β and neutralises its biological effect by blocking interaction with IL-1 receptors.. This makes it possible to prevent activation of the gene induced by IL-β and the production of inflammatory mediators. However, in the CIRT, another immunosuppressor such as methotrexate was unable to reduce levels of IL-β, IL-6 or PCR, and nor did it reduce the incidence of cardiovascular events in the recruited patients.23 A more detailed analysis of the patients included in the latter clinical trial revealed that their PCR levels before the start of treatment were within the normal range, so that the hypothesis is feasible that inhibition of the inflammation would only slow down the atherosclerotic process when a previous pro-inflammatory response had persisted over time beforehand. The studies published earlier also show that methotrexate is able to inhibit the development of the atherosclerotic lesion,24 so that it is possible that the capacity of methotrexate to reduce inflammation depends on the previous inflammatory state.
The importance and novelty of our study lies in the fact that it is, in our knowledge, the first study to evaluate the potential pro-inflammatory role that the auto/paracrine action of platelets plays in NLRP1 inflammasome expression in endothelial cells. It does so by evaluating the action of 2 drugs with an inhibiting effect on the inflammatory platelet response, while also comparing them in terms of a possible difference in their inhibiting power. We confirmed the involvement of inflammasomes in endothelial dysfunction.11,20,25 Assuming the importance of NLRP1 inflammasome in the endothelial dysfunction that triggers atherosclerosis, it is necessary to consider that we have here a new therapeutic target on which to centre when designing future treatment strategies for cardiovascular diseases.
Our study has several limitations. Firstly, the design of this study does not make it possible to elucidate the exact mechanisms by which clopidogrel and aspirin are able to promote the inhibition of platelet auto/paracrine action, thereby inhibiting NLRP1 inflammasome expression in endothelial cells. Nor does it evaluate the effect of these drugs on the secretion of IL-β and the subsequent caspase-1 activation through this. New basic research studies are required to clarify these mechanisms of action, as well as the biological effects of the same. Another limitation of this study is the small size of the sample, which restricts its statistical power. Nevertheless, the data from this crossed experimental design study of cellular tissues are sufficiently reliable to permit drawing exact conclusions. Lastly, we determined the RNA messenger expression of NLRP1 inflammasome by quantitative analysis with the polymerase chain reaction, without determining gene protein activity. Determining NLRP1 protein expression levels using immunohistochemical techniques or Western blot could offer additional information, although extracting these intracytosolic molecular complexes and performing a quantitative analysis of enzyme activity is not very efficient. In fact, evaluating NLRP1 inflammasome RNA messenger expression suing the technique employed in this study is a reliable means of quantifying NLRP1 production in cellular tissues in this particular study.
ConclusionsLow concentrations of aspirin and clopidogrel protect endothelial function thanks to the inhibition they produce on NLRP1 inflammasome activation. Platelet inhibition by the P2Y and COX routes causes a similar effect in the inhibition of proatherogenic NLRP1 inflammasome in arterial endothelial cells. Data from this study show that in HAEC cells the intracytosolic expression of NLRP1 inflammasome is attenuated by the inhibitory action of platelet auto/paracrine activity of aspirin and clopidogrel, thereby modulating the immuno-inflammatory process which triggers the inflammatory mechanisms that cause atherosclerosis.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Silvia Bleda, Joaquin de Haro, Isabel Sánchez, Ilsem Laime, Francisco Acin. Efecto del clopidogrel vs. aspirina en la función de la expresión del inflamasoma proaterosclerótico NLRP1 en células endoteliales. Estudio ECLOAS. Clin Investig Arterioscler. 2020;32:193–199.